Research Article
Volume 1 Issue 5 - 2017
The Interrelation of Insulin Resistance, Serum Adiponectin Level in Rheumatoid Arthritis Hypertensive Females with Subclinical Atherosclerosis and its Dynamics with the Endothelial Dysfunction Correction
State Establishment «Dnipropetrovsk Medical Academy of Health Ministry of Ukraine», Department of Innere medicine #2 Dnipro, Ukraine
*Corresponding Author: Oksana SIRENKO, State Establishment «Dnipropetrovsk Medical Academy of Health Ministry of Ukraine»,
Department of Innere medicine #2 Dnipro, Ukraine.
Received: May 18, 2017; Published: September 14, 2017
Summary
The study shows the results of comprehensive clinical and laboratory, instrumental, biochemical examination of 62 hypertensive females aged 45-65 years with rheumatoid arthritis, there were summarized data on the role of main and additional risk factors in the assessment of overall cardiovascular risk in these patients by studying their relationship with subclinical atherosclerosis presence. Subclinical manifestations of atherosclerosis in hypertensive patients with rheumatoid arthritis characterized by a carotid pool lesions predominance with significantly higher atherosclerotic plaques frequency definition in comparison with a group isolated rheumatoid arthritis, including unfavorable structure. However, cardiovascular risk structure by mSCORE in hypertensive patients with rheumatoid arthritis and subclinical atherosclerosis manifestations shows moderate risk dominating. The thesis shows the presence of atherosclerotic plaques in patients with hypertension combined with rheumatoid arthritis associates with endothelial dysfunction, hyperinsulinemia, age and duration of glucocorticoid therapy (based on logistic regression analysis). It was determined the role of adiponectin as a marker of atherosclerotic carotid arteries. It was estimated the effectiveness of complex therapy with oral L-Arginine solution in the normalization of endothelial dysfunction, insulin resistance reducing in hypertensive patients with rheumatoid arthritis.
Keywords: Hypertension; Rheumatoid arthritis; Subclinical atherosclerosis; Adiponectin; Cardiovascular risk factors
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease with unknown etiology, characterized by symmetrical erosive arthritis (synovitis) and a wide range of extraarticular (systemic) manifestations [1]. With the global prevalence of 0.24% RA causes a significant impact on rates of disability in the world [2]. As we know, disability is the major socio-economic impact of RA, during the first three years after the disease onset steady disability in 27% of RA patients has marked, and in 8-11 years occurs in approximately 85% of patients who require constant modern medical treatment, rehabilitation and often complex orthopedic surgery [3]. The direct costs (eg, medication, office visits, surgeries) associated with the treatment of RA have been estimated at $8.4 billion annually in the United States [4].
In recent decades, the RA treatment strategy radically changed the by early intensive treatment to achieve remission as a primary therapeutic goal [5]. Significant success in the RA treatment was reached by biological agents implementation into clinical practice. Data from 1993 to 2008 from the Nationwide Inpatient Sample, Healthcare Cost and Utilization Project showed the number of total knee replacements and total hip replacements performed annually increased, yet the number of procedures associated with RA declined [6].
Thus, in recent years RA mortality structure has changed. At present, the main cause of premature mortality in RA (more than 50% of cases) is atherosclerotic cardiovascular disease [7]. In 2009, the European League Against Rheumatism (EULAR) taskforce recommended screening, identification of CVD risk factors and CVD risk management in RA pts largely based on expert opinion [8]. In view of substantial new evidence, 2015/2016 years’ update was conducted [9] with notes that validated RA-specific CVD risk prediction models with a proven superiority over general population CVD risk prediction algorithms are currently lacking.
Thus, the limited traditional factors predictive role in RA pts, and the inconsistency of their effects requires the cardiovascular risk additional markers introduction into the clinical practice and research for ways of influencing them. Recent studies have shown the most common comorbidity condition in RA such as hypertension, insulin resistance, dyslipidaemia, adipose tissue metabolic disorders and obesity [10,11,12]. However, it is little known about association between this factors and subclinical manifestation of atherosclerosis in RA pts.Endothelial dysfunction is highly prevalent in RA and considered as a link between cardiometabolic factors and early stage of atherogenesis [13,14]. Endothelial dysfunction correction may be useful part of primary prevention in RA pts. In this way L-arginine aspartate demonstrated white adipose tissue and insulin resistance reducing possibility [15]. We aimed to evaluate the level of insulin resistance, serum adiponectinin females with rheumatoid arthritis in combination with hypertension, its interrelation with subclinical atherosclerosis and dynamics with the endothelial dysfunction correction.
