Research Article
Volume 1 Issue 6 - 2017
Effects of Different Photobiomodulation Dosimetries on Temporomandibular Dysfunction: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
1Graduation Student, Physiotherapy Course, Lutheran University of Brazil, Torres/RS, Brazil
2Physiotherapist, Torres/RS, Brazil
3Dental Surgeon, Professor, Master, Dentistry Course, Lutheran University of Brazil, Torres/RS, Brazil
4Physiotherapist, Professor, Master, Physiotherapy Course, Lutheran University of Brazil, Torres/RS, Brazil
5Physiotherapist, Professor, Doctor, Physiotherapy Course, Lutheran University of Brazil, Torres/RS, Brazil
2Physiotherapist, Torres/RS, Brazil
3Dental Surgeon, Professor, Master, Dentistry Course, Lutheran University of Brazil, Torres/RS, Brazil
4Physiotherapist, Professor, Master, Physiotherapy Course, Lutheran University of Brazil, Torres/RS, Brazil
5Physiotherapist, Professor, Doctor, Physiotherapy Course, Lutheran University of Brazil, Torres/RS, Brazil
*Corresponding Author: Marcelo Baptista Dohnert, Physiotherapist, Professor, Doctor, Physiotherapy Course, Lutheran University of Brazil, Torres/RS, Brazil.
Received: December 22, 2017; Published: December 27, 2017
Abstract
Introduction: changes involving temporomandibular joint, masticatory musculature and associated structures characterize temporomandibular dysfunction (TMD). The analgesic and anti-inflammatory effect produced by photobiomodulation has contributed to pain relief and functional improvement. However, the parameters to be used have not yet been well established.
Aim: To compare the efficacy of three different photobiomodulation dosimetries in the treatment of patients with TMD.
Methods: A randomized, double-blind, placebo-controlled clinical trial with 44 subjects divided into the groups 8 J/cm² (n = 11), 60 J/cm² (n = 11), 105 J/cm² (n = 11), and control (n = 11). Pain, symptom severity, and joint mobility were evaluated before and after a 10-session protocol of photobiomodulation with AlGaAs laser (830 nm), at a power density of 30 mW/cm².
Results: the mouth opening increased in the 8J/cm2 group from 10.49 ± 4.68 to 15.40 ± 6.43 degrees, and in the right protrusion from 9.80 ± 4.2 to 12.56 ± 5.40 degrees after the intervention protocol (p < 0.05). All groups significantly decreased pain (p < 0.05).
Conclusion: 830-nm laser photobiomodulation was effective in reducing TMD pain and symptoms at all doses tested. Only the doses of 8 J/cm2 were effective regarding maximal opening and protrusion of the mandible.
Keywords: Lasers; Physical Therapy Modalities; Low-Level Light Therapy; Temporomandibular Joint Disorders; Temporomandibular
Joint Dysfunction Syndrome
Abbreviations: TMD: Temporomandibular Dysfunction; TMJ: Temporomandibular Joint; VAS: Visual Analogue Scale
Introduction
Temporomandibular dysfunction (TMD) is the term used to characterize a set of abnormalities involving temporomandibular joint (TMJ), masticatory muscles and associated structures [1-3]. The proper functioning and normal range of TMJ movements are extremely important for the performance of vital functions such as chewing, swallowing, sucking, breathing, and speech [4].
When there is an imbalance in this system, a wide range of clinical problems arise. These musculoskeletal disorders develop very characteristic signs and symptoms, the main one being muscle and/or joint pain, which implies the limitation of mandibular movements [5,6]. In addition to these, other symptoms commonly appear, which may or may not be concomitant, such as cephalea, cervical pain, joint noise, muscle fatigue, dizziness, hearing loss, and tinnitus [1,5].
The causes for the onset of TMD are associated with multiple factors, including the presence of parafunctional habits, occlusal factors, inadequate postures, local traumas, and Biopsychosocial aspects such as stress, anxiety or depression [1,3,4,7,8].
Laser photobiomodulation has become a popular option in the treatment of musculoskeletal syndromes due to its analgesic, anti-inflammatory and regenerative action [1,9,10]. Moreover, this therapy has the great advantage of being of low cost and non-invasive [3,11].
Several studies have demonstrated the efficacy of laser in the treatment of TMD, mainly related to the immediate relief of pain after application and recovery of function [2,5,7,10-13]. Notwithstanding, the lack of consensus on which dosimetry to use is evident, and there are controversies in the results between studies that used different parameters [1,5,7,14].
