Review Article
Volume 1 Issue 5 - 2017
Comprehensive Review of Isolated Posterior Interosseous Nerve Palsy
Golden Jubilee National Hospital, Clydebank, United Kindom
*Corresponding Author: Nanjundappa S. Harshavardhana, Golden Jubilee National Hospital, Clydebank, United Kindom.
Received: September 19, 2017; Published: September 27, 2017
Abstract
Background: Isolated Posterior Interosseous nerve (PIN) palsy is an uncommon condition and its management concerning the role of surgery is controversial. Existing literature is sparse and treatment algorithm based on high level of evidence (LoE) is absent.
Objectives: To undertake a comprehensive literature review in English and formulate a treatment algorithm for management of isolated PIN palsy based on the current evidence to aid the healthcare professionals in making evidence-based decisions.
Methods: All published full-text articles in English language on isolated PIN palsy were retrieved using PubMed, MEDLINE, Google Scholar, Science Direct, CINAHL and Cochrane database of reviews. No Level of Evidence (LoE) I and II studies were identified that reported the comparison of operative vs. non-operative management of PIN palsy. This review is based on low LoE comprising largely of case series and case reports (i.e. LoE IV and IV) and two retrospective comparative studies (i.e. LoE III).
Results: Isolated PIN palsy is broadly categorised as: i) Compressive and ii) Non-compressive. The existing evidence supports surgical intervention for compressive PIN palsy. The commonly performed surgeries include: i) Extraneural neurolysis, ii) Neurorraphy and iii) Nerve grafting and iv) Tendon transfers. The prognosis was poor patients: i) Aged over fifty years, ii) Delay to surgery of over twelve months and iii) Long-standing compression with fascicular thinning. A course of non-operative treatment for six months was preferred for a majority of non-compressive PIN pathologies.
Conclusion: The existing evidence for surgical decompression of isolated PIN palsy is dictated by the i) Severity of palsy (i.e. mild, moderate and severe), ii) Rapidity of progression (i.e. acute vs. slow) iii) Underlying pathology (i.e. compressive vs. non-compressive), iv) Age of the patient at surgery (i.e. <50 vs. >50 years) and v) Delay to surgery. A simplified algorithm was generated from the existing literature to aid healthcare professionals to counsel patients presenting with this uncommon pathology regarding operative intervention.
Keywords: Isolated; Posterior interosseous nerve; Palsy; Neuropathy; Non-traumatic; Compressive; Non-compressive
Acknowledgements: To Mr. Iain McGraw, Consultant Orthopaedic Surgeon based at the Royal Alexandra Hospital, Paisley, U.K., for
asking me to undertake this review.
Introduction
The Posterior interosseous nerve (PIN) is a pure motor nerve that is one of the terminal branches of Radial nerve (posterior cord of brachial plexus) [1]. The other terminal branch is superficial radial nerve (SRN) which is purely sensory supplying the skin over the anterolateral aspect of distal forearm and dorsolateral aspect of base of thumb/hand. The PIN has two terminal branches namely i) Medial and shorter recurrent branch and ii) Lateral and longer descending branch [1]. The medial branch provides innervation to Extensor carpi radialis brevis (ECRB), Extensor digitorum communis (EDC) and Extensor carpi ulnaris (ECU). The lateral branch supplies innervation to Extensor indicis proprius (EIP), Extensor pollicis longus (EPL) and Brevis (EPB) and Abductor pollicis longus (APL). The lateral branch ends by providing proprioceptive innervation to the wrist joint [2].
PIN palsy is one of the peripheral entrapment neuropathies and its clinical presentation/signs overlap considerably with that of Radial tunnel syndrome (RTS) also known as the ‘Resistant tennis elbow’ [3]. The key differences in clinical presentation and signs between PIN palsy and RTS are summarized in Table 1. RTS is to be differentiated from Wartenberg syndrome, which is the compression of superficial radial nerve (SRN) at the posterior border of the Brachioradialis muscle as it transitions from deep to subcutaneous plane [4]. The roof of the Radial tunnel is formed by fibrous band between Brachialis/Brachioradialis and Extensor carpi radialis brevis (ECRB)/superficial head of supinator muscles. The floor of the tunnel is formed by anterior capsule of elbow joint and deep head of Supinator. Pain is the characteristic presentation without any motor weakness in RTS unlike in PIN palsy [5]. The ‘Rule of nine’ test reported by Loh., et al. and Werner’s criteria were found to be helpful aids in the diagnosis of RTS [6,7]. Interestingly in one study, the innervation of ECRB was found to arise from SRN in 43%, Radial N in 55% and PIN in 2% of patients, though the standard anatomical texts consider ECRB to be a muscle supplied by PIN [8,9]. The annual incidence of PIN palsy is 0.03% whilst that of RTS is 0.003% (i.e. the Radial tunnel syndrome [RTS] is 10 times less common than PIN palsy) [10].
| PIN Palsy | RTS |
| Inability to extend thumb and fingers | Tenderness over lateral aspect of elbow |
| Weakness in thumb abduction | Pain 4 cms distal to lateral epicondyle |
| Radial deviation of the wrist with extension | Pain aggravated by middle finger extension |
| Normal sensations in distal anterolateral forearm and dorsolateral aspect of hand | Pain aggravated by repetitive prono-supination movements |
| Pain is typically absent | No motor weakness in thumb or fingers |
Table 1: Differences between PIN palsy and Radial tunnel syndrome (RTS).
PIN palsy is usually iatrogenic following surgical interventions or secondary to trauma. The common non-traumatic and non-iatrogenic compressive causes of PIN palsy include: i) Myositis ossificans [11], ii) Mass lesions (soft tissue and bony–i.e. Intra/intermuscular lipoma, ganglion, hemangioma, synovial chondromatosis, Bicipital bursa and osteochondroma etc.) [12-17], iii) Pigmented villonodular synovitis (PVNS) [18], iv) Infections (Osteomyelitis, Septic arthritis of the elbow and Tuberculosis) [19-21] and v) Inflammatory arthropathy (Psoriatic arthritis and Rheumatoid arthritis [RA]) [22-24]. Rheumatoid pannus and synovial cyst in RA elbow were common causative agents and the mean duration from onset of RA to manifestation of PIN palsy was 8 years. The incidence of iatrogenic PIN palsy is up to 3.2% following distal biceps tendon repair [25]. PIN is prone for compressive neuropathy at the following anatomical areas:
- The leading edge of Extensor carpi radialis brevis (ECRB)
- Leash of Henry (Radial recurrent vessels)
- Proximal edge of Supinator muscle (i.e. the Arcade of Frosche)
- Within the Supinator muscle
- After it exits between the two heads of Supinator along its distal course.