Patients and Methods
Patients: The present study was conducted with approval from the Ethics Committee at State Establishment «Dnipropetrovsk Medical Academy of Health Ministry of Ukraine» according to the principles outlined in the Helsinki declaration.
All participants of research gave informed, written consent. 69 females from 45 to 65years with established diagnosis of RA (according to the 1988 American College of Rheumatology and 2010 American College of Rheumatology/EULAR criteria for RA) and HT (according to 2013 ESC Guidelines) were enrolled. Only female subjects were selected considering the gender structure of RA. All patients received stable therapy of RA more than 6 months. Рatients with verified diagnosis of ischemic heart, disease diabetes mellitus and disease activity of RA DAS28 > 3.2 haven’t included in the study.
The median systolic blood pressure was 136.5 [131.5; 142.9] mm Hg, diastolic-77,98 [74,1; 83.4] mm Hg. The RA activity index on the DAS28 scale was 2.6 [2.1; 2.9] points. 17 (40.5%) RA females in combination with hypertension have obesity of І-ІІ degree (BMI 30,0-39,9 kg/m2), 17 (40,5%) pts were with increased BMI 25.0-29.9 kg/m2) and 8 (19.0%)-with a normal BMI (18.5-24.9 kg/m2).
Basic disease-modifying RA treatment received 50 (80.6%) patients: methotrexate with the average dose 15 [10; 15] mg per week, the average duration of treatment with methotrexate 66 [48; 90] months. Glucocorticoids received 39 patients (67.2%), the average daily dose of methylprednisolone was 4 [2; 4,5] mg, the mean duration of glucocorticoid therapy-60 [30; 93] months. Antihypertensive treatment received 42 (72.4%) of HT patients: ACE inhibitors-30 (71.4%) patients, angiotensin II receptor antagonist–11 (26.2%), b-blockers- 8 (19.0%), diuretics-15 (35.7%). Statins received 30 (43.5%) enrolled patients.
Assessments:Height, weight, and waist and hip circumference were measured using standard approaches. Overall adiposity, abdominal obesity, and fat distribution were estimated by the body mass index (BMI), waist circumference, and waist-hip ratio, respectively. We assessed systolic blood pressure (SBP), diastolic blood pressure (DBP), disease activity for RA by the Disease Activity Score for 28 joints based on erythrocyte sedimentation rate (DAS28-ESR). C-reactive protein concentrations were determined using immunoturbidimetric methods. Standard laboratory blood tests of erythrocyte sedimentation rate, renal and liver function, hematological parameters, lipids, and glucose were performed. The glomerular filtration rate was estimated using the CKD-EPI formula. Cardiovascular risk was assessed by SCORE models adapted for patients with RA by introducing a 1.5 multiplication factor and total cholesterol/HDL cholesterol ratio according to EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis.
Insulin and adiponectin concentrations were measured at the baseline and after 4 weeks using solid-phase sandwich enzyme-linked immunosorbent assays (ELISA) (DRG, Germany and ASSAYPRO, USA respectively). Normal adiponectin concentration in blood serum was considered 3-14 ng/mL, insulin-2-25 µIU/mL. The level of insulin resistance, function of pancreas cells, peripheral tissue sensitivity to insulin index were calculated using the standard formula HOMA1 (E. Bonora, 1998), HOMA2 (Wallace T, 2004) using HOMA 2 Calculator Version 2.2.2. HOMA1-IR values above 2.77 and HOMA2-IR above 1.00 testified insulin resistance. Using both indices HOMA1, HOMA2 was due to the lack of clear guidance of the insulin resistance model using in patients with HT with RA. Determination of flow mediated vasodilation of brachial artery was performed at the baseline and after 4 weeks based on Celermajer’s recommendation (1992). The diameter of the brachial artery was measured with 7.5 MHz transducer of «Philips Envisor C». Duplex extracranial carotid arteries scaning was performed according to recommendations of the American Society of Echocardiography (2005). Scanning was performed on the unit «Philips Envisor C» in the presence of pulsed color Doppler and Tissue Doppler, using linear sensors 5, 7.5 MHz and 3.5 MHz convex transducer. The presence of subclinical atherosclerosis displays installed at intima-media thickening (> 0.9 mm) or imaging of atherosclerotic plaques (AP).
