Research Article
Volume 1 Issue 5 - 2017
Physiological Responses of Nestling Blue Macaw (Anodorhynchus hyacinthinus-Latham, 1790) Submitted to Physical Containment, Pant anal - Ms.
1Blue Macaw Institute-Pantanal/MS/Brazil
2University of Vila Velha-ES/Brazil
2University of Vila Velha-ES/Brazil
*Corresponding Author: Marina Drago Marchesi, Blue Macaw Institute-Pantanal/MS/Brazil.
Received: December 06, 2017; Published: December 09, 2017
Abstract
One of the most stressful management practices for free-living wild birds is physical containment. Physiological responses of the body facing stressful situations can be analyzed by measuring blood lactate. The present study determined the values of serum lactate in nestling Anodorhynchus hyacinthinus (n = 22) in the Pantanal - MS, undergoing containment and handling, and compared the findings with the age, weight and the presence or not of parents nearby during the handling of them. The samples were analyzed with Accutrend® Plus - Roche by reflectance photometry. The results obtained ranged from 4.7 mmol/L 11.2 mmol/L, and were independent of body weight, age, and presence or absence of the parents.
Keywords: Wild birds; Parrots; Stress management
Introduction
The blue macaw (Anodorhynchus hyacinthinus) is the largest representative of the Psittacidae family (Sick, 1997). From the conservation point of view, the popularity and charisma make the macaw a "flag species" of extreme importance for biodiversity (Nunes and Betini, 2002).
One of the ecosystem that houses a large number of bird species is the Pantanal (Nunes., et al. 2005) and even the endangered ones, such as the blue macaw (Anodorhynchus hyacinthinus), are seen with relative frequency, having their largest population inhabiting the Pantanal (Guedes 2009; Marchesi., et al. 2015).
A. hyacinthinus nestlings receive parental care for 12 to 18 weeks of age (Guedes, 2004), which makes them less vulnerable to stressors (De Bellis., et al. 1999; (Wright., et al. 2001), and reach late sexual maturity, like the other parrots (Wright., et al. 2001).
Information on the health conditions of wild birds helps to preserve the species, as well as providing an improvement of appropriate management techniques (Daszak, 2000). Lactate, a product of body metabolism (Stockham and Scott, 2008), is independent of weight (Papoti., et al. 2003) and is directly related to sexual maturity (Villar, 2001).
A better understanding of the physiological impacts resulting from capture and manipulation can identify factors that provide an overview to minimize stress during management (Harms., et al. 2003). The objective of this study was to determine the value of serum lactate levels in A. hyacinthinus nestling in the Pantanal, subjected to physical containment stress and venous puncture, correlating these values with age, weight and presence or not of the parents during management.
Material and Methods
Place of study
The study was conducted at the Blue Macaw Institute, located in the Caiman Ecological Refuge - REC (19º51'-19º58'S and 56º17'-56º24'W), Miranda, Pantanal-Mato Grosso do Sul/Brazil.
The study was conducted at the Blue Macaw Institute, located in the Caiman Ecological Refuge - REC (19º51'-19º58'S and 56º17'-56º24'W), Miranda, Pantanal-Mato Grosso do Sul/Brazil.
Capture of the nestling
The nestling were captured inside natural and artificial nests, made for A. hyacinthinus, by ascending technique with tree climbing equipment, and placed inside buckets to be transferred from the nest to the ground. During this process, some parents stayed close to the nestling, perched on neighboring trees, maintaining contact with them by means of vocalization.
The nestling were captured inside natural and artificial nests, made for A. hyacinthinus, by ascending technique with tree climbing equipment, and placed inside buckets to be transferred from the nest to the ground. During this process, some parents stayed close to the nestling, perched on neighboring trees, maintaining contact with them by means of vocalization.
Collection of material
After physical containment 1.5 ml of blood was collected from the ulnar vein with the aid of hypodermic needle 25 x 7 and 3 ml syringe. For the present study, a drop of blood was used, the excess of the material was destined to other researches.
After physical containment 1.5 ml of blood was collected from the ulnar vein with the aid of hypodermic needle 25 x 7 and 3 ml syringe. For the present study, a drop of blood was used, the excess of the material was destined to other researches.
Determination of blood lactate
A blood sample was placed on the disposable test strip to determine the value of serum lactate through the Accutrend® Plus - Roche apparatus. In 60 seconds the instrument measures the intensity of the test strip reaction by means of reflectance photometry, assuming the value in the sample. The statistical analysis used to determine the influence of weight and age on the lactate value was linear regression. For the influence of presence or absence of the parents on the lactate value, the t-test with a 95% confidence interval was performed.