In view of the above, this study aims to compare the efficacy of three different photobiomodulation dosimetries in the treatment of patients with temporomandibular dysfunction.
Material and Methods
This is a randomized, double-blind, placebo-controlled clinical trial. The study was approved by the Ethics Committee of the Lutheran University of Brazil, under number 2011-175H. Participants who met the eligibility criteria were informed about the procedures and signed the Free and Informed Consent Form.
Thirty-four subjects of both sexes with a clinical diagnosis of TMD participated in the study. They were referred by dental surgeons from the public and private services of the municipality of Três Cachoeiras/RS. Subjects with medication to control pain, with contraindications for laser therapy, such as suspicion of infections and/or tumors, and patients using orthodontic appliance or total dental prosthesis were excluded. The subjects were randomly assigned into 8 J/cm2 group, 60 J/cm2 group, 105 J/cm2 group, and placebo group. The evaluations were performed at two different time points, initially and soon after the end of the treatment protocol. Data collection was performed by a collaborating researcher, previously trained with the evaluation instruments and not knowledgeable of the group to which the subject belonged (Figure 1).
The Visual Analogue Scale (VAS) was used for measuring pain. Symptom severity was quantified by the Anamnestic Questionnaire of Fonseca., et al. [15], which allows classification as without TMD, mild TMD, moderate TMD, and severe TMD.
TMJ mobility was assessed through computerized biophotogrammetry. 13-mm spherical surface markers were used in the glabella, one centimeter below the anatomical process of the anterior nasal spine, in the mental protuberance, body and portion of the condylar process of the mandible. These points were used to guide joint angle measurements, quantified through the program Corel Draw X7® (Figure 2).
| Movement | Image Plan | Anatomical points | Measured angle |
| Opening | Lateral | Condyle of the mandible and angle of the mandible | Condyle of the mandible x mental protrusion x orthogonal axis "X" |
| Lateralization | Frontal | 1 cm below the anatomical process of the anterior nasal spine and mental protuberance | Nasal spine x mental protrusion x orthogonal axis "X" |
| Mandibular Protrusion | Lateral | Anterior nasal spine line and mental protrusion | Anterior nasal spine x protuberance x orthogonal plane "Y" |
| Mandibular Retraction | Lateral | Anterior nasal spine line and mental protrusion | Anterior nasal spine x protuberance x orthogonal plane "Y" |
Table 1: Anatomical points used for the angular analysis of the movement.
The images were taken by a 12.1-megapixel digital camera (brand Canon® Power Shot sx40 SH) with auto-zoom. The camera-object distance was fixed at 1.60 meters and leveled on a tripod 1 meter above the ground. The images were obtained simultaneously in the anterior frontal, right and left lateral planes. Movements of maximal mouth opening and occlusion, right and left lateralization of the mandible, and mandibular protrusion and retraction were recorded.
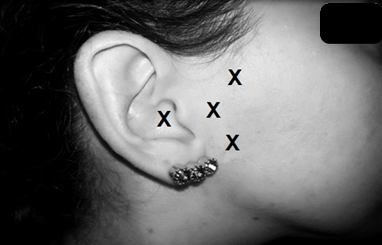
The photobiomodulation intervention protocol was performed three times a week, totaling 10 sessions, with three levels of energy density. The equipment used was a low-level aluminum gallium arsenide (AlGaAs) laser (brand Ibramed®, model Laserpulse Diamond Line) previously calibrated, with a wavelength of 830 nanometers (nm), power of 30 mW/cm2, and contact area of 0.01160 cm2 (Table 2). The placebo group received the application of laser therapy with the equipment turned on, but with zero intensity for 15 seconds at each point. In all groups, photobiomodulation was performed punctually and in contact with the surface, perpendicular to the skin, bilaterally. Four application points were used for each temporomandibular joint. The application points were in the preauricular region and in the external acoustic meatus (Figure 3).