Statistical analysis of the data was carried out using licensed software STATISTICA 6.1, SPSS version 22.0 and Microsoft Excel 2013. Due to the distribution of data using non-parametric statistical methods-quantitative characters were presented as median (Me) and interquartile range [25% 75%]. For comparison of two independent groups of criteria used U-Mann-Whitney and Pearson Square - to compare ratios. For a comparison of all three groups have used multiple comparison of univariate analysis of variance Kruskal-Wallis (Kruskal-Wallis ANOVA). Assessment of the relationship between pairs of independent features, expressed in quantitative value, performed by the coefficient of rank correlation P. Spearman - R. In addition, to determine the independence indices performed uni- and multivariate (binary logistic) regression analysis. To determine the diagnostic efficiency of adiponectin and IR used ROC-analysis, calculating the area under the ROC-curve (AUROC). Statistically significant differences were determined research results at the level of p < 0,05.
Study Design:Patients were devided into three groups: The 1st group made up 29 females with RA combined with hypertension, 2nd group–20 patients with RA, 3rd group–20 patients with hypertension. All groups have been matched by age, disease duration, received therapy. In the endpoint patients were prospectively randomly and blindly divided into study group patients, which received L-Arginine aspartate (Tivortin, "Yuriya-Farm", Kiev, Ukraine) in an oral solution 30 ml/day during 4 weeks (average period evaluating the effectiveness according to the literature) in addition to standard treatment, and control group–received placebo.
Results
HT females with RA characterized by significant 5 times higer SCORE level in compare with RA group (p = 0.02). Median of cardiovascular risk matched by mSCORE in HT females with RA was 4 [1.0; 5.5] %, the number of patients with low risk-12 (28.6%), with the average risk-17 (40.4%), high and very high risk-13 (31%). Simultaneously carotid atherosclerotic changes identified by ultrasound in 33 (78.6%) RA females in combination with hypertension (figure 1). AP were established in 23 (54.8%) pts, increased intima-media thickening-in 19 (45.2%) HT females with RA. The higher incidence of AP in patients with RA in combination with hypertension compare to controls also remained statistically significant with regard to the number of traditional cardiovascular risk factors. Determined that HT patients with RA and subclinical atherosclerosis manifestations had significantly higher levels of cardiovascular risk matched by mSCORE scale, but still interpreted to 70.5% as moderate risk (figure 1a). Thus 84% of moderate risk by mSCORE patients have carotid atherosclerosis estimated by US (figure 1b).
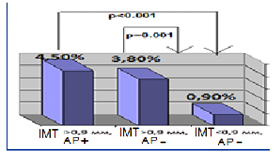
Figure 1a: Cardiovascular risk level matched by mSCORE in HT females with RA depending on subclinical atherosclerosis presence.
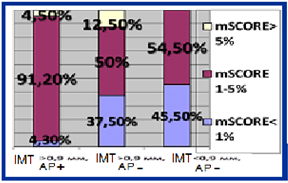
Figure 1b: Structure of cardiovascular risk matched by mSCORE in HT females with RA depending on subclinical atherosclerosis presence.
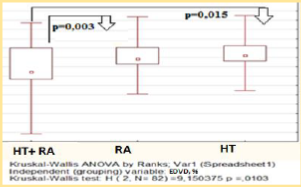
This results may indicate the importance of additional factors in the overall cardiovascular risk formation that is not reflected by traditional and modificated scales. Thus in our study endothelial function measured by EDVD was significantly lower among the HT females with RA – median level was 4,9 [3,3; 10,0] %, endothelial dysfunction has been established in 31 (73,8%) pts (p < 0.05) (figure 2). Significant correlation have been established between EDVD level and of DAS28 (R = -0.68, p < 0.05), SCORE level (R = -0,65, p < 0.001), waist circumference (R = 0.48, p < 0.05), GFR (R = 0,58, p < 0.05).
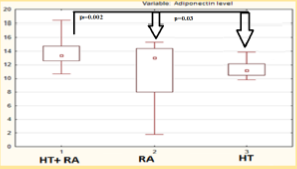
Median serum adiponectin concentration in HT females with RA was 13.8 [12.5; 14.8] mg/ml significantly higher in isolated HT, RA patients (Figure 3) and with subclinical atherosclerosis manifistations (p < 0,05). Serum adiponectin level was correlated with the intima-media thickness (R = 0,78, p < 0.05), DAS28 index (R = 0,41, p < 0.05), the level of cardiovascular risk by mSCORE (R = 0,44 p < 0.05), insulin level (R = 0,33, p < 0.05), the waist/hips ratio (R = 0 33, p < 0.05), HOMA2 index (R = 0,33, p < 0.05), EDVD level (R = -0,47, p < 0.05). The results of regression analysis showed factors associated with increased adiponectin level were insulin resistance (IR) (OR = 1,354, p = 0.04, 95% CI 1,10-2,6), RA activity matched by DAS28 (OR = 2,037, p = 0.049, 95% CI 1,50-2,3), glucocorticosteroid therapy presence (OR = 1,288, p = 0.048, 95% CI 1,06-1,35).
