Research Article
Volume 5 Issue 1 - 2020
Synthesize and Characterization of N-Benzalcefuroxime Which is Derived from
a β-Lactamantibiotic Cefuroxime with the Screening of Antimicrobial,
Anti-Inflammatory and Anti-Diabetic Potentials
a β-Lactamantibiotic Cefuroxime with the Screening of Antimicrobial,
Anti-Inflammatory and Anti-Diabetic Potentials
Department of Pharmacy, University of Science and Technology Chittagong (USTC), Bangladesh-4202
*Corresponding Author: Farhana Hoque, Department of Pharmacy, University of Science and Technology Chittagong (USTC), Chittagong-4202, Bangladesh.
Received: June 4, 2020; Published: June 13, 2020
Abstract
Cefuroxime is a β-lactam antibiotic. The Schiff base ligand N-benzalcefuroxime was synthesized from cefuroxime and benzaldehyde. This compound was characterized by elemental analysis, some spectral analyses (e.g. NMR, UV and IR) and some other physic-chemical techniques. The synthesized compound was screened for antibacterial activity by disc diffusion method and it showed very good antibacterial activity against some pathogenic bacteria. Ligands were found to be more active against both organisms. The SB ligand can be used against the diseases which are caused by these bacteria. Anti-inflammatory activity of SB ligand was good. The SB ligand showed no anti-diabetic activities. These ligands can be used as a starting materials for the complexation reaction. From the studies of anti-inflammatory and anti-diabetic activities, the compound showed good anti-inflammatory activity and no anti-diabetic activity.
Keywords: Keywords: Schiff base; cefuroxime; benzaldehyde; antibacterial; anti-inflammatory
Introduction
Organic synthesis is a special branch of chemical synthesis dealing with the synthesis of organic compounds. In the total synthesis of a complex product it may take multiple steps to synthesize the product of interest. Organic synthesis is a special branch of chemical synthesis dealing with the synthesis of organic compounds. Schiff bases are very important intermediate compounds for the synthesis of various bioactive compounds. They contain the azomethine group (-C=N-). These are usually formed by the consideration of an active carbonyl compound.[1] The electrophilic carbon atoms of aldehydes and ketones can be targets of nucleophilic attack by amines. This type of compound is known as an imine or Schiff base.2They can be referred to as imines.[3,4] Several Schiff bases have been reported. They possess different pharmacological activities such as antimicrobial, anti-inflammatory, antidepressant, antipyretic etc. Acylation of Schiff bases by acid anhydrides, acid chlorides and acyl cyanides is initiated by attack at the nitrogen atom and leads to net addition of the acylation agent to the carbon-nitrogen double bond. Reactions of this type have been put to good use in natural product synthesis. Several Schiff base ligands possess different pharmacological activities such as antimicrobial, anti-inflammatory, anti-depressant, anti-pyretic etc.[5] Schiff bases are common enzymatic intermediates where an amine, such as the terminal group of a lysine residue reversibly reacts with an aldehyde or ketone of a cofactor or substrate. The common enzyme cofactor PLP forms a Schiff base with a lysine residue and is transaldiminated to the substrate.[6] Cefuroxime, sold under the brand name Zinacef among others, is an antibiotic used to treat and prevent a number of bacterial infections such as pneumonia, meningitis, otitis media, sepsis, urinary tract infections and Lyme disease. It is used by mouth or by injection into a vein or muscle. Cefuroxime is the second generation of Cephalosporins. The cephalosporins are a class of β-lactam antibiotics originally derived from the fungus Acremonium, which was previously known as Cephalosporium”. Together with cephamycins, they constitute a subgroup of β-lactam antibiotics called cephems. Cephalosporins were discovered in 1945, and first sold in 1964. The antibacterial spectrum of cephalosporins resembles that of cefuroxime. Most gram negative and gram-positive microorganisms are sensitive to cephalosporins, but there is significant regional variation in susceptibility of Enterobacteriaceae to cephalosporin because of the spread of resistance [7]. Cefuroxime features Beta-lactamase stability Cefuroxime is stable to most beta-lactamases Cefuroxime can be considered for the therapy of urinary tract infections caused by beta-lactamases producing E.coli [8]. Considering the above facts, a schiff base was synthesized out of cefuroxime and benzaldehyde [9]. Under suitable condition, in the coordination complexes, the atoms or ions of nearly all of the metals can serve as central acceptors. The charged ions or neutral molecules surrounding the central metal ion are called the ligands, which contain at least one donor atom [10]. The free ligands and their metal complexes were screened for antimicrobial activities against Bacillus cerens, Escheria coli, Pseudomonas aeruginosa, Staphylococcus aureus and Candiadaalbicans [11,12]. The anti-inflammatory and antimicrobial properties of this schiff base evaluated in hope to establish it as an effective therapeutic agent [13].
