Case Report
Volume 3 Issue 1 - 2019
Tumoral Calcinosis in a 19 Year Old Male: A Case Report and Review of Literature
1Lecturer Trauma and Orthopedics, Faculty of Medicine Cairo University; Clinical Fellow Trauma and Orthopaedics, Royal London Hospital
2Professor of Trauma and Orthopedics, Faculty of Medicine Cairo University
3Assistant Lecturer of Trauma an Orthopedics Faculty of Medicine Cairo University
4Resident of Trauma and Orthopedics, Faculty of Medicine Cairo University
2Professor of Trauma and Orthopedics, Faculty of Medicine Cairo University
3Assistant Lecturer of Trauma an Orthopedics Faculty of Medicine Cairo University
4Resident of Trauma and Orthopedics, Faculty of Medicine Cairo University
*Corresponding Author: Begad Hesham Mostafa Zaky Abdelrazek, Lecturer Trauma and Orthopedics, Faculty of Medicine Cairo University; Clinical Fellow Trauma and Orthopaedics, Royal London Hospital.
Received: May 29, 2019; Published: June 11, 2019
Abstract
Introduction:Tumoral calcinosis is a rare condition characterized by periarticular calcific deposits. Patients typically demonstrate normocalcemia and hyperphosphatemia.
Patients and Methods: We present a case of multiple joint tumoral calcinosis in a 19 year old male. The condition started in the left elbow after a traumatic event 9 years ago, managed successfully with surgical resection. Eight years later the patient developed slowly growing masses in both hips and the right elbow.
He presented to our institute with limitation of movement and impairment in activity of daily living (ADL). Imaging and metabolic profile reached the diagnosis of hyperphosphatemic tumoral calcinosis (HP TC). Medical treatment was commenced to which he was non-compliant. Surgical resection of the mass from the left hip was performed through a posterior approach.
Results: Histopathology confirmed the diagnosis. Post-operatively pain and range of motion (ROM) of the left hip was markedly improved. Medical treatment was continued for 6 months.
Key-Words: Calcium; Hyperphosphatemia; FGF23; Peri-articular; Tumoral; Idiopathic
Introduction
It is a rare condition, more commonly seen in young males of African descent [1]. It usually appears in age less than 20 years [2]. Historically tumoral calcinosis (TC) was known as Lipocalcinoma granuloma [3]. The term used today was introduced in the early 1940s and was used to describe calcium carbonate deposition. It was first described by Inclan who distinguished TC from metastatic and dystrophic calcifications and established hyperphosphatemia is part of the disease process [4]. It wasn't until later that calcium phosphate and pyrophosphate were identified from the deposits [5].
Pattern of inheritance is controversial, mainly identified to be autosomal dominant where different genetic mutations have been implicated. Recently, light is being shed on autosomal recessive post-translation mutations in N-acetyl galactoseaminyltransferase-3 (GALNT3) [6] and fibroblast growth factor receptor-23 (FGF23) [7] genes.
Metabolic studies showed increased 1, 25 (OH)2 and phosphate levels, typically with normal calcium and parathormone levels [8,9]. The increase phosphate is expected to cause hypocalcemia, which is not a finding in TC. This could be explained by up regulated renal calcium resorption from the distal convoluted tubules under the effect of increased phosphate. The high phosphate levels form calcium phosphate complexes that deposit in the soft tissues [10].
Despite the peculiar metabolic and histologic characteristics of TC, the term is still frequently used as a misnomer by physicians to describe any mass of calcification [11]. The condition originally described by Incan became known as primary tumoral calcinosis, whilst secondary tumoral calcinosis was attributable to an underlying condition as renal failure or hyperparathyroidism.
Pathogenesis of primary tumoral calcinosis is not yet proven. Different theories were put forward; (a) Repetitive micro-trauma and reparative dysfunction, (b) Periarticular forces, histiocytic aggregates, (c) Hemorrhage from micro-trauma inducing a reparative response [12]. Inclan highlighted hyperphosphatemia as a peculiar metabolic characteristic of the condition [4]. However, several reviews showed that hyperphosphatemia occurs in only a few of patients.