PIN palsy is characterized by motor weakness predominantly affecting the fingers/thumb extension and relative sparing of wrist extension. However, there is radial deviation of the wrist with attempted wrist extension (owing to weak ECU). Other rare non-traumatic causes that have been reported in literature included Schwannoma, Neurofibroma, Pseudogout, as Para-neoplastic neurological syndrome secondary to gastric adenocarcinoma and following manipulation (frictional massage and acupuncture of the elbow) [26-30]. Non-compressive PIN palsy is largely due to vasculitic or neurogenic causes (i.e. Neuralgic amyotrophy) [31-32]. The existing literature is muddled with lack of clarity owing to the overlapping nature of spectrum in clinical presentation between PIN palsy and RTS and some cases of Neuralgic amyotrophy (NA) being reported as PIN palsy. Confusion about the nomenclature further adds to the woes in making evidence-based recommendations. More recently, consensus seems to be emerging to label as PIN palsy if the motor weakness were to be the primary presenting feature in the absence of pain/paraesthesia [33]. Patients with PIN palsy also tend to have weakness in thumb abduction in-addition to finger/thumb extension.
Our objectives were therefore to undertake a comprehensive review of all published evidence on isolated non-traumatic PIN palsy with a view to propose a treatment algorithm, draft evidence-based recommendations to aid clinicians in offering informed choices to patients. An attempt was also made to provide a brief synopsis of relevant applied surgical anatomy and clear the confusion that exists about the nomenclature of PIN palsy.
Methods
All published literature on PIN palsy was retrieved by the author using the keywords: Isolated, Posterior interosseous nerve, Palsy, Neuropathy, Non-traumatic, Compressive, Non-compressive and their combinations thereof in English language up to 31st January 2017. The search engines used were MEDLINE and PubMed (1980 onwards), CINAHL (1982 onwards), Cochrane database of reviews (1992 onwards), Science Direct (1997 onwards) and Google Scholar (2004 onwards). For ease of interpretation and undertaking an in-depth narrative review, only full-text articles that were published in English language were included. The initial search yielded 214 studies that were evaluated against stringent inclusion criteria (Table 2). Following the review of all full-text articles (FTA), 55 studies were met the inclusion criteria and were analysed for this review. The FTA were then classified into high Level of Evidence (LoE) vs. low LoE studies (i.e. LoE I & II vs. LoE III, IV & V) to facilitate undertaking this review, drafting the surgical algorithm and making evidence-based recommendations.
The key answers one was looking for in the existing literature were for the following questions:
- What is the anatomy of PIN and what controversy exists surrounding its diagnosis and nomenclature?
- What are the forms of PIN palsy and its pathophysiology?
- What classification system exists and how is PIN palsy diagnosed and investigated?
- What are the surgical treatment options? What are the results / outcomes of surgery?
- Is there a treatment algorithm that would guide healthcare professionals in managing PIN palsy?
- What are the common causes of iatrogenic PIN palsy? What practical tips exist to minimize them?
As there were no high LoE studies and only two retrospective comparative studies (i.e. LoE III) that lacked uniformity regarding: i) Nomenclature and etiology, ii) Patient selection and indications for surgery, iii) Duration of follow-up and iv) Outcome measures used to report results, no narrative review or meta-analysis could be undertaken. This is merely a systematic review of largely observational studies of low LoE (i.e. LoE III, IV & V studies) [34]. An objective attempt was made to formulate treatment algorithm by eliminating any type of bias.
| Inclusion | Exclusion |
| Non-traumatic PIN palsy | Traumatic PIN palsy |
| Isolated PIN palsy | Radial tunnel syndrome (RTS) and combined palsies/mixed presentations |
| Diagnostic work-up and investigations of PIN palsy | Publications in language other than English |
| Clinical outcomes of PIN palsies treated both operatively and non-operatively | |
| Clinical studies reporting surgical interventions and outcomes of PIN palsy | |
| All case series and case reports |
Table 2: Inclusion criteria for inclusion in this narrative review.
Results
A. Anatomy of Posterior interosseous nerve (PIN)
PIN is the larger of the two terminal branches of Radial N. The most common site of PIN compression is at the Arcade of Frosche (AoF), i.e. the leading edge of the supinator muscle that is fibrous in 80% of individuals and membranous in remaining 20% [35]. In a cadaveric study involving 60 elbows, Ozkan., et al. undertook a detailed anatomical study of PIN in AoF. The anatomical specifications with reference to vital landmarks were measured with callipers and are summarized in Table 3 that highlights the vulnerability of PIN during surgical exposures.
PIN is the larger of the two terminal branches of Radial N. The most common site of PIN compression is at the Arcade of Frosche (AoF), i.e. the leading edge of the supinator muscle that is fibrous in 80% of individuals and membranous in remaining 20% [35]. In a cadaveric study involving 60 elbows, Ozkan., et al. undertook a detailed anatomical study of PIN in AoF. The anatomical specifications with reference to vital landmarks were measured with callipers and are summarized in Table 3 that highlights the vulnerability of PIN during surgical exposures.
| No | Parameter | Distance | |
| Mean | Range | ||
| 1 | Lateral intermuscular septum to division of Radial nerve | 92 mm | 85–120 mm |
| 2 | Length of PIN to Arcade of Frosche (AoF) | 46 mm | 35–65 mm |
| 3 | Distance from radial head to AoF | 21 mm | 17–30 mm |
| 4 | Length of PIN branch to ECRB to division of PIN in AoF | 28 mm | 20–45 mm |
| 5 | Supinator branch to division in AoF | 21 mm | 15–28 mm |
Table 3: Key distance of PIN from important anatomical landmarks.
B. Controversy with nomenclature
PIN palsy and Radial tunnel syndrome (RTS) are distinct but overlapping/inter-related pathologies. The PIN is not compressed in the radial tunnel and the predominant finding/presentation in RTS is pain and paraesthesia esp. with extension of middle finger against resistance (as Extensor carpi radialis brevis [ECRB] is affected), that was described as pathognomonic sign [36]. The key presentation in PIN palsy is mainly weakness in fingers and thumb extension (i.e. Extensor digitorum communis [EDC], Extensor pollicis longus [EPL] and Extensor indicis proprius [EIP]) and ulnar deviator (i.e. Extensor carpi ulnaris [ECU]) [1,9]. The site of compression in PIN palsy is distal to the RTS. The anatomy of RTS and key structures are illustrated in Figure 1.