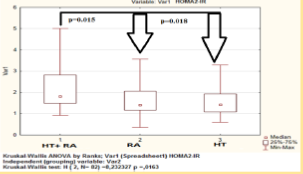
IR was estimated in 21 (50%) and 36 (85.7%) of HT females with RA matched by HOMA1-IR and HOMA2-IR respectively, the median level was 3.3 [2.6; 5.5] and 1.8 [1.5; 2.8] respectively (Figure 4). The presence of atherosclerotic changes in patients with RA in combination with hypertension was associated with IR (Table 1). НОМА1-2R level was correlated with waist circumference (R = 0.48, p < 0.05), DAS28 (R = 0.36, p < 0.05), EDVD (R = -0.43, p < 0.05), GFR (R = -0.34, p < 0.05) and association with adiponectin level (R = 0,27, р < 0,05). According to our data use of the model HOMA2-IR, perhaps more fully reflects the presence of insulin resistance and its association in hypertensive females with RA. Data demonstrates increased functional activity of the pancreas insular part, and significant decrease in peripheral insulin receptors sensitivity in this patients group.
| Indicators | Subclinical Atherosclerosis + | Subclinical Atherosclerosis - | Р* |
| Insulin level | 20,3 [12,8; 24,7] | 11,8 [11,4; 12,5] | 0,014 |
| Adiponectin level | 15,3 [12,8; 17,7] | 13,3 [11,8; 14,7] | 0,003 |
| НОМА1-IR | 4,4 [2,7; 5,6] | 2,7 [2,5; 2,8] | 0,051 |
| НОМА2-IR | 2,6 [1,6; 3,2] | 1,5 [1,4; 1,6] | 0,011 |
| HOMA2-%B | 168,8 [122,2; 221,7] | 135,8 [111,4; 155,8] | 0,230 |
| HOMA2-%S | 38,7 [32,0; 57,3] | 66 [63,2; 67,2] | 0,014 |
* - differences between groups p < 0.05 (by Mann-Whitney test)
Table 1: Insulin, insulin resistance, adiponectin levels in patients with rheumatoid arthritis in combination with hypertension depending on subclinical atherosclerosis presence.
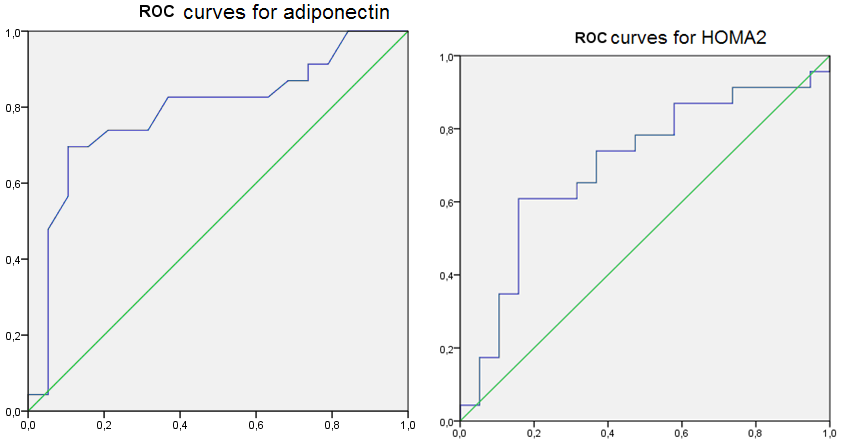
These results created conditions to assess the diagnostic efficacy of serum adiponectin level and IR determine in HT females with RA. For this, we used the ROC-analysis (Figure 5), the results of which stated good quality diagnostic model as AUROC for adiponectin was 0.787 (95% CI 0,642-0,932, p < 0.05) and 0.700 (95% CI 0,536-0.864, p < 0.05) for the HOMA2 index.