Smack., et al. introduced a new classification which stratifies TC into subgroups based on pathogenesis and phosphate levels; (a) primary normo-phosphatemic tumoral calcinosis (NP TC), (b) primary hyperphosphatemic (HP) and (c) secondary tumoral calcinosis [13]. HP TC is associated with the autosomal inherited post-translation mutations of FGF-23 gene (encoding phosphaturic protein) which normally decreases phosphate reabsorption and 1, 25 (OH)2 synthesis in renal proximal convoluted tubules [7,14] and GLANT3 gene [15]. A case report by Roberts MS., et al. presented what the authors claim is the first autoimmune HP TC with early onset in boy less than 10 years old. High levels of FGF-23 levels along with anti-FGF-23 autoantibodies were detected in the patient's serum [16].
Recently, HP TC is considered as a variant of Hyperostosis Hyperphosphatemia syndrome, having the same genotype and similar metabolic imbalances [17]. NP TC lacks metabolic imbalance and is found to be associated with non-functional/inactive SAMD9; a tumor suppressor and anti-inflammatory protein [18].
TC more commonly affects the hips and the elbows and is rare in the hands and the knee [2]. Masses are essentially lobular painless and firm to hard, usually in close relation to extensor tendons and demonstrate high rate of local recurrence if inadequately removed [4]. Grossly they are cystic, with yellowish chalky content. Microscopically, consists central calcium granule deposits with surrounding multinucleated giant cells and epithelioid cells [19].
Radiologically, TC has characteristic features, yet it may closely mimic appearance of other diagnoses. On Plain radiographs, it can be seen as multi-lobed cysts around joints. It is better evaluated in axial cuts of a CT scan which may show sedimentation sign due to calcium deposition in layers and hence fluid levels [20]. Less active slowly growing masses are more homogenous. Usually lacks osseous invasion. Typically demonstrates a heterogeneous high signal intensity on T2-weighted MRI, with focal areas of signal drop-out owing to calcific deposits and low signal intensity in T1 pulse sequence. Bone scan usually shows an increased uptake [21].
Management of tumoral calcinosis may be difficult, partly due to high incidence of recurrences, particularly with inadequate resection of poorly defined lesions [22-24] but also because some lesions may be encased with a fibrous layer impermeable to ions, rendering them resistant to phosphate restriction treatment [12]. A combination of surgical resection and anti-hyperphosphatemic treatment yields the best results [25, 26]. Recently ultrasound guided percutaneous fragmentation and aspiration has demonstrated significant symptomatic improvement [27].
We present a case of idiopathic HP TC that presented to and was managed at our institute. An informed consent and approval for publication were obtained from the patient prior to submission.
Patients and Methods
A 19 year old male presented to our outpatients in June 2018 with pain and limitation of movement in right elbow and both hip joints. The condition started 9 years ago at the age of 10 years; 2 months after a traumatic injury to the left elbow, which was managed non-operatively in a cast. The mass in the left elbow was resected successfully at another medical center with no recurrence. Two years ago he started to complain of pain and swelling in the right elbow, and both hips. He reports that the masses were slowly growing in size, which has started to cause limping and interferes with activity of daily living (ADLs). He works as a carpenter, right hand dominant, had to quit work because of his pain.
Physical examination of the hip demonstrated bilateral limitation of hip movement more marked on the left side. Left hip range of motion (ROM) 0° adduction (ADD), 40° abduction (ABD), 80° flexion (F), 30° internal rotation (IR) and 10° external rotation (ER). Right hip ROM: ADD 10°, ABD 60°, F 110°, IR 40°, ER 20°. Trendelenburg's test was positive bilaterally and waddling wide-based gait was noted. Ober's test denoting ilio-tibial band tightness was positive on the left side. No neurovascular compromise was identified.