PIN palsy and Radial tunnel syndrome (RTS) are distinct but overlapping/inter-related pathologies. The PIN is not compressed in the radial tunnel and the predominant finding/presentation in RTS is pain and paraesthesia esp. with extension of middle finger against resistance (as Extensor carpi radialis brevis [ECRB] is affected), that was described as pathognomonic sign [36]. The key presentation in PIN palsy is mainly weakness in fingers and thumb extension (i.e. Extensor digitorum communis [EDC], Extensor pollicis longus [EPL] and Extensor indicis proprius [EIP]) and ulnar deviator (i.e. Extensor carpi ulnaris [ECU]) [1,9]. The site of compression in PIN palsy is distal to the RTS. The anatomy of RTS and key structures are illustrated in Figure 1.
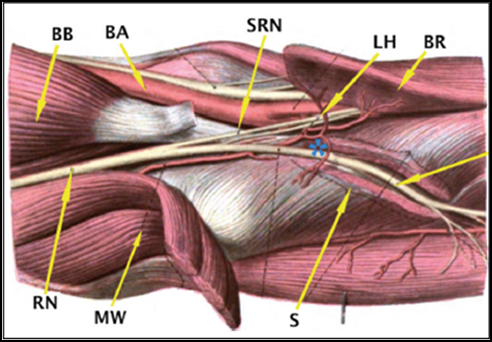
BB: Biceps Brachii
BA: Brachial Artery
SRN: Superficial Radial Nerve
LH: Leash of Henry
BR: Brachioradialis
RN: Radial Nerve
MW: Mobile Wad
S: Supinator
*: Posterior interosseous nerve (PIN)
Figure 1: Anatomy of Radial tunnel and that of Posterior Interosseous nerve (PIN).
BA: Brachial Artery
SRN: Superficial Radial Nerve
LH: Leash of Henry
BR: Brachioradialis
RN: Radial Nerve
MW: Mobile Wad
S: Supinator
*: Posterior interosseous nerve (PIN)
Figure 1: Anatomy of Radial tunnel and that of Posterior Interosseous nerve (PIN).
C. Forms of PIN palsy
The PIN palsy can be broadly divided into: i) Traumatic vs. Non-traumatic and ii) Compressive vs. Non-compressive palsies. Depending on the duration of progression of PIN palsy, it is also classified as Acutely progressive (usually lasts one month or less) vs. Slow progressive PIN palsy (where deterioration of muscle function progressed beyond one month) [37]. The compressive PIN palsy is characterized by the mechanical compression secondary to a growth/lesion/constriction that causes anatomical/morphological changes in the fascicles whilst the non-compressive etiologies are largely: i) Vasculitic and ii) Neurogenic. The differentiation of compressive vs. non-compressive PIN palsy is critical as operative intervention has a proven role in compressive PIN palsy and its role in non-compressive etiologies is controversial.
The PIN palsy can be broadly divided into: i) Traumatic vs. Non-traumatic and ii) Compressive vs. Non-compressive palsies. Depending on the duration of progression of PIN palsy, it is also classified as Acutely progressive (usually lasts one month or less) vs. Slow progressive PIN palsy (where deterioration of muscle function progressed beyond one month) [37]. The compressive PIN palsy is characterized by the mechanical compression secondary to a growth/lesion/constriction that causes anatomical/morphological changes in the fascicles whilst the non-compressive etiologies are largely: i) Vasculitic and ii) Neurogenic. The differentiation of compressive vs. non-compressive PIN palsy is critical as operative intervention has a proven role in compressive PIN palsy and its role in non-compressive etiologies is controversial.
D. Etiology of PIN palsy
The most common non-traumatic cause of compressive PIN palsy was either a bony or soft tissue growth with the commonest culprit being an inter or intramuscular lipoma which is best detected by a magnetic resonance imaging (MRI) scan (Figure 2). Repetitive prono-supination movements causes a pressure of 40-50 Torr with resultant stretching of supinator muscle leading to PIN compressive neuropathy as observed by Werner., et al. [38]. Long-term computer use and manual work was also associated with causation of PIN palsy whose incidence is only likely to rise in near future [39]. Palsies secondary to traumatic (i.e. fractures or fracture-dislocations) conditions was excluded from this review.
The most common non-traumatic cause of compressive PIN palsy was either a bony or soft tissue growth with the commonest culprit being an inter or intramuscular lipoma which is best detected by a magnetic resonance imaging (MRI) scan (Figure 2). Repetitive prono-supination movements causes a pressure of 40-50 Torr with resultant stretching of supinator muscle leading to PIN compressive neuropathy as observed by Werner., et al. [38]. Long-term computer use and manual work was also associated with causation of PIN palsy whose incidence is only likely to rise in near future [39]. Palsies secondary to traumatic (i.e. fractures or fracture-dislocations) conditions was excluded from this review.
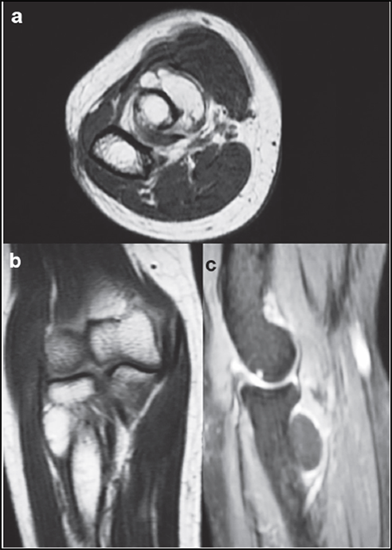
a: Axial image
b: Coronal image
c: Sagittal image
Figure 2: T2 weighted MRI images of a large intermuscular lipoma causing PIN compression.
b: Coronal image
c: Sagittal image
Figure 2: T2 weighted MRI images of a large intermuscular lipoma causing PIN compression.
E. Pathophysiology of non-compressive PIN palsy
Non-compressive PIN palsy was either due to ischemic insult (i.e. vasculitic) or secondary to neuralgic amyotrophy (NA). NA is an acute inflammatory neuropathy affecting one or multiple nerves of the Brachial plexus having varying mechanisms and phenotypes [40]. The characteristic presenting feature was the sudden onset of pain followed by paraesthesia or/and motor deficit of the upper extremities. Single nerve involvement affecting the Anterior interosseous nerve (AIN) was most common in 35% of the patients followed by PIN in up to one-fourth of individuals as reported by Akane., et al. [40]. Pain was typically absent in up to 10% of patients in the index publication of Parsonage., et al. though Akane., et al. found this to be the case in almost 43% of the affected individuals. Parsonage Turner syndrome (PTS) is a form of NA secondary to viral infection affecting the Anterior interosseous nerve with bilateral presentation [41]. It was largely a self-limiting condition with good outcome by 6-12 months. Diffuse axonal demyelination with fibrosis and inflammatory cell infiltration was observed on histology. Isolated PIN palsy due to NA has a favourable prognosis in at least one-third of patients whilst the remaining two-thirds had persistent neuropathic symptoms. Interestingly such patients were found to have hourglass-like fascicular constriction of nerves despite absence of any compressive element. Pan., et al. postulated inflammation and edema to be the primary factors that caused adhesions, scarring and local fixation of fascicles leading to their eventual thinning, constriction and torsion [42]. Suarez et al had identified inflammatory cell infiltration of CD8+ and T lymphocyte cells on histological evaluation [43]. Neurolysis was recommended for severe constrictions and operative outcomes were better than conservative treatment for such cases of NA. The differential diagnosis of such rare presentations included i) Brachial plexopathy, ii) Cervical radiculopathy and iii) Hereditary neuropathy with liability for pressure palsies.