| Energy density of 8 J/cm² per point | Energy density of 60 J/cm² per point | Energy density of 105 J/cm² per point | |
| Wavelength | 830 nm | 830 nm | 830 nm |
| Radiation energy per point | 0.96 J | 7.2 J | 12.64 J |
| Power Equipment | 30 mW | 30 mW | 30 mW |
| Mode of emission | Continuous | Continuous | Continuous |
| Spot size | 0.11600 cm2 | 0.11600 cm2 | 0.11600 cm2 |
| Power Density | 2.59 W/cm² | 2.59 W/cm² | 2.59 W/cm² |
| Total irradiated points | Eight (four for each side) | Eight (four for each side) | Eight (four for each side) |
| Frequency of irradiation | 3 x week/10 sessions | 3 x week/10 sessions | 3 x week/10 sessions |
| Total irradiation time per point (sec) | 32 seconds | 240 seconds | 420 seconds |
| Total energy density per session | 64 J/cm² | 480 J/cm² | 840 J/cm² |
| Total radiation energy per session | 7.68 J | 57.6 J | 101.12 J |
| Total irradiation time per session (sec) | 266 seconds | 1,920 seconds | 3,360 seconds |
| Total energy density accumulated in 10 sessions | 640 J/cm² | 4,800 J/cm² | 8,400 J/cm² |
| Total cumulative radiation energy in 10 sessions | 76.8 J | 576 J | 1011.2 J |
| Total irradiation time in 10 sessions | 2,586 seconds | 19,200 seconds | 33,600 seconds |
Sec = seconds.
Table 2: Parameters used in different groups of photobiomodulation.
Table 2: Parameters used in different groups of photobiomodulation.
The SPSS (Statistical Package for the Social Sciences) software, version 22.0, was used to analyze the results. Initially, a descriptive analysis of the variables, expressed as absolute number, mean and standard deviation was performed. The study groups were statistically analyzed by the Student's t-test for parametric intragroup analyses. ANOVA was used for intergroup analyses, followed by the Tukey post hoc test. For those data that did not present a normal distribution, the Wilcoxon test was used for intragroup analysis and the Kruskal-Wallis test for analysis between groups. The level of significance established for the statistical test is p < 0.05.
Results and Discussion
Thirty-four subjects participated in the study, of which 90.9% were female. The age was 31.9 ± 12.9 years (ranging from 15 to 59 years). 95.4% were white. The mean time of pain was 77.2 ± 68.7 months. Temporomandibular dysfunction was bilateral in 65.9% of the subjects. The groups were homogeneous regarding age, gender, time of pain, occupation, and affected TMJ (Table 3).
The prevalence of the female sex observed in this study corroborates the literature, since it has been described that women present greater TMD symptomatology than men [16-18].
| Group | |||||
| Variable | 8 J/cm² (n = 11) | 60 J/cm² (n = 11) | 105 J/cm² (n = 11) | Placebo (n = 11) | p value |
| Age, years (n ± sd) # | 35.82 ± 13.77 | 27.73 ± 9.75 | 34.82 ±15.28 | 29.45 ± 12.45 | 0.42 |
| Gender, M/F& | 0/11 | 1/10 | 2/9 | 1/10 | 0.53 |
| Skin color, n (%)& | 0.55 | ||||
| White | 10 (90.9) | 11 (100) | 11 (100) | 10 (90.9) | |
| Black | 1 (9.1) | 0 (0) | 0 (0) | 1 (9.1) | |
| Time of pain, months (n ± sd) # | 38.59 ± 75.75 | 14.00 ± 21.65 | 14.00 ± 91.60 | 7.55 ± 6.56 | 0.42 |
| Occupation, (n ± sd) & | 0.39 | ||||
| Diarist | 2 (18.2) | 0 (0) | 0 (0) | 0 (0) | |
| Housewife | 2 (18.2) | 2 (18.2) | 2 (18.2) | 0 (0) | |
| Student | 1 (9.1) | 2 (18.2) | 2 (18.2) | 5 (45.5) | |
| Vendor | 0 (0) | 2 (18.2) | 0 (0) | 0 (0) | |
| Other | 6 (55.5) | 5 (45.4) | 7 (63.6) | 6 (55.5) | |
| Affected TMJ, n (%)& | 0.82 | ||||
| Right | 1 (9.1) | 2 (18.2) | 1 (9.1) | 1 (9.1) | |
| Left | 1 (9.1) | 1 (9.1) | 0 (0) | 0 (0) | |
| Bilateral | 9 (81.8) | 8 (72.7) | 10 (90.9) | 10 (90.9) | |
#One-way ANOVA & Chi-square test.
Table 3: Characterization of the initial study sample (n = 44).
Table 3: Characterization of the initial study sample (n = 44).