Materials and Methods
Chemicals and reagents: All chemicals were of analytical grade and used as received. All solvents were purified using conventional methods. API (cefuroxime) was collected in pure state.
Physical measurement: IR spectra were registered on a Shimadzu Infrared Spectrophotometer using potassium bromide disc, within the range of 4000-400 cm-1 at the University of Chittagong. UV-visible spectrum was recorded on a UV-visible spectrophotometer at the University of Science and Technology Chittagong (USTC). 1HNMR spectra were registered on a Bruker NMR spectrophotometer using deuterated chloroform as solvent, at BCSIR laboratories, Dhaka. Melting point was determined on an electro-thermal melting point apparatus and TLC was performed using conventional TLC plates at the University of Science and Technology Chittagong. All chemical tests were performed in the University of Science and Technology Chittagong, Bangladesh.
Antimicrobial screening: Two pathogenic bacteria Staphylococcus aureus (gram positive) and Escherichia coli (Gram negative) were collected from the Department of Microbiology, University of Chittagong, Bangladesh. Nutrient agar was used as a culture media for bacteria while potato dextrose agar was employed in antifungal studies. The Schiff base was dissolved in methanol. The in-vitro antimicrobial activity of the Schiff base was assessed by disc diffusion method. The diameter of the zone of inhibition produced by the compound was compared with the parent drug, cefuroxime.
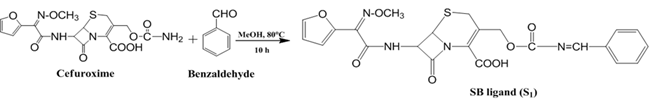
Preparation of Schiff base ligand derived from cefuroxime and benzaldehyde: Cefuroxime and benzaldehyde (1:1) were taken in a round bottom flask along with solvent. The reaction was carried out at 80oC for 10 hours. The reaction was monitored by TLC. Then the product was allowed to condense and collected by filtration. This was purified byre-crystallization. The product was dried in desiccators over anhydrous silica gel.
Results and Discussions
Rf value measurement: The Rf values of ligand and reactants were measured by using the solvent n-hexane and methanol (ratio is 5:2). Rf value of benzaldehyde was 0.93, schiff base was 0.23 and cefuroxime was 0.02.
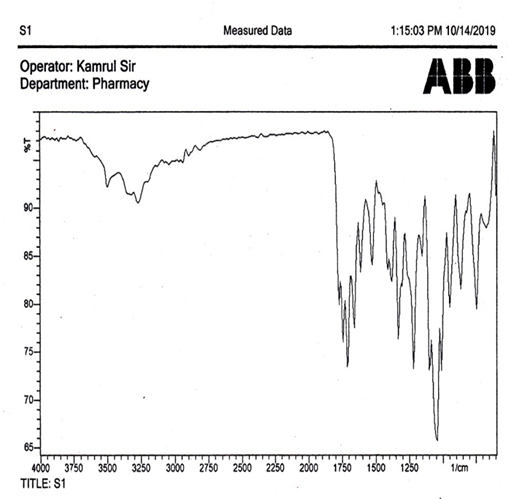
Infrared spectroscopy: The results of infrared spectral analysis are shown in (Table 1). The SB ligand displays the characteristic bands at 1600 cm-1 forazomethine (-N=CH-) group which is absent for the cefuroxime.