Non-compressive PIN palsy was either due to ischemic insult (i.e. vasculitic) or secondary to neuralgic amyotrophy (NA). NA is an acute inflammatory neuropathy affecting one or multiple nerves of the Brachial plexus having varying mechanisms and phenotypes [40]. The characteristic presenting feature was the sudden onset of pain followed by paraesthesia or/and motor deficit of the upper extremities. Single nerve involvement affecting the Anterior interosseous nerve (AIN) was most common in 35% of the patients followed by PIN in up to one-fourth of individuals as reported by Akane., et al. [40]. Pain was typically absent in up to 10% of patients in the index publication of Parsonage., et al. though Akane., et al. found this to be the case in almost 43% of the affected individuals. Parsonage Turner syndrome (PTS) is a form of NA secondary to viral infection affecting the Anterior interosseous nerve with bilateral presentation [41]. It was largely a self-limiting condition with good outcome by 6-12 months. Diffuse axonal demyelination with fibrosis and inflammatory cell infiltration was observed on histology. Isolated PIN palsy due to NA has a favourable prognosis in at least one-third of patients whilst the remaining two-thirds had persistent neuropathic symptoms. Interestingly such patients were found to have hourglass-like fascicular constriction of nerves despite absence of any compressive element. Pan., et al. postulated inflammation and edema to be the primary factors that caused adhesions, scarring and local fixation of fascicles leading to their eventual thinning, constriction and torsion [42]. Suarez et al had identified inflammatory cell infiltration of CD8+ and T lymphocyte cells on histological evaluation [43]. Neurolysis was recommended for severe constrictions and operative outcomes were better than conservative treatment for such cases of NA. The differential diagnosis of such rare presentations included i) Brachial plexopathy, ii) Cervical radiculopathy and iii) Hereditary neuropathy with liability for pressure palsies.
F. Investigations for diagnosing PIN palsy
Electrodiagnostics (i.e. electromyography (EMG) and nerve conduction studies (NCS) studies in patients with RTS are typically normal and rarely helpful. Patients with PIN palsy may have reduction in compound muscle action potential (CMAP) amplitude and nerve conduction velocity (NCV) [44]. Denervation potentials are suggestive of incomplete PIN palsy. Magnetic resonance Neurorraphy (MRN) is a novel tool that is increasingly being used in diagnosing such iatrogenic/non-compressive PIN palsies and EMG/NCS might be of complimentary value in aiding with the diagnosis [45]. The EMG and NCS study are often normal in the first few weeks to three months following the injury and adds to diagnostic value only at 3-6 months. Though MRN is a promising diagnostic tool, further studies in humans are needed to establish and define its actual role in early phases of PIN palsy.
Electrodiagnostics (i.e. electromyography (EMG) and nerve conduction studies (NCS) studies in patients with RTS are typically normal and rarely helpful. Patients with PIN palsy may have reduction in compound muscle action potential (CMAP) amplitude and nerve conduction velocity (NCV) [44]. Denervation potentials are suggestive of incomplete PIN palsy. Magnetic resonance Neurorraphy (MRN) is a novel tool that is increasingly being used in diagnosing such iatrogenic/non-compressive PIN palsies and EMG/NCS might be of complimentary value in aiding with the diagnosis [45]. The EMG and NCS study are often normal in the first few weeks to three months following the injury and adds to diagnostic value only at 3-6 months. Though MRN is a promising diagnostic tool, further studies in humans are needed to establish and define its actual role in early phases of PIN palsy.
G. Classification system
Three main classification systems have been reported to describe PIN palsy: i) the Ochi classification (Table 4), ii) the Wu classification (Table 5) and iii) the Hirachi classification (Table 6). The Ochi classification system is a generic compressive neuropathy classification system that is applicable to both anterior interosseous nerve (AIN) and PIN palsy that distinguishes between the presence vs. absence of edema and takes into consideration the type and pattern of fascicular constriction. Ochi identified three main patterns of AIN/PIN compression [46]. The Wu classification system further sub-divides Ochi type II palsy into mild, moderate and severe forms. It therefore from a surgical perspective has greater weightage given the prognostic/outcomes significance [47]. Hirachi differentiated the PIN palsies into three main sub-types based on the key muscles involved and presentation of functional deficits in his review of traumatic PIN palsies. Hirachi type I was a complete PIN palsy whilst types II & III affected the recurrent (i.e. medial) and descending (i.e. lateral) motor branches respectively [48]. Isolated selective palsy of descending branch of PIN with dropped thumb and index finger has been reported secondary to a pseudoneuroma and four years following plate fixation of forearm fracture (due to prominent implants) [49,50]. The mechanism proposed to explain such a selective presentation was repetitive prono-supination with disturbed blood supply that caused constriction and adhesions of the nerve’s branch.
Three main classification systems have been reported to describe PIN palsy: i) the Ochi classification (Table 4), ii) the Wu classification (Table 5) and iii) the Hirachi classification (Table 6). The Ochi classification system is a generic compressive neuropathy classification system that is applicable to both anterior interosseous nerve (AIN) and PIN palsy that distinguishes between the presence vs. absence of edema and takes into consideration the type and pattern of fascicular constriction. Ochi identified three main patterns of AIN/PIN compression [46]. The Wu classification system further sub-divides Ochi type II palsy into mild, moderate and severe forms. It therefore from a surgical perspective has greater weightage given the prognostic/outcomes significance [47]. Hirachi differentiated the PIN palsies into three main sub-types based on the key muscles involved and presentation of functional deficits in his review of traumatic PIN palsies. Hirachi type I was a complete PIN palsy whilst types II & III affected the recurrent (i.e. medial) and descending (i.e. lateral) motor branches respectively [48]. Isolated selective palsy of descending branch of PIN with dropped thumb and index finger has been reported secondary to a pseudoneuroma and four years following plate fixation of forearm fracture (due to prominent implants) [49,50]. The mechanism proposed to explain such a selective presentation was repetitive prono-supination with disturbed blood supply that caused constriction and adhesions of the nerve’s branch.