All intervention groups, including the placebo group, demonstrated a significant reduction of pain in the VAS from pre- to post-intervention, with no differences between groups. In the 8 J/cm2 group, the initial average was 6.45 ± 2.50, decreasing to 1.88 ± 1.64 in the final evaluation (p = 0.00). In the 60 J/cm2 group, the initial pain score was 6.11 ± 2.22, decreasing to 2.70 ± 2.00 (p = 0.00). In the 105 J/cm2 group, the initial pain level was 4.91 ± 1.51, decreasing to 2.09 ± 1.97 at the end of the study (p = 0.00). Finally, in the placebo group, the initial VAS value was 5.55 ± 2.06, decreasing to 3.70 ± 2.11 at the end of the study (p = 0.01) (Figure 3).
A literature review conducted by Aparicio., et al. demonstrated a placebo effect of photobiomodulation on pain in 40% of the reviewed studies [1]. The authors believe that TMD patients are susceptible to placebo effects because of the psychological component involved, and that the desire to feel better seems to influence physiological processes, thus achieving a favorable outcome [1]. Likewise, Shukla and Muthusekar, in their systematic review, found that of the 13 selected studies, seven demonstrated superiority of laser therapy over the placebo effect, while the other six did not demonstrate significant differences between the intervention and placebo groups in relation to pain [19]. Magri., et al. verified the efficacy of laser therapy on pain intensity by VAS in 61 women with myofascial pain, using as a therapy a laser with 780-nm wavelength, continuous emission mode, energy density of 5 J/cm2 at three points in the masseter muscle, and 7.5 J/cm2 at three other points in the anterior temporalis muscle, totaling eight sessions [20]. There was a reduction in pain in both groups, with no differences between the laser therapy and the placebo group [20]. Unlike these results, Mazzeto., et al. evaluated the pain symptoms and mandibular movements of 40 patients with TMD, using parameters similar to those in this study (AlGaAs laser, 830nm, continuous mode at five points around TMJ, power of 40mW, and energy density of 5 J/cm2 per point) [21]. Significant improvements in painful symptoms were observed by VAS only in the group receiving photobiomodulation, whereas the placebo group did not present significant results [21].
The mechanism of action of photobiomodulation is not yet fully understood [22,23]. It is known that photobiomodulation can influence the synthesis and release of several substances involved in analgesia [24]. The theories report that there is a release of endogenous opioids, increased urinary excretion of glucocorticoids, increased ATP production, stimulation of local microcirculation, and decreased cell hypoxia [1,21]. Other authors further affirm increased level of beta-endorphins, reduced bradykinin expression, and release of histamine associated with increased lymphatic flow and blood circulation, controlling the inflammation process and inducing muscle relaxation [2,5]. Freitas and Hamblim described that the effects of photobiomodulation are mainly due to increased oxidative metabolism in mitochondria [23]. One of the most important chromophores is the enzyme cytochrome c oxidase, which absorbs light in the region close to the infrared spectrum [23]. The main hypothesis is that photons dissociate inhibitory nitric oxide from this enzyme, leading to an increase in electron transport and ATP production [23]. Another hypothesis is the activation of light-sensitive ion channels, which allow calcium to enter the cell, triggering signaling pathways through reactive oxygen species (ROS), cyclic AMP, nitric oxide and Ca2+, leading to activation of transcription factors, which may increase the expression of genes related to protein synthesis, cell migration and proliferation, anti-inflammatory signaling, anti-apoptotic proteins, and antioxidant enzymes [23].
Regarding the severity of TMD symptoms, evaluated through the Fonseca questionnaire, the results found were similar to the pain level responses by VAS. A significant decrease was observed in all groups from pre- to post-intervention, including the placebo group. The 8 J/cm2 group had an initial mean score of 80.63 ± 14.25 points, and 31.25 ± 31.36 points (p = 0.001) at the end. The 60 J/cm2 group showed an initial score of 71.67 ± 12.74 points, and a final score of 31.25 ± 19.22 points (p = 0.000). The values of the 105 J/cm2 group decreased from 63.89 ± 19.32 points to 27.22 ± 23.06 points (p = 0.000). The placebo group obtained an initial score of 62.78 ± 26.47 points, and a final score of 34.44 ± 18.78 points (p = 0.012) (Figure 4).
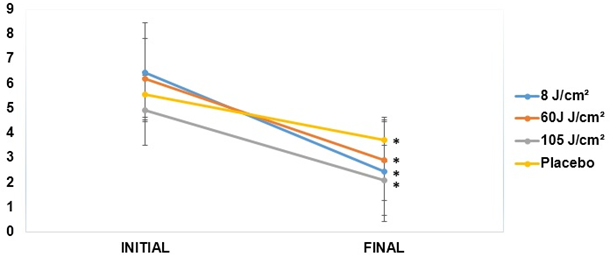
*p < 0.05 relative to the initial evaluation of the same group. Student's t-test.