| Compound | -N=CH-(cm-1) | Ar-H (cm-1) | -C=O(cm-1) | -OH(cm-1) | ArC=C(cm-1) | -OCH3(cm-1) |
| Cefuroxime | ---- | 3350 (w) | 1700 (s) | 2450 (w) | 1470 (m) | 1160 (s) |
| SB ligand | 1600 (s) | 3250 (w) | 1700 (s) | 2854 (w) | 1550, 1450 (m) | 1150 (s) |
Table 1: IR spectral analysis of the SB ligand N-benzalcefuroxime
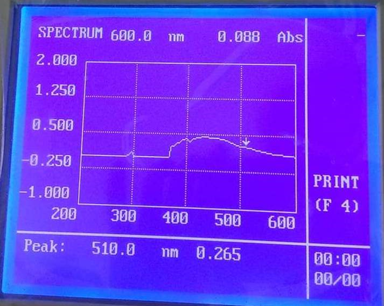
UV-Visible Spectroscopy: The electronic absorption spectra of the schiff base was recorded at room temperature using methanol as solvent. The wavelength maximum of schiff base was different from cefuroxime. For the prepared schiff base the wavelength maximum was found at 295, 405 and 510 nm; and for the cefuroxime wavelength maximum was found at 395, 335 and 405.
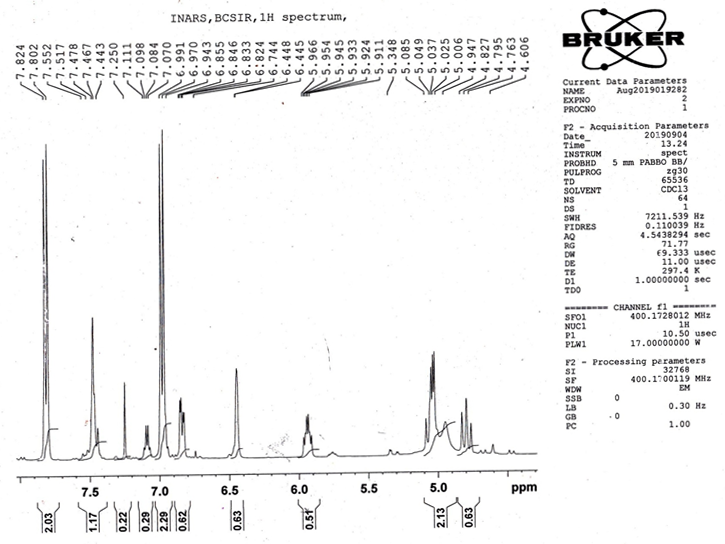
1H NMR Spectral Analysis: The observed chemical shifts for the different types of protons in the 1HNMR spectrum are presented in (Table 3). The 1HNMR spectrum of the prepared Schiff base ligand indicated a singlet at 7.8ppm owing to the presence of an azomethine proton (CH=N). Another significant multiplet signal was observed at 6.5–7.9 ppm for arayl protons. A broad signal was found at 2.5ppm for amide protonand a singlet signal was found at 2.1 ppm for methoxy (OCH3) proton.
| Compound | Chemical shift (in ppm) | Assignment of protons |
| N-benzalcefuroxime | 10.0 | S, 1H, [-COOH] |
| 1.5 | S, 4H, [-CH] | |
| 2.1 | S,1H,[-OCH3] | |
| 7.8 | S, 1H, [-N=CH-] | |
| 6.5-7.9 | S, 5H, [AR-H] | |
| 2.5 | S, 1H, [-CONH] |
Table 2: The 1H NMR spectral analysis of SB ligand N-benzalcefuroxime
Melting Point Determination: The synthesized Schiff base demonstrated a characteristic melting point which is different from cefuroxime.