According to the study second phase results it was estimated increased mean EDVD by 23.2% on the whole (p < 0.001) after 4 weeks of treatment with L-Arginine, in compare with standard therapy–on 20.2% (p = 0.03). Paradoxical vasoconstriction was corrected in all patients. Endothelial function normalizing was achieved in 25 (65.8%) of L-Arginine group of patients, i.e. the number of patients with a normal level of EDVD was increased by 34.2% in this group, that significantly higer than in standart therapy group (p < 0.001). Table 2 shows after 4 weeks of L-Arginine aspartate supplementation EDVD was significantly higher than in standard therapy group (p < 0.001). The level of EDVD to end of the observation in the study subgroups was 10.7 [10.3; 11.0] % in HT patients with RA, 9.2 [9.1; 10.5] % in RA patients, 9.7 [9.5; 10.7] % in HT patients, i.e. EDVD was increased in this groups on 58.5% (р = 0.003), 17.4% (p = 0.03), 13.4% (p = 0.04) respectively. Preferential increase number of patients with a normal level of EDVD was observed in HT patient with RA–on 33.6% (p < 0.001), in RA patients–on 5.4% (p = 0.05), in HT patients – on 4.8% (p = 0.07). It should be noted, that in HT patients with RA was maximal number of patients with corrected endothelial function-12 (80.0%), in RA group–5 (45.5%), in HT group–7 (58.3%).
The level of insulin to the end of the study was lower on 9.3% (p = 0.004), insulin resistance–on 7.9% (p = 0.004) matched by НОМА1-IR and on 18.7% (p = 0.002) matched by НОМА2-IR among patients received L-Arginine aspartate. Thus the insulin resistance correction in this patients was mainly due to increase the sensitivity of insulin receptors, as evidenced by the dynamics of indicators HOMA2-%B, HOMA2-%S – -4.3% (р = 0,003) and +15.5% (р < 0,001) respectively. In the standard treatment group these parameters in the dynamics weren’t differ significantly. The level of insulin resistance matched by НОМА1-IR, НОМА2-IR to end of the observation in the study subgroups was respectively 3.0 [2.6; 3.6], 1.3 [1.0; 1.8] in HT patients with RA, 2.6 [2.2; 3.2], 1.3 [1.1; 1.8] in RA patients, 2.3 [2.0; 2.8], 1.2 [1.0; 1.7] in HT patients. Thus pronounced dynamics of reducing insulin resistance was observed by index НОМА2-IR in HT patients with RA–reducing by 22.8% (p = 0.001) (figure 6). The inclusion of L-arginine aspartate contributed to the most significant increase HOMA2-%S on reaching EDVD ≥ 10% – on 32.8 % compare to patients with EDVD < 10% (table 3). In patients with endothelial dysfunction correction observed a significant decrease of adiponectin in compare to preserved endothelial dysfunction patients on 11.2 % (p = 0.04).
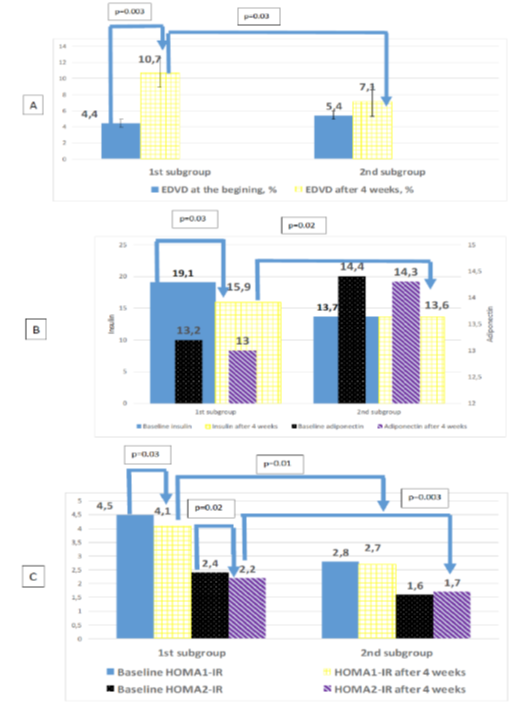
Figure 6: Dynamics levels of endothelium dependent vasodilatation (A), adiponectin, insulin (B), insulin resistance (C) after 4 week in hypertensive patients with rheumatoid arthritis.
| EDVD after 4 weeks ≥ 10% N = 35 | EDVD after 4 weeks < 10% N = 34 | Р | |
| Adiponectin, ng/ml | 12,7 [11,8; 13,8] | 14,3 [13,1; 15,3] | 0,04 |
| Insulin, µIU/mL | 10,0 [5,7; 15,9] | 11,3 [7,6; 21,1] | 0,06 |
| НОМА1-IR | 2,7 [2,6; 4,2] | 4,3 [3,8; 5,2] | 0,07 |
| НОМА2-IR | 1,5 [1,3; 1,9] | 2,3 [2,0; 2,6] | 0,04 |
| HOMA2-%B | 138,0 [124,0; 168,0] | 180,0 [146,5; 215,0] | 0,04 |
| HOMA2-%S | 58,0 [38,5; 64,0] | 39,0 [27,5; 42,0] | 0,02 |
Table 2: The levels of adiponectin, insulin, insulin resistance depending on the degree of endothelial function correction.