Physical examination of the right elbow revealed a firm mass causing limitation in extension ROM, with loss of 10° of terminal extension. There was however, no evidence of neurovascular compromise. No red flags were identified, no symptoms suggestive of infective or malignant process. No history or signs of renal disease or hyperparathyroidism as an underlying cause of secondary calcific deposits.
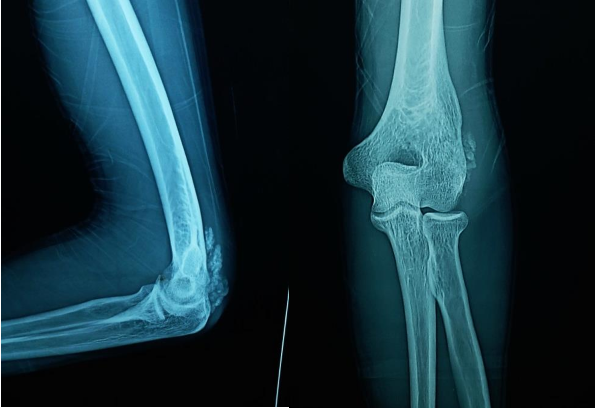
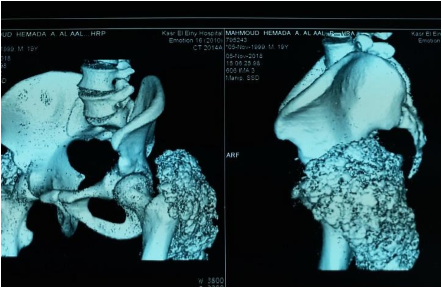
Investigations in the form of imaging and blood tests were done. Plain radiographs pelvis anteroposterior (AP) view (Figure 1a), elbow AP and lateral (Figure 1b) views showed periarticular fluffy calcific deposits. Computed Tomography (CT) scan of the pelvis and both hips (Figure 2a) with 3D reconstruction (Figure 2b) revealed the characteristic sedimentation sign of heterogeneous cystic multi-lobed masses lacking osseous erosion. The lesion appeared to be larger on the left side as compared to the right. Metabolic profile was remarkable for Hyperphosphatemia (5.9; Normal level according to lab reference: 2.5-4.9), calcium, Parathormone were however within normal range.
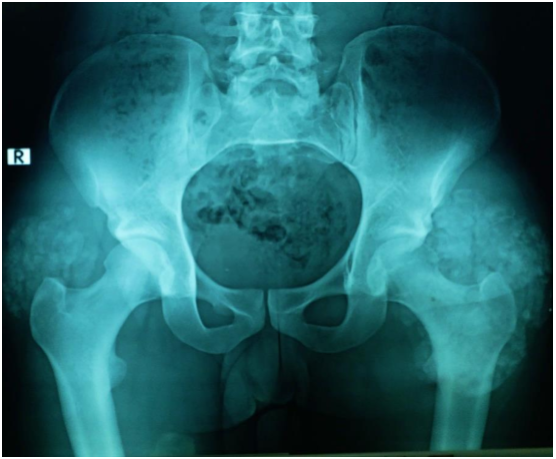
Figure 1a: AP view of the pelvis showing calcific deposits in both hips, greater in size in the left hip.
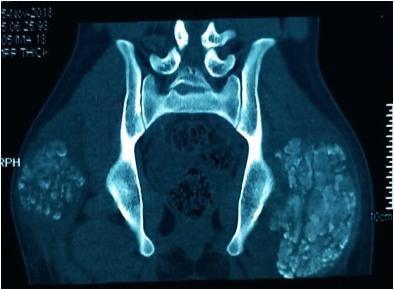
Figure 2a: Coronal CT cuts of the pelvis and both hip joints showing characteristic sedimentation sign.