Antibacterial Activity: The target compound was screened for antibacterial activity against two species of Gram-positive bacteria Staphylococcus aureus and Gram-negative bacteria Escherichia coli. Results of the disc diffusion studies are depicted in (Table 4). The diameters of zone of inhibition (in mm) of the standard drug cefuroxime was determined against Escherichia coli and Staphylococcus aureus.
| Test Organism | Diameter of zone of inhibition (mm) | |
| Cefuroxime[standard] (mm) 30mg/mL | N-benzalcefuroxime(mm) 30mg/mL | |
| Staphylococcus aureus (+ve) | 14 | 18 |
| Escherichia coli (-ve) | 13 | 14 |
Table 3: Antibacterial activity of the SB Ligand N-benzalcefuroxime
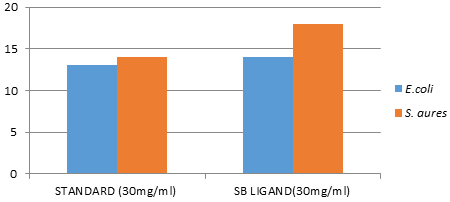
Figure 5: Graphical presentation of anti-bacterial activity for the SB ligand {N-benzalcefuroxime (SB) and standard cefuroxime)
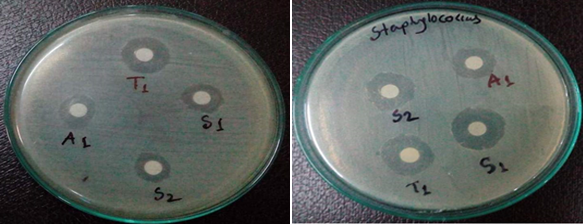
Figure 6: Left is for antibacterial activity for the SB ligand S1 (N-benzalcefuroxime) and standard cefueoxime (A1) against Escherichia coli; Right is for the bacteria Staphylococcus aureus
The diameters of zone of inhibition (in mm) of the standard drug cefuroxime was determined against Escherichia coli and Staphylococcus aureus. The N-benzalcefuroxime was found to be more active against Escherichia coli & Staphylococcus aureus than standard (cefuroxime).
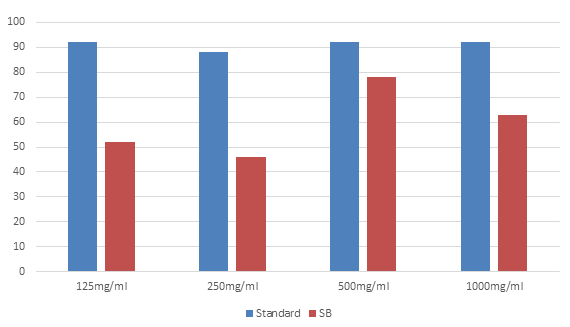
Anti-Inflammatory Activity: It was performed by measuring the absorbance of the treatment groups and converting it into % inhibition of protein denaturation. In the present study for the in-vitro anti-inflammatory activity test, the SB ligand showed good anti-inflammatory effect. The SB ligand showed good anti-inflammatory activity and the activity is highest in 500µg/ml. (See table 4, 5 and 6).
The statistical data were obtained significant below the p-value < o.5< (p<0.001) Percent inhibition of protein denaturation was calculated as:
| Sample No | 125 µg/ml | 250 µg/ml | 500 µg/ml | 1000 µg/ml |
| 1 | 0.018 | 0.0165 | 0.0115 | 0.011 |
| 2 | 0.017 | 0.0163 | 0.0118 | 0.008 |
| 3 | 0.019 | 0.0169 | 0.0112 | 0.015 |
Negative control =0.146
Table 4: Spectroscopic Determination of Anti-inflammatory Activity of Standard (diclofenac)
| Sample No | 125 µg/ml | 250 µg/ml | 500 µg/ml | 1000 µg/ml |
| 1 | 0.063 | 0.080 | 0.022 | 0.056 |
| 2 | 0.070 | 0.077 | 0.039 | 0.050 |
| 3 | 0.078 | 0.035 | 0.035 | 0.052 |
Negative control =0.146
Table 5: Spectroscopic Determination of Anti-inflammatory Activity of SB ligand (N-benzalcefuroxime)
| Test group | 125 µg/ml | 250 µg/ml | 500 µg/ml | 1000 µg/ml |
| Standard | 0.018 | 0.0165 | 0.0115 | 0.011 |
| % inhibition of Standard | 92% | 88% | 92% | 92% |
| SB ligand | 0.070 | 0.078 | 0.032 | 0.053 |
| % inhibition of SB ligand | 52% | 46% | 78% | 63% |
Table 6: Tabulation for In-vitro Anti-inflammatory Activity
Graphical Representation
Anti-Diabetic Activity: The A1C test provides the assessment of the overall control of diabetes [8]. The prepared SB Ligand showed no anti-diabetic activity.