| Cardiometabolic risk factors | Study patients (n = 69) | |||
| +L-Arginine aspartate (n = 38) | -L-Arginine aspartate (n = 31) | |||
| Baseline | After 4 weeks | Baseline | After 4 weeks | |
| EDVD, % | 7,6 [4,8; 7,8] | 9,9 [9,3; 11,1]** | 7,4 [4,6; 7,7] | 7,9 [4,8; 7,6]# |
| EDVD < 10% (%) | 68,4 | 34,2** | 70,9 | 67,7# |
| Adiponectin, ng/ml | 12,8 [11,2; 12,0] | 12,6 [10,8; 11,8] | 12,1 [11,4; 12,3] | 12,0 [11,5; 12,2] |
| Insulin, µIU/mL | 12,9 [10,6; 13,8] | 11,7 [9,9; 13,2]* | 12,7 [12,5; 13,1] | 12,5 [12,3; 12,9] |
| НОМА1-IR | 3,8 [2,7; 5,1] | 3,5 [2,5; 4,8]* | 3,7 [2,5; 5,2] | 3,7 [2,4; 5,0] |
| НОМА1-IR > 2.77 (%) | 57,9 | 50,0 | 64,5 | 61,2 |
| НОМА2-IR | 1,6 [1,3; 1,8] | 1,3 [0,9; 1,5]* | 1,5 [1,2; 2,0] | 1,4 [1,0; 1,9] |
| НОМА2-IR > 1.00 (%) | 68,4 | 47,4* | 64,5 | 58,1 |
| HOMA2-%B | 132,6 [111,9; 134,6] | 122,6 [97,9; 129,7]* | 131,0 [100,4; 135,6] | 129,8 [102,4; 133,4] |
| HOMA2-%S | 61,1 [41,4; 66,8] | 67,3 [38,6; 72,8]* | 62,2 [40,6; 58,7] | 63,1 [45,6; 57,1]# |
* significant differences between groups in dynamics p < 0.05 (by Wilcoxon test) ** p < 0.001
# - significant differences between the L-Arginine aspartate group and the standart treatment
group p < 0.05 (by Mann-Whitney test)
Table 3: Dynamics endothelium dependent vasodilatation (EDVD), adiponectin, insulin, insulin resistance levels after 4 weeks in study patients.
Discussion
Thus, in HT females with RA observed increased frequency of subclinical manifistation of atherosclerosis, including carotid AP presence with instability signs. However, cardiovascular risk structure by mSCORE in hypertensive females with RA and subclinical atherosclerosis manifestations shows moderate risk dominating. This results correspond to the latest data that mSCORE (the EULAR modification with 1.5 coefficient applied after meeting the above criteria) underestimated the risk in low- and moderate risk patients, while most of the RA patients were classified into these two groups. Furthermore, existing datas showed mSCORE overestimated the risk in high-risk RA patients. Comparative research has shown that the SCORE equation and its modification by the EULAR experts are insufficient to indicate precisely the populations requiring risk reduction. In the study led by Arts [16], four risk equations were compared (FHS, SCORE, RRS, QRISK-2), proving that none of the algorithms was able to correctly classify RA patients into specific risk groups [17]. These study results support the need for population specific cardiovascular risk stratification models with the consideration of vascular imaging and, potentially, the use of novel CVD risk biomarkers in HT patients with RA. Equally important, the reported study results reinforces the need for more awareness in daily clinical practice of increased cardiovascular risk in HT females with RA and the notion that much more research is necessitated in this field. Our study results correspond to the Gonzáles-Gay., et al. [18], ultrasound of carotid arteries could be recommended to the majority of RA patients, especially in patients classified in the moderate CV risk group with additional risk factors.
It should be noted associative link between the presence of atherosclerotic vascular lesions with increased levels of adiponectin in patients with hypertension combined with RA was not correlated with the data obtained for the general population [19]. The similar results were obtained Dessein., et al. isolated in patients with RA [20]. Perhaps this indicates a non-linear relationship between atherosclerotic vascular lesions and circulating levels of adiponectin in conditions combined current RA and hypertension, which requires further detailed study. Also established IR presence associated with increased adiponectin level indicating a complex and possibly specific combination of RA and HT exchange relationship between muscle and adipose tissue also needs further study.