Based on the history, physical findings, metabolic profile and imaging studies; a diagnosis of idiopathic HP TC was made. The patient was started on antacid (mucogel), which acts as a phosphate lowering agent and phosphate restricting diet was advised. The patient was not strictly compliant to medical treatment. After obtaining an informed written consent, surgical resection of mass from the hip was planned, starting with the more symptomatic left side and larger mass.
The procedure was performed under general anesthesia in the lateral decubitus. A posterior approach was planned. Dissection was carefully performed deeply. The sciatic nerve was identified, dissected from distal to proximal and protected. A chalky white yellowish cystic mass was seen (Figure 3). The mass was found deep to the gluteus Maximus (GM) and had a thin capsule attached to the muscle and the trochanteric bursa. For complete visualization of the extent of the lesion; the insertion of the GM was partially divided at the gluteal tuberosity. The mass was dissected from the surrounding healthy tissue and resected en bloc, measured around 15 cm (Figure 4). A drain was secured and the wound was closed in layers.
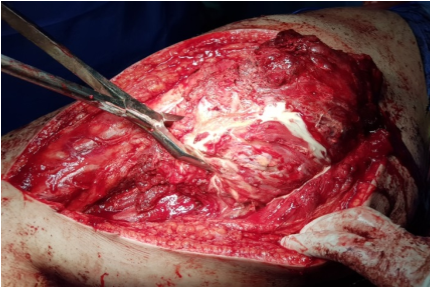
Figure 3: Chalky white yellowish mass identified intraoperative, with attachment to GM and trochanteric bursa.
The mass was sent for culture and sensitivity and histopathological examination. Culture and sensitivity showed no growth, while histopathology revealed multiple lobules of calcific material surrounded by histiocytic giant cells containing few small psammomatous calcifications; confirming diagnosis of TC (Figure 5). Post-operatively plain radiographs were obtained in the AP and lateral views to confirm complete resection of the mass. Phosphate levels were obtained and did not show a drop from pre-operative levels. The patient was instructed to fully weight bear and perform full ROM hip activities as comfort and pain allows. More strict compliance to dietary restrictions and medications was advised. Pain in the left hip dropped significantly post-operatively. The ROM of the left hip also showed marked improvement from pre-operative range.
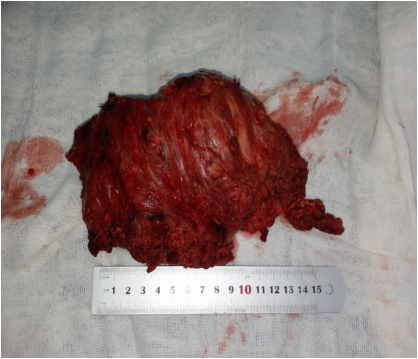
Figure 5: Microscopic appearance of the lesion showing multinucleated giant cells and psammomatous calcifications.
Discussion
Tumoral calcinosis is essentially an idiopathic diagnosis, many theories were put forward explaining the underlying pathogenesis. Trauma is not typically a known predisposing factor. Early onset TC was reported with autoimmune pathogenesis [16]. TC may occur secondary to chronic kidney disease, primary hyperparathyroidism, or milk-alkali syndrome. In our patient trauma was the exciting factor. It is hence, considered a diagnosis of exclusion. Masses have been described as painless. [28], however this patient's main complaint and reason to seek medical care was unrelenting pain. Imaging studies have typical features, usually plain radiographs and CT scan are sufficient. Magnetic resonance and bone scan are only undertaken for atypical cases or for academic purposes.
Being a rare condition, we don’t have enough literature based on clinical trials to study, compare and report outcomes of different management options. Furthermore, compliance of patients to medical treatment is individualized and poses a bias to accuracy of reporting success of medical treatment. The variability in terms of size of the lesions, symptoms and metabolic status in a narrow studied population of individuals reported, renders management of each case individualized based on those variables [29].