Figure 3: Analysis of joint pain in the initial and final TMJ assessed by VAS.
Figure 3: Analysis of joint pain in the initial and final TMJ assessed by VAS.
The use of functional questionnaires to assess TMD is poorly described in the literature [2,15]. The Fonseca questionnaire was developed according to the Helkimo’s anamnestic index and is one of the few instruments available in Portuguese to characterize the severity of TMD symptoms [15]. There are still few studies that use these questionnaires or functional scales to compare results before and after photobiomodulation, as was done in this study. They end up being more used for diagnostic purposes, with the aim of presenting the most prevalent symptoms [16].
In the assessment of temporomandibular joint mobility, evaluated by biophotogrammetry, the subjects in the 8 J/cm2 group demonstrated a significantly lower ADM than those of the 105 J/cm2 group on both sides, and placebo group on the right side (p < 0.05). However, at the end of the study, all groups showed similar results (Table 4). In the retraction movement on the right side, the initial mean of the 8 J/cm2 group was significantly higher than that of the 60 J/cm2 and placebo groups (p < 0.05) (Table 4).
The 8 J/cm2 group was the only one that demonstrated a significant increase in the maximal mouth opening movement bilaterally from pre- to post-intervention (p < 0.05) (Figure 5). This result was also observed in the protrusion movement on the right side (p < 0.05) (Table 4).
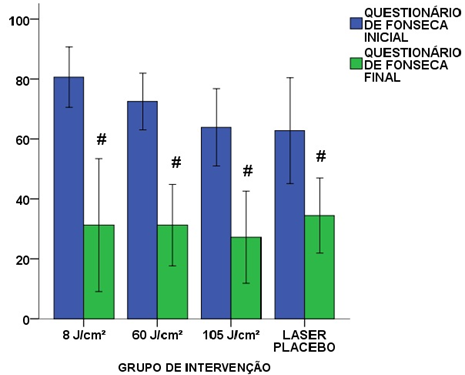
#p < 0.05 relative to the initial evaluation of the same group. Student’s t-test.
Figure 4: Results of the initial and final Fonseca questionnaire in the different study groups.
Figure 4: Results of the initial and final Fonseca questionnaire in the different study groups.
Catão., et al. used an AlGaAs laser with wavelength of 830 nm, power of 40 mW, and dose applied per point of 4 J/cm2 in 20 patients with TMD, being applied at five points in each temporomandibular joint [16]. The authors found a significant increase in mouth opening (p = 0.028) after treatment [16]. Similarly, Mazzetto., et al. also showed a significant improvement in right and left mandibular movements in the group treated with an active dose of AlGaAs laser (830 nm) and energy density of 5 J/cm2 per point in five points around the TMJ, when compared to the placebo group [21].
Salmos Brito., et al. evaluated the effects of 12 photobiomodulation sessions in 58 patients with acute and chronic TMD using an AlGaAs laser at five points around the TMJ, in continuous mode, with a wavelength of 830 nm, beam output power of 40 mW, diameter of 6 mm, point energy density ranging from 1.5 to 2 J/cm2, and total energy density for each side of 8 J/cm2, applied to patients in the acute, subacute or chronic phases [11]. There was a significant reduction in pain intensity and improvement in maximal mouth opening after treatment [11]. Among the groups, the acute phase presented better results when compared to the chronic phase [11].
[25] Rohlig., et al. reported improvement in mandibular movements after application of 10 laser sessions with wavelength of 820 nm, 3 J/cm2, and output power of 300 mW at trigger points of masticatory muscles [25]. A total of 40 patients were included, being divided into intervention group and control group.