Current data support a complex relationship of endothelial dysfunction and cardiometabolic factors in HT, RA presence. According to our data, endothelial function was significantly lower in comorbidity of HT and RA and was associated mainly with abdominal obesity, inflammation activity and IR. Perhaps this may indicate a relationship with metabolic factors such as the adipose tissue exchange. Thus, as endothelial dysfunction is one of the early stages of atherosclerosis, it is reasonable to consider that substances secreted by adipose tissue may influence directly or indirectly (for instance by induction of microinflammation) the function of endothelial cells [21].
Present study shows that the correction and expectly normalizing of endothelial dysfunction in HT patients with RA with L-Arginine aspartate supplementation may caused benefit effects on range of cardiometabolic factors. Current obtained data may indicate the key role of endothelial function in reducing the sensitivity of peripheral insulin receptors and adiposity dysfunction in HT patients with RA, which requires more detailed study. As such, assessments of endothelial function on example of L-Arginine aspartate supplimentation could prove to be useful tools in the identification and monitoring of cardiometabolic risk in HT patients with RA. It should be noted that the increase in insulin sensitivity under the influence of L-arginine is also noted in other studies. Thus, R. Lucotti et al. [22] in a randomized, double-blind study involving 64 patients with CVD without diabetes after coronary artery grafting bypass orally for 6 months L-arginine at a dose of 6.4 g/day, established EDVD increase (p < 0.01), decrease in insulin resistance (p < 0.05) and increased adiponectin levels (p <0.01). In a study conducted Piatti P M. et al. oral administration of 9 grams/day of L-arginine for 1 month in patients with type 2 diabetes has led to a significant increase in forearm blood flow (36%), normalization initially reduced cGMP level, decrease in systolic blood pressure by 14% and improved sensitivity to insulin [23]. In 2011 Dong, J.Y., et al. published meta-analysis of 11 randomized, double-blind, placebo-controlled trials used oral L-arginine supplementation showed possible insulin resistance reducing effect [24].
Including L-Arginine aspartat in complex therapy in HT patients with RA appear to have added benefits with respect to endothelial function, but whether these benefits translate into improved outcomes remains unknown. Answers to these questions gained through future investigations may give us the knowledge to develop and implement novel interventions and strategies to reduce the morbidity and mortality of HT patients with RA. The dynamics EDVD increase, decrease HOMA index may indicate confirmation that endothelial dysfunction is the "cornerstone" of not only HT patients with RA progression, but also the development of metabolic changes that accompany it.
Limitations
However, the results of this study should be interpreted with caution because of several limitations. Therefore, only female subjects were chosen for this study, and consequently the results can only be applied to the female gender. It should be noted that pts had stable therapy of RA more than 6 mounths and low disease activity. In this way L-arginine suplimentation effects may differ in RA pts with higher inflamation level or therapy changes. The study was carried out in a 4-weeks period, and as that time was not enough for adequate evaluation of hard end points, such as insulin resistance and adiponectin normalisation, the study assesses only intermediate end points. However, it cannot be established from the present study whether the changes observed were permanent or reversible. In this regard, more prolonged L-arginine supplimentation study in hypertensive patients with rheumatoid arthritis requires.
However, the results of this study should be interpreted with caution because of several limitations. Therefore, only female subjects were chosen for this study, and consequently the results can only be applied to the female gender. It should be noted that pts had stable therapy of RA more than 6 mounths and low disease activity. In this way L-arginine suplimentation effects may differ in RA pts with higher inflamation level or therapy changes. The study was carried out in a 4-weeks period, and as that time was not enough for adequate evaluation of hard end points, such as insulin resistance and adiponectin normalisation, the study assesses only intermediate end points. However, it cannot be established from the present study whether the changes observed were permanent or reversible. In this regard, more prolonged L-arginine supplimentation study in hypertensive patients with rheumatoid arthritis requires.
Conclusions
- Hypertensive females with rheumatoid arthritis are characterized by high frequency of subclinical atherosclerosis do not fully reflected by a modified SCORE. Analysis of additional risk factors allowed to reveal in hypertensive patients with rheumatoid arthritis the presence of signs of endothelial dysfunction and increased adiponectin, insulin resistance level. Good quality diagnostic model for adiponectin and HOMA2 index were determined and hence, they could be taken into account when evaluating cardiovascular risk in this pts.