| Ochi type | Description | |
| Type I | Edema and colour change in the epineurium without fascicular deformity | |
| Type II | Fascicular constriction present | |
| A–Recess type | 1: < 25% fascicular thinning | |
| B–Recess-bulging type | 2: 25 to 50% fascicular thinning | |
| C–Rotation type | 3: < 75% fascicular thinning | |
| D–Rotation-bulging type | ||
| Type III | Swelling in the fascicule with no evidence of edema in the epineurium | |
Table 4: The Ochi classification.
| Wu type | Description (Ochi type II) |
| Mild | Ochi Type IIABCD-1 |
| Moderate | Ochi Type IIABCD-2 |
| Severe | Ochi Type IIIABCD-3 |
Table 5: The Wu classification.
| Hirachi type | Description (Ochi type II) |
| Type I | Complete palsy with weakness in extension of wrist and all fingers + weakness in thumb extension and abduction |
| Type II | Palsy due to selective involvement of Recurrent terminal branch resulting in selective weakness of middle, ring and little finger extension. |
| Type III | Palsy due to selective involvement of Descending terminal branch resulting in weakness of thumb abduction and extension of index finger. |
Table 6: The Hirachi classification.
H. Treatment options and outcomes
Non-operative management with expectant watch for 3-6 months is the initial treatment of choice for all forms of PIN palsy. It constitutes the main treatment strategy for non-compressive PIN palsy of vasculitic and neurogenic pathologies. Operative treatment is primarily reserved for compressive PIN palsy and key interventional procedures undertaken that is described in literature include:
Non-operative management with expectant watch for 3-6 months is the initial treatment of choice for all forms of PIN palsy. It constitutes the main treatment strategy for non-compressive PIN palsy of vasculitic and neurogenic pathologies. Operative treatment is primarily reserved for compressive PIN palsy and key interventional procedures undertaken that is described in literature include:
- Extraneural neurolysis (i.e. decompression)
- Interfascicular neurolysis (IFN)
- Neurorraphy (NY) and nerve grafting (NG)
- Salvage procedures like Tendon transfers (TT) combined with either IFN, NY or NG.
The literature is sparse with lack of consistency to report/compare the superiority of one operative modality vs. another (i.e. IFN vs. NY/NG). Only three clinical studies have reported surgical outcomes with in a cohort of >25 patients with isolated non-traumatic PIN palsy since the beginning of this Millennium (i.e. 2000 onwards).
One of the first publications with large case series was published by Kim., et al. who reported surgical results in 45 patients with PIN palsies and the authors used LSUHSC grading system to report their outcomes [51]. Only 25 patients had non-traumatic etiolgoy and this select cohort was included in this review. Twenty-one had entrapment neuropathy all underwent neurolysis whilst four patients with extrinsic compression due to tumours had resection of lesions (Schwannoma, Neurofibroma, Ganglion cyst and Lipoma). All 25 patients had good recovery to Louisiana state university health science centre (LSUHSC) Grade III or above and the authors advocated Neurorraphy or nerve grafting (i.e. NY and NG) when the nerve action potential was absent. They also recommended cautious monitoring with serial evaluation for spontaneous recovery in cases of blunt injuries and contusions (i.e. lesion in-continuity).
Ochi., et al. reported results of 50 patients who were treated for spontaneous PIN palsy. Thirty-eight patients were treated surgically whilst 12 were managed non-operatively [52]. This was the first LoE III study in English language that had compared surgical vs. non-operative treatments. The mean follow-up was 21 months (range: 5.5–221 months) and MRC grading of EDC was used to report surgical results. IFN was undertaken in 27 patients whilst Extraneural neurolysis was performed in three cases. Good outcomes were observed in young patients (defined as those aged < 50 years) and when the duration to surgery was < 7 months (esp. so in patients aged over 50 years). They observed best results when tendon transfer (i.e. Palmaris longus to Extensor pollicis longus) was coupled with IFN esp. in older patients (i.e. > 50 years). An isolated tendon transfer (TT) was advocated primarily as a salvage procedure following either failed neurolysis or Neurorraphy and in patients aged > 70 years.
More recently, Wu., et al. reported outcomes 41 patients with spontaneous PIN palsy of whom 24 were treated surgically and 17 managed non-operatively [47]. All patients had hourglass-like fascicular constriction of the PIN proven on ultrasound. The breakdown of operative modality in these patients included IFN (10), NY (8) and NG (6). Good recovery was seen in 20 patients as reported by medical research council (MRC) muscle power grading of EDC, ECU and EPL. They advocated IFN or NY for mild to moderate cases of PIN palsy and NY +/- NG for severe cases of PIN palsy. Outcomes were better in the surgically treated group than those treated non-operatively by almost two fold (i.e. 83.3% vs. 42.9% success) for similar severity of PIN palsy of > 3 months duration.
RA elbow most commonly causes entrapment neuropathy of Ulnar and Radial nerves. PIN involvement in RA is uncommon and is usually incomplete [53,54]. The presentation could be either unilateral or bilateral and the pathogenesis is largely compressive secondary to either pannus or synovial cyst [24,55]. Published studies recommend control of RA disease process with pharmacotherapy (Methotrexate being the key medication) and Biologics despite complete finger drop. Steroid infiltration was also associated with recovery and surgery was reserved for refractory cases despite best disease control [56]. Westkaemper., et al. reported 100% success with surgery whilst non-operative treatment was successfully in only 43% of the instances [57]. Surgical options undertaken were synovectomy, excision of synovial cyst/pannus and resection of radial head in-addition to neurolysis.
Suffices to say that the evidence exists to perform IFN and NY/NG for mild and moderate compressive PIN palsies and TT reserved for severe compressions associated with muscle wasting. The prognosis was also influenced by: i) Age at surgery (< 50 years vs. > 50 years), ii) Type of progression (i.e. rapid vs. slow progression) and ii) Delay to surgery (< one year vs. > one year). Existing evidence reports poor outcomes in patients aged over fifty years with a delay to surgery of more than twelve months in patients < 50 years. Nerve grafting and Neurorraphy was also not recommended in patients aged over 70 years as axonal regeneration, cortical mapping and rehabilitation was poor/unpredictable with tendon transfer (TT) being the preferred surgical option in them. Co-existent C8 (Ulnar nerve) palsy was also found to be associated with poor prognosis [58].