Light parameters and applied doses are fundamental in photobiomodulation [2,23]. It is known that if the parameters are applied incorrectly, the treatment will probably be ineffective [23]. Knowledge of the potential effects of irradiation parameters, including wavelength, pulse rate, power, energy, and energy density is essential in the treatment of a given condition [26]. Very low or very high doses may not promote significant effects, and, above all, excessive light may lead to unwanted inhibitory effects [23]. At low doses (up to 2 J/cm2), photobiomodulation stimulates proliferation, while at higher doses (16 J/cm2 or higher), photobiomodulation is suppressive [23]. The use of low intensity laser with a high fluency (above 80 J/cm2) overstimulates mitochondrial chromophores, which, in turn, activates the mitochondrial apoptosis pathway, altering the cell cycle, inhibiting cell proliferation and even causing cell death [23].
| Group | |||||
| Variable | 8 J/cm² (n = 11) | 60 J/cm² (n = 11) | 105 J/cm² (n = 11) | Placebo (n = 11) | P value |
| Right opening, degrees | |||||
| Initial | 10.49 ± 4.68 | 15.01 ± 5.65 | 17.63 ± 4.84 | 16.56 ± 5.96 | 0.02#& |
| Final | 15.40 ± 6.43 | 17.91 ± 6.97 | 16.28 ± 6.82 | 16.02 ± 6.85 | 0.86 |
| P value | 0.01 | 0.13 | 0.39 | 0.65 | |
| Left opening, degrees | |||||
| Initial | 10.81 ± 5.46 | 13.46 ± 7.00 | 17.09 ± 6.51 | 15.76 ± 5.26 | 0.05# |
| Final | 15.93 ± 7.77 | 16.06 ± 9.55 | 15.38 ± 7.78 | 15.44 ± 6.70 | 0.99 |
| P value | 0.03 | 0.25 | 0.07 | 0.77 | |
| Right lateralization, degrees | |||||
| Initial | 6.23 ± 3.19 | 6.38 ± 4.36 | 8.04 ± 4.16 | 7.24 ± 3.56 | 0.60 |
| Final | 7.28 ± 3.32 | 7.79 ± 4.58 | 10.63 ± 4.80 | 8.17 ± 4.67 | 0.25 |
| P value | 0.39 | 0.23 | 0.11 | 0.75 | |
| Left lateralization, degrees | |||||
| Initial | 6.93 ± 3.96 | 6.73 ± 4.01 | 8.78 ± 3.77 | 7.24 ± 3.56 | 0.59 |
| Final | 8.46 ± 5.24 | 7.35 ± 4.23 | 9.90± 7.08 | 8.93 ± 2.36 | 0.70 |
| P value | 0.26 | 0.50 | 0.43 | 0.09 | |
| Right protrusion, degrees | |||||
| Initial | 9.80 ± 4.24 | 10.38 ± 5.24 | 10.42 ± 4.26 | 12.09 ± 4.99 | 0.74 |
| Final | 12.56 ± 5.40 | 11.05 ± 4.88 | 10.77 ± 5.21 | 11.66 ± 5.79 | 0.88 |
| P value | 0.05 | 0.58 | 0.85 | 0.97 | |
| Left protrusion, degrees | |||||
| Initial | 13.02 ± 6.76 | 11.51 ± 6.98 | 9.42 ± 4.53 | 8.64 ± 3.78 | 0.32 |
| Final | 12.49 ± 5.40 | 11.04 ± 6.90 | 8.99 ± 4.56 | 10.95 ± 2.88 | 0.54 |
| P value | 0.99 | 0.68 | 0.76 | 0.17 | |
| Right retraction, degrees | |||||
| Initial | 10.76 ± 7.44 | 4.34 ± 3.48 | 3.31 ± 2.06 | 5.29 ± 3.67 | 0.02$& |
| Final | 9.16 ± 5.80 | 7.15 ± 5.33 | 6.21 ± 3.67 | 6.88 ± 4.58 | 0.35 |
| P value | 0.70 | 0.19 | 0.22 | 0.64 | |
| Left retraction, degrees | |||||
| Initial | 10.19 ± 5.80 | 3.62 ± 2.79 | 4.91 ± 4.98 | 5.59 ± 5.44 | 0.09 |
| Final | 7.59 ± 5.06 | 6.17 ± 4.76 | 5.72 ± 2.75 | 6.9 ± 3.42 | 0.82 |
| P value | 0.71 | 0.09 | 0.92 | 0.79 | |
$p < 0.05 comparing the 8 J/cm² group with the 60 J/cm² group;
#p < 0.05 comparing the 8 J/cm² group with the 105 J/cm² group;
&p < 0.05 comparing the 8 J/cm² group with the placebo group.
One-way ANOVA. .
Table 4: Results of initial and final joint mobility in each study group (n = 44).
#p < 0.05 comparing the 8 J/cm² group with the 105 J/cm² group;
&p < 0.05 comparing the 8 J/cm² group with the placebo group.
One-way ANOVA. .
Table 4: Results of initial and final joint mobility in each study group (n = 44).