Conclusion
Synthesis of Schiff base ligands (SB) was performed by using condensation reaction method (combination). An important feature of this synthesis was that, readily available starting materials were used under relatively mild conditions. The N-benzalcefuroxime (SB) was prepared from cefuroxime and benzaldehyde. These ligands have been characterized on the basis of IR, 1HNMR, UV-spectral data. The product was purified by recrystallization process. This synthetic product was confirmed by checking mp, TLC, Rf value, colour, chemical test, several spectral data etc. Anti-microbial activities against Escherichia coli and Staphylococcus aureus were determined. Ligands were found to be more active against both organisms. The SB ligand can be used against the diseases which are caused by these bacteria. Anti-inflammatory activity of SB ligand was good. The SB ligand showed no anti-diabetic activities. These ligands can be used as a starting materials for the complexation reaction.
References
- Khed AM and Marwani HM. "Synthesis, Spectral, Thermal Analyses and Molecular Modeling of Bioactive Cu(II)-complexes with 1,3,4-thiadiazole Schiff Base Derivatives. Their Catalytic Effect on the Cathodic Reduction of Oxygen". International Journal of Electrochemical Science 7: (2012): 10074-10093.
- Simion A. "Synthesis of Imines, Diimines and Macrocyclic Diimines as Possible Ligands, in Aqueous Solution". Journal of the Chemical Society, Perkin Transactions. DOI: 10.1039/b102749m.
- Alan D and Wilkinson A. “IUPAC Compendium of Chemical Terminology”. The Gold Book 2 (1997).
- Ay, E. "Synthesis and Characterization of Schiff Base 1-Amino-4-methylpiperazine Derivatives". CBU Journal of Science 12.3 (2014): 375-3925.
- Philip MA, Thomas OA, John Z. JAMA Internal Medicine 1 (2003).
- Eliot AC and Krisch JF. “Pyridoxal Phosphate Enzymes: Mechanistic, Structural, and Evolutionary Considerations”.Annual Review of Biochemistry 73 (2004): 383-415.
- Robert H. Rubin., et al “Trimethoprim-Sulfamethoxazole”. The New England Journal of Medicine (1980): 1-20.
- Melander, A., et al. "Sulphonylurea Antidiabetic Drugs. An Update of Their Clinical Pharmacology and Rational Therapeutic Use". Drugs 37.1 (1989): 58-72.
- Rani Neeraj., et al. “Synthesis and Comparative Fungitoxicity of Benzalbenzylamines and Benzalanilines”. Pesticide Research Journal 18.2 (2006): 129-132.
- Vogel Al, Tatchell AR, Furnish BS, Hannaford Al. “Vogel's Textbook of Practical Organic Chemistry”. 5 (1997).
- Laurence B, Keith P, Donald B, Lain B. “Goodman and Gilman's Manual of Pharmacology and Therapeutics(2001): 727-733.
- Elzzahany EA., et al. “Synthesis, Characterization and Biological Activity of Some Transition MetalComplexes with Schiff Bases Derived from 2-Formylindole, Salicyladehyde, and N-amino Rhodanine”. Australian Journal of Basic and Applied Sciences 2.2 (2008): 210-220.
- Pinky J, Jeremiah S, Mallory C, Jane WN. Nigerian Journal of Paediatrics (2016):1-25.
Citation: Farhana Hoque., et al. “Synthesize and Characterization of N-Benzalcefuroxime Which is Derived from a β-Lactamantibiotic Cefuroxime with the Screening of Antimicrobial, Anti-Inflammatory and Anti-Diabetic Potentials”. Chronicles of Pharmaceutical Science 5.1 (2020): 52-58.
Copyright: © 2020 Farhana Hoque., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution,
and reproduction in any medium, provided the original author and source are credited.
































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.