The discrepancy between the concerns of the patients and surgeons poses another challenge to treatment of TC. While surgeons are concerned about phosphate levels and metabolic imbalance; patients are concerned about their symptoms of limping, discomfort or even pain, this may partly explain poor compliance to medical treatment [30].
Efficacy of medical treatment in isolation is still questionable. Antacids and phosphate restrictive diet, lower the phosphate absorption. If not successful, some propose long-term carbonic anhydrase inhibitor to stimulate phosphate excretion. [25] Others have recently thrown light on the use of sodium thiosulphate to shrink the lesions [31].
In the case report of Mirian C.H. Janssen [1] medical treatment using antacids and phosphate restrictive diet was successful to shrink the mass 8 cm within 2 years. Evolving novel theories behind the pathogenesis, have led to emerging medical remedies for the condition. Based on a case report of autoimmune early onset HP TC, the authors believe immunomodulatory therapy may play a role in management.
Although recurrence after surgical resection is not uncommon; in our patient complete resection of his elbow lesion was effective with no recurrence. This coupled by the fact he was non-compliant to medical treatment, encouraged us to opt for surgical excision of the left hip mass. In multiple joints affection, a sound rationale would be to start with most affected joint in terms of lesion size and magnitude of symptoms.
A well-planned surgical approach offering adequate exposure, proper identification of the extents of the mass and any soft tissue attachment; allows sufficient marginal resection. Some may rely on obtaining intra-operative images to ensure adequate removal, we think the lesion may have soft tissue attachments and extensions beyond what the visible radio-opaque mass seen on X-rays. Neurovascular structures in close proximity to the lesion, must be identified, dissected and protected throughout the operative field prior to removal of the lesion.
Conclusion
Tumoral calcinosis is a rare condition, whose pathogenesis is still not clearly understood, despite the several theories put forward to describe the disease process. Lesions commonly affect young men and may occur in multiple joints. Metabolic imbalance remains the baseline abnormality in the majority of cases. Medical treatment may help lower phosphate levels and potentially shrink the size of lesions. However, surgical resection if properly planned and performed is a sound safe treatment modality that usually provides cure.
References
- Janssen MC and de Sévaux RG. “Tumoral calcinosis”. Journal of Inherited Metabolic Disease 33.1 (2010): 91-92.
- Fathi I and Sakr M. “Review of tumoral calcinosis: A rare clinico-pathological entity”. World Journal of Clinical Cases 2 (2014): 409–414.
- Duret M. “Tumeurs Multiples et Singulieres des bourses Sereuses”. Bull Mem Soc Anat Paris 74 (1899): 725-773.
- Inclan A., et al. “Tumoral calcinosis”. Journal of the American Medical Association 121.7 (1943): 490-495.
- Harkess James W and Hans J Peters. "Tumoral calcinosis: a report of six cases." JBJS 49.4 (1967): 721-731.
- Topaz O., et al. “Mutations in GALNT3, encoding protein involved in O-linked glycosylation, cause familial tumoral calcinosis”. Nat Genet 36.6 (2004): 579-581.
- Benet-Pages A., et al. “An FGF23missense mutation causes familial tumoral calcinosis with hyperphosphatemia”. Hum Mol Genet 14 (2005): 385-390.
- Mitnick PD., et al. “Calcium and phosphate metabolism in tumoral calcinosis”. Annals of internal medicine 92.4 (1980): 482-7.
- Zerwekh JE., et al. “Tumoral calcinosis: Evidence for concurrent defects in renal tubular phosphorus transport and in 1α, 25-dihydroxycholecalciferol synthesis”. Calcified tissue international 32.1 (1980): 1-6.
- Janssen MC and De Sévaux RG. “Tumoral calcinosis”. Journal of inherited metabolic disease 33.1 (2010): 91-2.
- Olsen KM and Chew FS. “Tumoral calcinosis: pearls, polemics, and alternative possibilities”. Radiographics 26.3 (2006): 871-85.