Although studies are still unclear as to the definition of the best protocol for photobiomodulation treatment in TMD, there already seems to be a consensus that the use of laser provides benefits when applied and administered correctly [2,27]. The present study demonstrated positive effects regarding TMD pain and symptoms at the different energy densities used, as well as in the placebo group. Notwithstanding, only in the 8 J/cm2 group there was a significant improvement of joint mobility for mandibular opening and right protrusion, which leads us to believe that this dose may be more efficient in the treatment of TMD. In addition, the application time spent for irradiation of this dose is lower, which optimizes the performance of other therapeutic techniques.
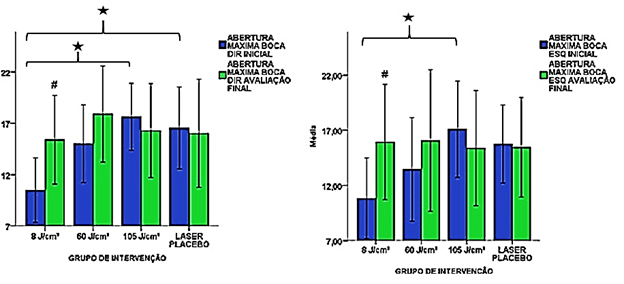
#p < 0.05 relative to the initial evaluation of the same group. Student's t-test.
*p < 0.05 relative to the same evaluation of the other group. One-way ANOVA.
Figure 5: Results of the initial and final right and left mandibular opening movement in the different study groups.
*p < 0.05 relative to the same evaluation of the other group. One-way ANOVA.
Figure 5: Results of the initial and final right and left mandibular opening movement in the different study groups.
Different wavelengths have been used in the photobiomodulation approach in TMD [4]. However, the most common situation in therapeutic use has been wavelengths in the range of infrared light spectrum, located in the electromagnetic spectrum between 780 and 904 nm, due to its increased penetration [27-29]. The energy density and optical properties of the tissue are also considered essential factors that could influence the treatment of TMD [2]. Notwithstanding, to date, studies have not reached a definitive scientific conclusion about the frequency and duration of the sessions to be performed [30]. The number of sessions differs considerably between studies, ranging from a single session to 20 applications [2]. Controversies over the effectiveness of photobiomodulation in TMD are believed to be due to disagreement over which dosimetry to use [30]. Many studies have not reported all the parameters that were used, which makes it difficult to compare their results [8,10,16]. Describing the parameters used is essential for this study to become reproducible.
Conclusion
The results of this study demonstrated a significant reduction of TMD pain and symptoms in all the photobiomodulation protocols used, including the placebo group. However, only the 8 J/cm2 group showed a positive effect on the maximal opening and mandible protrusion movements.
References
- Aparicio JH., et al. “The use of low level laser therapy in the treatment of temporomandibular joint disorders: Review of the literature”. Med Oral Patol Oral Cir Bucal 18.4 (2013): 603-612.
- Herpich CM., et al. “Analysis of laser therapy and assessment methods in the rehabilitation of temporomandibular disorder: a systematic review of the literature”. Journal of Physical Therapy Science 27.1 (2015): 295-301.
- Godoy CHL., et al. “Evaluation of effect of low-level laser therapy on adolescents temporomandibular disorder: study protocol for a randomized controlled trial”. Trials 14.229 (2013): 1-6.
- Demirkol N., et al. “Effectiveness of occlusal splints and low-level laser therapy on myofascial pain”. Lasers in Medical Science 30.3 (2015): 1007-1012.
- Herpich CM., et al. “Effects pf phototherapy on muscle activity and pain in individuals with temporomandibular disorder: a study protocol for a randomized controlled trial”. Trials 15.491 (2014): 1-8.
- Gonçalves DA., et al. “Symptoms of temporomandibular disorders in the population: an epidemiological study”. Journal of Orofacial Pain 24.3 (2010): 270–278.
- Petrucci A., et al.“Effectiveness of Low-Level Laser Therapy in Temporomandibular Disorders: A Systematic Review and Meta-Analysis”. Journal of Orofacial Pain 25.4 (2011): 298-307.
- Rodrigues JH., et al. “Evaluation of pain, jaw movements, and psychosocial factors in elderly individuals with tempormandibular disorder under laser phototherapy”. Lasers Medical Science 30.3 (2015): 953-959.
- Assis TO., et al. “O uso do laser na reabilitação das desordens temporomandibulares”. Fisioter em Movimento 25.2 (2012): 453-459.