- Oral supplementation of L-arginine causes multiple beneficial effects on the complex of cardiometabolic factors including: endothelial dysfunction, peripheral insulin resistance, adipose tissue exchange in hypertensive females with rheumatoid arthritis.
References
- Burgers L., et al. “Longterm outcome of Rheumatoid Arthritis defined according to the 2010-classification criteria”. Annals Rheumatic diseases 73.2 (2013): 428-432.
- Cross M., et al. “Global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study”. Annals Rheumatic Diseases 73.7 (2014): 1316–1322.
- Yelin E. “Work disability in rheumatic diseases”. Current Opinion in Rheumatology 9.2 (2007): 91-96.
- Birnbaum H., et al. “Societal costs of rheumatoid arthritis patients in the US”. Current Medical Research and Opinion 26.1 (2010): 77-90.
- Smolen J., et al. “Treating rheumatoid arthritis to target: recommendations of an international task force”. Annals Rheumatic Diseases 69.4 (2010): 631–637.
- Hekmat K., et al. “Decrease in the incidence of total hip arthroplasties in patients with rheumatoid arthritis—results from a well defined population in south Sweden”. Arthritis Research & Therapy 13.2 (2011): R67.
- Avina-Zubieta J., et al. “Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies”. Arthritis & Rheumatology 59.12 (2008): 1690-1697.
- Peters MJ., et al. “EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis”. Annalsof the Rheumatic Diseases69.2 (2010): 325-331.
- Agca R., et al. EULAR recommendations for cardiovascular update inflammatory joint disorders: 2015/2016 rheumatoid arthritis and other forms of disease risk management in patients with Annals of the Rheumatic Diseases 0 (2016): 1-12.
- F Lago., et al. “Cardiometabolic comorbidities and rheumatic diseases: focus on the role of fat mass and adipokines”. Arthritis Care and Research 63.8 (2011): 1083–1090.
- Ferraz Amaro., et al. “Insulin resistance and rheumatoid arthritis” Reumatología Clínica 7.2 (2011): 124–129.
- Panoulas VF., et al. “Hypertension in rheumatoid arthritis”. Rheumatology 47.9 (2008): 1286–1298.
- Sitia S., et al. “From endothelial dysfunction to atherosclerosis”. Autoimmunity Reviews 9.12 (2010): 830–834. https://www.ncbi.nlm.nih.gov/pubmed/20678595
- Versari D., et al. “Endothelial dysfunction as a target for prevention of cardiovascular disease”. Diabetes Care 32(Suppl 2) (2009): 314–321.
- Piatti PM., et al. “Long term oral L–arginine administration improves peripheral and hepatic insulin sensitivity in Type 2 diabetic patients”. Diabetes Care 24.5 (2001): 875-880.
- Arts EEA., et al. “Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis”. Annals of the Rheumatic Diseases 74.4 (2015): 668–674.
- Krzysztof Bonek. “Cardiovascular risk assessment in rheumatoid arthritis – controversies and the new approach”. Reumatologia 54.3 (2016): 128–135.
- Gonzбlez-Gay MA., et al. “Carotid ultrasound in the cardiovascular risk stratification of patients with rheumatoid arthritis: when and for whom?” Annals of the Rheumatic Diseases 71.6 (2012): 796-798.
- Young Hee Rho., et al. “Adipocytokines, Insulin Resistance and Coronary Atherosclerosis in Rheumatoid Arthritis”. Arthritis & Rheumatology 62.5 (2010): 1259–1264.
- H Dessein., et al. “Rheumatoid arthritis impacts on the independent relationships between circulating adiponectin concentrations and cardiovascular metabolic risk”. Mediators of Inflammation 20 (2013): 461849.
- He M., et al. “Endothelial dysfunction in rheumatoid arthritis. The role of monocyte chemotactic protein-1-induced protein”. Arteriosclerosis, Thrombosis, and Vascular Biology 33.6 (2013): 1384–1391.
- Lucotti P., et al. “Beneficial effects of a long-term oral L-arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients”. American Journal of Physiology - Endocrinology and Metabolism 291.5 (2006): 906–912.
- Dong JY., et al. “Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials”. American Heart Journal 162.6 (2011): 959-965.
Citation:
Olexandr KURYATA and Oksana SIRENKO. “The Interrelation of Insulin Resistance, Serum Adiponectin Level in Rheumatoid Arthritis Hypertensive Females with Subclinical Atherosclerosis and its Dynamics with the Endothelial Dysfunction Correction”. Orthopaedic Surgery and Traumatology 1.5 (2017): 162-173.
Copyright: © 2017 Olexandr KURYATA and Oksana SIRENKO. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.