- Slavin RE., et al. “Familial tumoral calcinosis. A clinical, histopathologic, and ultrastructural study with an analysis of its calcifying process and pathogenesis”. The American journal of surgical pathology 17.8 (1993): 788-802.
- Smack D., et al. “Proposal for pathogenesis-based classification of tumoral calcinosis”. Int J Dermatol 35.4 (1996): 265-271.
- Bacchetta J and Salusky IB. “Evaluation of hypophosphatemia: lessons from patients with genetic disorders”. Am J Kidney Dis 59 (2012): 152–159.
- Specktor P., et al. “Hyperphosphatemic familial tumoral calcinosis caused by a mutation in GALNT3 in a European kindred”. Journal of human genetics 51.5 (2006): 487-90.
- Roberts MS., et al. “Autoimmune hyperphosphatemic tumoral calcinosis in a patient with FGF23 autoantibodies”. The Journal of clinical investigation 128.12 (2018): 5368-5373.
- Joseph L., et al. “Familial tumoral calcinosis and hyperostosis–hyperphosphataemia syndrome are different manifestations of the same disease: novel missense mutations in GALNT3”. Skeletal radiology 39.1 (2010): 63.
- Sprecher E. “Familial tumoral calcinosis: from characterization of a rare phenotype to the pathogenesis of ectopic calcification”. Journal of Investigative Dermatology 130.3 (2010): 652-60.
- Chew FS and Crenshaw WB. “Idiopathic tumoral calcinosis”. AJR Am J Roentgenol 158 (1992): 330.
- Hug I and Guncaga J. “Tumoral calcinosis with sedimentation sign”. The British journal of radiology 47.562 (1974): 734-6.
- Martinez S., et al. “Imaging of tumoral calcinosis: new observations”. Radiology 174 (1990): 215-222.
- Kirk TS and Simon MA. “Tumoral calcinosis. Report of a case with successful medical management”. Journal of Bone and Joint Surgery 63.7 (1981): 1167-9.
- Mozaffarian G., et al. “Treatment of tumoral calcinosis with phosphorus deprivation”. Annals of internal medicine 77.5 (1972): 741-5.
- Davies M., et al. “Tumoral Calcinosis: Clinical and Metabolic Response to Phosphorus Deprivation”. QJM: An International Journal of Medicine 63.3 (1987): 493-503.
- Yamaguchi T., et al. “Successful treatment of hyperphosphatemic tumoral calcinosis with long-term acetazolamide”. Bone 16.4 (1995): S247-50.
- Lufkin EG., et al. “Phosphorus excretion in tumoral calcinosis: response to parathyroid hormone and acetazolamide”. The Journal of Clinical Endocrinology & Metabolism 50.4 (1980): 648-53.
- Aina R., et al. “Calcific shoulder tendinitis: treatment with modified US-guided fine-needle technique”. Radiology 221.2 (2001): 455-61.
- McClatchie S and Bremner AD. “Tumoral calcinosis—an unrecognized disease”. Br Med J 1.5637 (1969): 142-55.
- Claramunt-Taberner D., et al. “Hyperphosphatemic tumoral calcinosis caused by FGF23 compound heterozygous mutations: what are the therapeutic options for a better control of phosphatemia?” Pediatric Nephrology 33.7 (2018): 1-5.
- Shroff R., et al. “Mechanistic insights into vascular calcification in CKD”. J Am Soc Nephrol 24 (2013): 179-189.
- Jost J., et al. “Topical sodium thiosulfate: a treatment for calcifications in hyperphosphatemic familial tumoral calcinosis?” J Clin Endocrinol Metab 101.7 (2016): 2810–2815.
Citation: Begad Hesham Mostafa Zaky Abdelrazek., et al. “Tumoral Calcinosis in a 19 Year Old Male: A Case Report and Review of Literature”. Orthopaedic
Surgery and Traumatology 3.1 (2019): 4-11.
Copyright: © 2019 Begad Hesham Mostafa Zaky Abdelrazek., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.