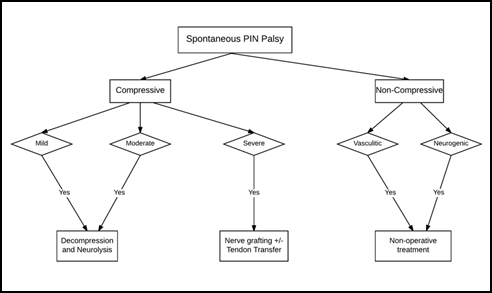
I. Treatment algorithm
A simplified treatment algorithm in management of an isolated PIN palsy based on existing evidence is illustrated as a flow chart in Figure 3. Adherence to this algorithm is helpful in optimizing the surgical outcomes. Another algorithm based on the rapidity of progression of PIN palsy with preferred treatment options for acute and slow-progressing palsies is summarized in Figure 4.
A simplified treatment algorithm in management of an isolated PIN palsy based on existing evidence is illustrated as a flow chart in Figure 3. Adherence to this algorithm is helpful in optimizing the surgical outcomes. Another algorithm based on the rapidity of progression of PIN palsy with preferred treatment options for acute and slow-progressing palsies is summarized in Figure 4.
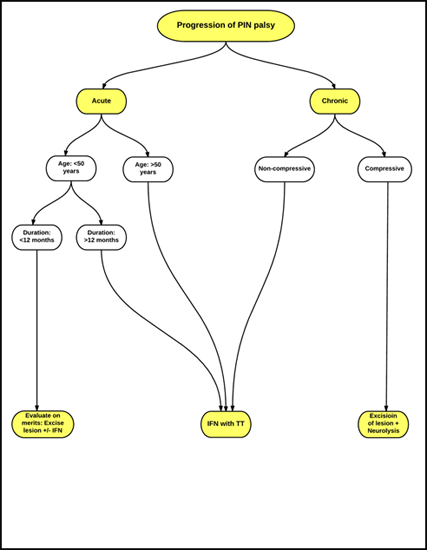
IFN: Interfascicular Neurolysis
TT: Tendon Transfer
Figure 4: Treatment recommendation for PIN palsy based on rapidity of progression.
TT: Tendon Transfer
Figure 4: Treatment recommendation for PIN palsy based on rapidity of progression.
J. Iatrogenic PIN palsy
Iatrogenic palsy is largely a ‘lesion in continuity’ (i.e. neuropraxia or axonotmesis–Sunderland type I and II types) due to excessive/vigorous retraction. It was seen following radial head/neck fracture fixations, open reduction of a radial head dislocation and open repair of distal biceps tendon rupture [59,60]. It was also reported following elbow arthroscopy as the anterolateral portal put this nerve at risk [61]. Proximal ulna fixation (esp. with long bicortical screw) put the PIN at risk of iatrogenic injury and PIN palsy was also reported with external fixator application for elbow instability (long ulna pins) [62]. In paediatric population, the incidence of PIN palsy in Monteggia fractures varied from 30 to 43% and was most commonly seen with Bado type II fracture patterns (i.e. lateral dislocation of radial head) [63]. Watchful wait with expectant treatment and use of supportive splints and physiotherapy is the mainstay of treatment. Exploration of the PIN with neurolysis is largely reserved for non-recovering cases beyond six months. EMG/NCS might was found to be of value prior to surgical intervention in such cases. Pronating the forearm and gentle retraction while addressing radial head surgeries (fixation/replacement) minimize such iatrogenic risks. Avoiding a long bicortical screw while fixing proximal ulna and supination of forearm during screw insertion also reduces PIN injury. Placing the anterolateral portal proximally minimizes the risk of PIN palsy.
Iatrogenic palsy is largely a ‘lesion in continuity’ (i.e. neuropraxia or axonotmesis–Sunderland type I and II types) due to excessive/vigorous retraction. It was seen following radial head/neck fracture fixations, open reduction of a radial head dislocation and open repair of distal biceps tendon rupture [59,60]. It was also reported following elbow arthroscopy as the anterolateral portal put this nerve at risk [61]. Proximal ulna fixation (esp. with long bicortical screw) put the PIN at risk of iatrogenic injury and PIN palsy was also reported with external fixator application for elbow instability (long ulna pins) [62]. In paediatric population, the incidence of PIN palsy in Monteggia fractures varied from 30 to 43% and was most commonly seen with Bado type II fracture patterns (i.e. lateral dislocation of radial head) [63]. Watchful wait with expectant treatment and use of supportive splints and physiotherapy is the mainstay of treatment. Exploration of the PIN with neurolysis is largely reserved for non-recovering cases beyond six months. EMG/NCS might was found to be of value prior to surgical intervention in such cases. Pronating the forearm and gentle retraction while addressing radial head surgeries (fixation/replacement) minimize such iatrogenic risks. Avoiding a long bicortical screw while fixing proximal ulna and supination of forearm during screw insertion also reduces PIN injury. Placing the anterolateral portal proximally minimizes the risk of PIN palsy.
Conclusion
The management of spontaneous and non-traumatic isolated PIN palsy is unclear with little consensus about its nomenclature, investigation of choice, operative modality and postoperative rehabilitation program. No high LoE studies (i.e. Randomized controlled or prospective comparative studies) exist in English literature to draw conclusions. Undertaking a systematic review with meta-analysis was not feasible as the two LoE III studies had heterogeneous cohort of patients. Suffices to say that there is emerging consensus with few diagnostic criteria and tests to distinguish PIN palsy from RTS.
Diagnosis of PIN palsy is largely clinical and detected with motor weakness of long finger extensors. Ultrasonography (USG) may detect the changes in fascicular architecture and compressive lesions, but is user dependant with poor intra and inter-observer reliability. Magnetic resonance imaging (MRI) is the investigation of choice for compressive lesions that aids in surgical excision. Electrodiagnostics (i.e. EMG and NCS) are less helpful in early phases and was invaluable at three to six months (esp. in iatrogenic cases). MR Neurorraphy is novel tool and its value in early phase of PIN palsy is not known. In summary, the key points in summarizing the existing literature are:
- Patients with PIN palsy aged < 50 years do better in-comparison to those over 50 years.
- Delay to surgery of less than seven months was associated with good prognosis
- Interfascicular neurolysis (IFN) is the recommended procedure of choice for mild to moderate PIN palsies.
- Nerve grafting (NG)/Neurorraphy is recommended for severe palsies and the Sural nerve was the donor autograft of choice.
- Tendon transfers (TT) is a salvage procedure reserved for severe and long-standing cases associated with muscle wasting. Palmaris longus (PL) to EPL was the TT of choice.
- TT is usually combined with IFN or/and NY/NG to optimize outcomes (esp. if the duration is > 12 months in patients < 50 years or > 7 months in patients > 50 years).
References
- Standring S. “Gray's Anatomy E-Book: The Anatomical Basis of Clinical Practice. Elsevier Health Sciences 2015.