A blood sample was placed on the disposable test strip to determine the value of serum lactate through the Accutrend® Plus - Roche apparatus. In 60 seconds the instrument measures the intensity of the test strip reaction by means of reflectance photometry, assuming the value in the sample. The statistical analysis used to determine the influence of weight and age on the lactate value was linear regression. For the influence of presence or absence of the parents on the lactate value, the t-test with a 95% confidence interval was performed.
Results
Samples of 22 nestlings aged between 50 and 100 days (Table 1), weighing between 950 and 1500g (Table 2) and lactate values between 4.0 and 12 mmol/L (Table 3) were obtained. During the physical containment and management procedure, in eight different nests, the parents were nearby and the other 14 were absent.
| Age (days) | Number of nestlings |
| 50-70 | 7 |
| 71-80 | 9 |
| 81-100 | 6 |
| Total | 22 |
Table 1: Number of nestlings studied and their respective age ranges (days).
| Weight (g) | Number of nestlings |
| 950-1000 | 2 |
| 1001-1200 | 9 |
| 1201-1400 | 5 |
| 1401-1500 | 6 |
| Total | 22 |
Table 2: Number of nestling studied and their respective weight ranges (g).
| Lactate (mmol/L) | Number of nestlings |
| 4,0- 6,9 | 9 |
| 7,0-10,0 | 7 |
| 10,1-12,0 | 6 |
| Total | 22 |
Table 3: Number of nestlings studied and their respective lactate intervals (mmol/L).
The influence of the variable weight (g) in relation to lactate (mmol/L), when the square R is equivalent to 0.05, was not relevant, that is, there is no significant relationship between the variables weight and lactate in the present study (Figure 1).
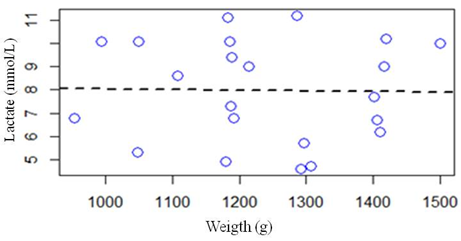
Figure 1: Linear regression analysis between a lactate response variable (mmol/L) and weight variable (g).
The influence of the variable age (days) in relation to lactate (mmol/L), when the square R is equivalent to 0.03, is shown in figure 2. The points in the graph indicate that there is also no significant relationship between the variables, that is, the value of lactate is independent of the age of the nestlings.
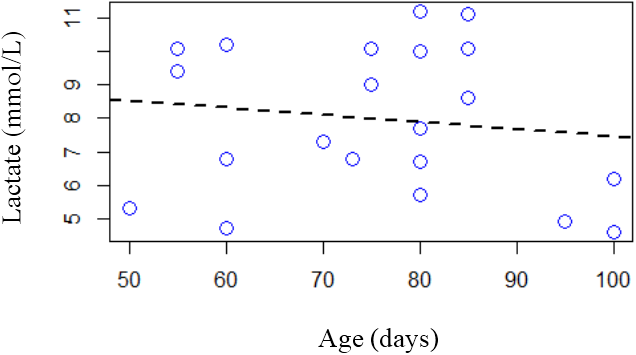
Figure 2: Linear regression analysis between the lactate response variable (mmol/L) and the variable age (days).
The lactate concentration of the nestlings, related to the presence or not of the parents during the management, did not have significant variation (figure 3).
Discussion
In a study of wistar rats, Papoti., et al. (2003) stated that animals with reduced body condition due to submition to hypoproteic diets had lower lactate concentrations when subjected to exercise load compared to animals with good body conditions and with normoproteic diet, that presented better performance and higher levels of lactate.
In the present study, the values of serum lactate were independent of the weight of the nestlings, corroborating with the findings of Papoti., et al. (2003), since the animals evaluated presented normal weight for the specie and slight variations regarding lactate levels.
Serum lactate values, found in A. hyacinthinus nestlings, did not show significant changes when compared to their age, according to Villar (2001), who states that sexual maturation leads to increased production of lactate in the body. Psittacidae are birds of late sexual maturity (Wright., et al. 2001) and the birds in the present study were still very young, completely dependent on their parents and nest.
The stress factor, promoted by physical restraint and venous blood collection in the nestling of the present study, was not dependent on the presence or not of the parents, because when exposed to physical containment, all nestlings presented similar values of serum lactate, independent of deprivation paternal and maternal, characterizing a finding contrary to the statements of De Bellis., et al. 1999; Newport., et al. 2002; Gluckman., et al. 2005.