- Ahrari F., et al. “The efficacy of low-level laser therapy for the treatment of myogenous temporomandibular joint disorder”. Lasers Medical Science 29.2 (2014): 551-557.
- Salmos-Brito JAL., et al. “Evaluation of low-level laser therapy in patients with acute and chronic temporomandibular disorders”. Lasers Medical Science 28.1 (2013): 57-64.
- Ayyldiz S., et al. “Evaluation of Low-Level Laser Therapy in TMD Patients”. Case Reports in Dentistry (2015): 1-6.
- Melchior MO., et al. “Low-level lasertherapy associated to occlusal splint to treat temporomandibular disorder: controlled clinical trial”. Revista Dor18.1 (2017): 12-7.
- Yueh LH., et al. “Fluence-dependent effects of low-level laser therapy in myofascial trigger spots on modulation of biochemicals associated with pain in a rabbit model”. Lasers Medical Science 30.1 (2015): 209-216.
- Chaves TC., et al.“Principais instrumentos para avaliação da disfunção temporomandibular, parte I: índices e questionários; uma contribuição para a prática clínica e de pesquisa”. Fisioter Pesq 15.1 (2008): 92-100.
- Catão MHCV., et al. “Avaliação da eficácia do laser de baixa intensidade no tratamento das disfunções temporomandibulares: um estudo clínico randomizado”. Rev CEFAC 15.6 (2013): 1601-1608.
- Correia LMF., et al. “Evaluation of body painful areas in patients with muscular temporomandibular disorder: a retrospective study”. Rev Dor 16.4 (2015): 249-253.
- Campi LB., et al. “Influence of biopsychosocial approaches and self-care to control chronic pain and temporomandibular disorder”. Rev Dor 14.3 (2013): 219-222.
- Shukla D and Muthusekhar MR. “Efficacy of low‑level laser therapy in temporomandibular disorders: A systematic review”. National Journal of Maxillofacial Surgery 7.1 (2016): 62-67.
- Magri LV., et al. “Effectiveness of low-level laser therapy on pain intensity, pressure pain threshold, and SF-MPQ indexes of women with myofascial pain”. Lasers Medical Science 32.2 (2017): 419-428.
- Mazzetto MO., et al. “Measurements of jaw movements and TMJ pain intensity in patients treated with GaAlAs laser”. Braz Dental Journal 21.4 (2010): 356-360.
- Madani AS., et al. “Low-level laser therapy for management of TMJ osteoarthritis”. Cranio 32.1 (2014): 38-44.
- Freitas LF and Hamblim MR. “Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy”. IEEE J Sel Top Quantum Electron 22.3 (2016): 1-37.
- Marini I., et al. “Effects of superpulsed low-level laser therapy on temporomandibular joint pain”. The Clinical Journal of Pain 26.1 (2010): 611-616.
- Rohlig BG., et al. “Evaluation of orofacial function in temporomandibular disorder patients after low-level laser therapy”. Acta Odontologica Scandinavica71.1 (2013): 1112-1117.
- Enwemeka CS. “The Relevance of Accurate Comprehensive Treatment Parameters in Photobiomodulation”. Photomedicine and Laser Surgery 29.12 (2011): 783-784.
- Maia MLM., et al. “Effect of low-level laser therapy on pain levels in patients with temporomandibular disorders: a systematic review”. Journal of Applied Oral Science 20.6 (2012): 594-602.
- Herpich CM., et al. “Immediate and short-term effects of phototherapy on pain, muscle activity, and joint mobility in women with temporomandibular disorder: a randomized, double-blind, placebo-controlled, clinical trial”. Disability and Rehabilitation11 (2017): 1-7.
- Pereira TS., et al. “Efficacy of red and infrared lasers in treatment of temporomandibular disorders – a double-blind, randomized, parallel clinical trial”. Cranio J Craniomandib Pract 32.1 (2014): 51-56.
- Chen J., et al. “Efficacy of low-level laser therapy in the treatment of TMDs: a meta-analysis of 14 randomised controlled trials”. Journal of Oral Rehabilitation 42.1 (2015): 291-299.
Citation:
Marcelo Baptista Dohnert., et al. “Effects of Different Photobiomodulation Dosimetries on Temporomandibular Dysfunction:
A Randomized, Double-Blind, Placebo-Controlled Clinical Trial”. Orthopaedic Surgery and Traumatology 1.6 (2017): 242-252.
Copyright: © 2017 Marcelo Baptista Dohnert., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.