- Rosenbaum R. “Disputed Radial Tunnel Syndrome”. Muscle Nerve 22.7 (1999): 960-967.
- Roles NC and RH Maudsley. “Radial Tunnel Syndrome: Resistant Tennis Elbow as a Nerve Entrapment”. Journal of Bone and Joint Surgery British 54.3 (1972): 499-508.
- Lanzetta M and G Foucher. “Entrapment of the Superficial Branch of the Radial Nerve (Wartenberg's Syndrome). A Report of 52 Cases”. International Orthopaedics 17.6 (1993): 342-345.
- Stanley J. “Radial Tunnel Syndrome: A Surgeon's Perspective”. Journal of Hand Therapy 19.2 (2006): 180-184.
- Loh YC., et al. “A New Clinical Test for Radial Tunnel Syndrome--the Rule-of-Nine Test: A Cadaveric Study”. Journal of Orthopaedic Surgery (Hong Kong) 12.1 (2004): 83-86.
- Werner CO. “Lateral Elbow Pain and Posterior Interosseous Nerve Entrapment”. Acta orthopaedica scandinavica 50.sup174 (1979): 1-67.
- Prasartritha TP Liupolvanish and A Rojanakit. “A Study of the Posterior Interosseous Nerve (Pin) and the Radial Tunnel in 30 Thai Cadavers”. Journal of Hand Surgery (American Volume) 18.1 (1993): 107-112.
- Plate AM and SM Green. “Compressive Radial Neuropathies”. Instructional course lectures 49 (2000): 295-304.
- Moradi A., et al. “Radial Tunnel Syndrome, Diagnostic and Treatment Dilemma”. The Archives of Bone and Joint Surgery 3.3 (2015): 156-62.
- Kitagawa YT., et al. “Myositis Ossificans of the Supinator Muscle Causing Posterior Interosseous Nerve Palsy: A Case Report”. Hand Surgery 15.2 (2010): 115-117.
- Colasanti R., et al. “Delayed Diagnosed Intermuscular Lipoma Causing a Posterior Interosseous Nerve Palsy in a Patient with Cervical Spondylosis: The “Priceless” Value of the Clinical Examination in the Technological Era”. Giornale Di Chirurgia 37.1 (2016): 42-45.
- Tuygun H., et al. “Partial Paralysis of the Posterior Interosseous Nerve Caused by a Ganglion”. Journal of Hand Surgery (European Volume) 33.4 (2008): 540-541.
- Busa R., et al. “Acute Posterior Interosseous Nerve Palsy Caused by a Synovial Haemangioma of the Elbow Joint”. Journal of Hand Surgery 20.5 (1995): 652-654.
- Yanagisawa H., et al. “Posterior Interosseous Nerve Palsy Caused by Synovial Chondromatosis of the Elbow Joint”. Clinical Radiology 56.6 (2001): 510-514.
- Spinner RJ., et al. “Posterior Interosseous Nerve Compression Due to an Enlarged Bicipital Bursa Confirmed by Mri”. The Journal of Hand Surgery: British & European Volume 18.6 (1993): 753-756.
- Madhavan P and IJ Leslie. “Intracapsular Chondroma of the Elbow Producing a Posterior Interosseous Nerve Palsy” The Journal of Hand Surgery: British & European Volume 23.1 (1998): 107-108.
- Kohyama K., et al. “Posterior Interosseous Nerve Palsy Secondary to Pigmented Villonodular Synovitis of the Elbow: Case Report and Review of Literature”. Orthopaedics & Traumatology: Surgery & Research 99.2 (2013): 247-251.
- Spinner RJ and M Spinner. “Tardy Posterior Interosseous Nerve Palsy Due to Childhood Osteomyelitis: A Case Report”. Journal of Hand Surgery (American Volume) 22.6 (1997): 1049-1051.
- Kato H., et al. “Acute Posterior Interosseous Nerve Palsy Caused by Septic Arthritis of the Elbow: A Case Report”. Journal of Hand Surgery (American Volume) 28.1 (2003): 44-47.
- Chen Wun-Schen and Hock-Liew Eng. “Posterior Interosseous Neuropathy Associated with Tuberculous Arthritis of the Elbow Joint: Report of Two Cases”. The Journal of hand surgery 19.4 (1994): 611-13.
- Ali E., et al. “Inflammatory Posterior Interosseous Nerve Palsy in a Patient with Psoriatic Arthropathy”. Journal of Plastic, Reconstructive & Aesthetic Surgery 64.8 (2011): e205-207.
- Fernandez AM and ML Tiku. “Posterior Interosseous Nerve Entrapment in Rheumatoid Arthritis”. Seminars Arthritis Rheum 24.1 (1994): 57-60.
- Ishikawa H and K Hirohata. “Posterior Interosseous Nerve Syndrome Associated with Rheumatoid Synovial Cysts of the Elbow Joint”. Clinical Orthopaedics and Related Research 254 (1990): 134-139.
- Nigro Phillip T., et al. “Prognosis for Recovery of Posterior Interosseous Nerve Palsy after Distal Biceps Repair”. Journal of Shoulder and Elbow Surgery 22.1 (2013): 70-73.
- Ichikawa J., et al. “Posterior Interosseous Nerve Palsy Due to Schwannoma: Case Report”. Journal of Hand Surgery (American Volume) 33.9 (2008): 1525-1528.
- Lallemand RC and RO Weller. “Intraneural Neurofibromas Involving the Posterior Interosseous Nerve”. Journal of neurology, neurosurgery, and psychiatry 36.6 (1973): 991-996.
- Taniguchi Y., et al. “Posterior Interosseous Nerve Syndrome Due to Pseudogout”. Journal of Hand Surgery British 24.1 (1999): 125-127.
- Ochi K., et al. “Polyarthritis and Posterior Interosseous Nerve Palsy Associated with Gastric Carcinoma”. Rheumatology International 32.8 (2012): 2557-2559.
- Wu YY., et al. “Posterior Interosseous Nerve Palsy as a Complication of Friction Massage in Tennis Elbow”. American Journal of Physical Medicine & Rehabilitation 89.8 (2010): 668-671.
- Hashizume H., et al. “Posterior Interosseous Nerve Paralysis Related to Focal Radial Nerve Constriction Secondary to Vasculitis”. The Journal of Hand Surgery: British & European Volume 18.6 (1993): 757-760.
- Yang JS., et al. “Neuralgic Amyotrophy Manifesting as Mimicking Posterior Interosseous Nerve Palsy”. Journal of Korean Neurosurgical Society 58.5 (2015): 491-3.
- Cravens G and D G Kline. “Posterior Interosseous Nerve Palsies”. Neurosurgery 27.3 (1990): 397-402.