Conclusion
The serum lactate value in free-living blue macaws nestlings ranged from 4.7 to 11.2 mmol/L in birds exposed to stress conditions due to manipulation and physical containment, showing no significant variations when compared to weigth, age and presence or not of parents during management.
References
- Daszak P., et al. “Emerging infectious disease of wildlife: threats to biodiversity and human health”. Science 287.5452 (2000): 443-449.
- De Bellis MD., et al. “A.E. Bennett Research Award. Developmental traumatology. Part II: brain development”. Biology Psychiatry 45.10 (1999): 1235-1236.
- FEITOSA, F.L.F. Semiologia Veterinária: a arte do diagnóstico. Editora Roca, São Paulo, 807p, 2004.
- Furtado E., et al. “Análise do consumo de oxigênio, freqüência cardíaca e dispêndio energético, durante as aulas do Jump Fit”. Revista Brasileira de Medicina do Esporte 10.5 (2004): 371-375.
- Gluckman PD., et al. “Environmental influences during development and their later consequences for health and disease: implications for the interpretation of empirical studies”. Proceedings of the Royal Society - Biological Sciences 272.1564 (2005): 671-677.
- Guedes NMR. “Araras Azuis: 15 anos de estudos no Pantanal”. In: IV SIMPÓSIO SOBRE RECURSOS NATURAIS E SÓCIO-ECONÔMICOS DO PANTANAL, 2004, Corumbá. Anais...Corumbá: Embrapa Pantanal, 2004: 53-62.
- Guedes NMR. “Sucesso reprodutivo, mortalidade e crescimento de filhotes de araras-azuis Anodorhynchus hyacinthinus (Aves, Psittacidae) no Pantanal, Brasil”. Tese de Doutorado, Universidade Estadual Paulista (Unesp), Botucatu, SP (2009).
- Harms CA., et al. “Venous blood gases and lactates of wild loggerhead sea turtles (Caretta caretta) following two capture techniques”. Journal of Wildlife Diseases 39.2 (2003): 366-374.
- Marhesi MD., et al. “Relationship between weight, age and hatching success and the concentration of heavy metals in nestling blue macaw (Anodorhynchus hyacinthinus Latham, 1790) in the Pantanal,Mato Grosso do Sul”. Pesquisa Veterinária Brasileira 35.6 (2015): 569-572.
- Newport DJ., et al. “Parental depression: animal models of an adverse live event”. American Journal of Psychiatry 159.8 (2002): 1265-1283.
- Nunes MFC and Betini GS. “Métodos de estimativa e abundância de psitacídeos. In: GALETTI, M.; PIZO, M. A (Ed.). Ecologia e conservação de psitacídeos no Brasil”. Belo Horizonte: Melopsittacus Publicações Científicas, (2002): 99-112.
- Nunes AP., et al. “Aves da Fazenda Nhumirim, Pantanal da Nhecolândia. Corumbá, MS”. Empresa Brasileira de Pesquisa Agropecuária (2005).
- Papoti M., et al. “Máxima fase estável de lactato durante a natação em ratos recuperados de desnutrição proteica”. Motriz 9.2 (2003):103-110.
- Sick H. Ornitologia Brasileira. Nova Fronteira, Rio de Janeiro, 912 (1997).
- Stockham SL and Scott MA. Monovalent Eletrolites and osmolarity. In: STOCKHAM, S. L.; SCOTT, M. A. (Ed.). Fundamentals of Veterinary Clinical Pathology. Ames: Blackwell Publishing, (2008): 495-558.
- Vila LG “Midazolam no estresse por contenção em aves silvestres”. Tese de Doutorado, Universidade Federal de Goiás. Goiás, GO (2015).
- Villar R. “Efeitos da Idade na Aptidão Física em Meninos Praticantes de futebol de 9 a 15 Anos”. Motriz 7.2 (2001): 93-98.
- Wright TW., et al. “Nest Poaching in Neotropical Parrots”. Conservation Biology (15.3): 710-720.
Citation:
Marina Drago Marchesi., et al. “Physiological Responses of Nestling Blue Macaw (Anodorhynchus hyacinthinus-Latham, 1790)
Submitted to Physical Containment, Pant anal - Ms.”. Multidisciplinary Advances in Veterinary Science 1.5 (2017): 192-197.
Copyright: © 2017 Marina Drago Marchesi., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.