- Obremskey WT., et al. “Level of Evidence in Orthopaedic Journals”. The Journal of Bone and Joint Surgery 87.12 (2005): 2632-2638.
- Ozkan M., et al. “Anatomic Study of Posterior Interosseous Nerve in the Arcade of Frohse”. Journal of Shoulder Elbow Surgery 8.6 (1999): 617-620.
- Lister GD., et al. “The Radial Tunnel Syndrome”. Journal of Hand Surgery American volume 4.1 (1979): 52-59.
- Ochi K., et al. “Slow Progression Predicts Poor Prognoses in Patients with Spontaneous Posterior Interosseous Nerve Palsy”. Journal of Plastic Surgery and Hand Surgery 47.6 (2013): 493-497.
- Werner CO., et al. “Direct Recording of Local Pressure in the Radial Tunnel During Passive Stretch and Active Contraction of the Supinator Muscle”. Archives of Orthopaedic and Trauma Surgery 96.4 (1980): 299-301.
- Jepsen Jørgen Riis. “Upper Limb Neuropathy in Computer Operators? A Clinical Case Study of 21 Patients”. BMC Musculoskeletal Disorders 5.1 (2004): 26.
- Akane M., et al. “Anterior Interosseous Nerve and Posterior Interosseous Nerve Involvement in Neuralgic Amyotrophy”. Clinical Neurology and Neurosurgery 151 (2016): 108-12.
- Turner JW and MJ Parsonage. “Neuralgic Amyotrophy (Paralytic Brachial Neuritis); with Special Reference to Prognosis”. Lancet 273.6988 (1957): 209-212.
- Pan Y., et al. “Hourglass-Like Constrictions of Peripheral Nerve in the Upper Extremity: A Clinical Review and Pathological Study”. Neurosurgery 75.1 (2014): 10-22.
- Suarez GA., et al. “Immune Brachial Plexus Neuropathy: Suggestive Evidence for an Inflammatory-Immune Pathogenesis”. Neurology 46.2 (1996): 559-61.
- Fardin P., et al. “Posterior Interosseous Nerve Neuropathy. Clinical and Electromyographical Aspects”. Electromyography and Clinical Neurophysiology 32.4-5 (1992): 229-234.
- Eppenberger Patrick., et al. “Magnetic Resonance Neurography”. Neuroimaging Clinics 24.1 (2014): 245-256.
- Ochi K. “Surgical Findings in the Nerve Fascicules of Spontaneous Anterior/Posterior Interosseous Nerve Palsy Patients”. Peripheral Nerve20 (2009): 36-45.
- Wu P., et al. “Surgical and Conservative Treatments of Complete Spontaneous Posterior Interosseous Nerve Palsy with Hourglass-Like Fascicular Constrictions: A Retrospective Study of 41 Cases”. Neurosurgery75.3 (2014): 250-257.
- Hirachi, K, et al. “Clinical Features and Management of Traumatic Posterior Interosseous Nerve Palsy”. Journal of Hand Surgery 23.3 (1998): 413-417.
- Hirayama T and Y Takemitsu. “Isolated Paralysis of the Descending Branch of the Posterior Interosseous Nerve. Report of a Case”. The Journal of Bone and Joint Surgery. American Volume 70.9 (1988): 1402-1403.
- Lal H., et al. “Tardy Palsy of Descending Branch of Posterior Interosseous Nerve: Sequela to Plate Osteosynthesis of Forearm Bones”. Journal of Hand Surgery (American Volume) 35.2 (2010): 274-276.
- Kim DH., et al. “Surgical Treatment and Outcomes in 45 Cases of Posterior Interosseous Nerve Entrapments and Injuries”. Journal of Neurosurgery 104.5 (2006): 766-777.
- Ochi K., et al. “Fascicular Constrictions in Patients with Spontaneous Palsy of the Anterior Interosseous Nerve and the Posterior Interosseous Nerve”. Journal of Plastic Surgery and Hand Surgery 46.1 (2012): 19-24.
- Muramatsu., et al. “Peripheral Neuropathies of the Forearm and Hand in Rheumatoid Arthritis: Diagnosis and Options for Treatment”. Rheumatology international 28.10 (2008): 951.
- Naito M., et al. “Simultaneous Bilateral Posterior Interosseous Nerve Palsy Caused by Rheumatoid Synovitis of the Elbows”. Modern Rheumatology 24.6 (2014): 1005-1010.
- Dang Alan C and Craig M Rodner. “Unusual Compression Neuropathies of the Forearm, Part I: Radial Nerve”. The Journal of hand surgery 34.10 (2009): 1906-1914.
- White SH., et al. “Posterior Interosseous Nerve Palsy in Rheumatoid Arthritis”. The Journal of Bone and Joint Surgery. British Volume 70.3 (1988): 468-471.
- Westkaemper JG., et al. “Posterior Interosseous Nerve Palsy in a Patient with Rheumatoid Synovitis of the Elbow: A Case Report and Review of the Literature”. Journal of Hand Surgery American volume 24.4 (1999): 727-731.
- Tadokoro N., et al. “C8 Radiculopathy; Clinical Data from 23 Patients”. Chubu Seikei Saigai Zasshi (Jpn) 54 (2011): 1089-1090.
- Stepanovich MT and CJ Hogan. “Posterior Interosseous and Ulnar Nerve Motor Palsies after a Minimally Displaced Radial Neck Fracture”. Journal of Hand Surgery American volume 37.8 (2012): 1630-1633.
- Stearns KL., et al. “Permanent Posterior Interosseous Nerve Palsy Following a Two-Incision Distal Biceps Tendon Repair”. Orthopaedics 27.8 (2004): 867-868.
- Kelly E W., et al. “Complications of Elbow Arthroscopy”. The Journal of Bone and Joint Surgery. American Volume 83-A.1 (2001): 25-34.
- Tomaino MM., et al. “Posterior Interosseous Nerve Palsy Following Placement of the Compass Elbow Hinge for Acute Instability: A Case Report”. The Journal of Bone and Joint Surgery. American Volume 24.3 (1999): 554-560.
- Ruchelsman DE., et al. “Persistent Posterior Interosseous Nerve Palsy Associated with a Chronic Type I Monteggia Fracture-Dislocation in a Child: A Case Report and Review of the Literature”. Hand (N Y) 4.2 (2009): 167-172.
Citation:
Nanjundappa S. Harshavardhana. “Comprehensive Review of Isolated Posterior Interosseous Nerve Palsy”. Orthopaedic
Surgery and Traumatology 1.5 (2017): 182-194.
Copyright: © 2017 Nanjundappa S. Harshavardhana. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.