Research Article
Volume 5 Issue 1 - 2020
Unifying Mechanism Involving Electron Affinity of Conjugated Imine as Anti-Cancer Agents and Miscellaneous Drugs: Reactive Oxygen Species, Oxidative Stress, and Electron Transfer
1Department of Chemistry and Biochemistry, San Diego State University, San Diego, CA, USA
2Library and Information Access, San Diego State University, San Diego, CA, USA
2Library and Information Access, San Diego State University, San Diego, CA, USA
*Corresponding Author: Peter Kovacic, Department of Chemistry and Biochemistry, San Diego State University, San Diego, CA, USA.
Received: May 30, 2020; Published: June 11, 2020
Abstract
In 2000, a unifying mechanism placed emphasis on electron transfer (ET), reactive oxygen species (ROS), and oxidative stress (OS). ET agents include imines, quinones, ArNO2, and metals. These functionalities or their metabolites are frequently found in anti-cancer and other agents. ET agents display reduction potential in the physiological range. This mechanism can also be applied to the iminium form.
As part of the present work, calculations of electron affinity (EA) were performed on the conjugated imines, which exhibit anti-cancer and other activity. EA is part of ET. Related aspects of the physiological action are addressed as well. A multifaceted approach appears desirable. A Structure–activity relationship (SAR) study was performed involving EA of the imine groups and the degree of conjugation.
Keywords: Imines; Electron Affinity; Anticancer; Reactive Oxygen Species; Oxidative Stress
Abbreviations: ET: Electron transfer; ROS: Reactive oxygen species; OS: Oxidative stress; AO: Antioxidant; EA: Electron affinity
Introduction
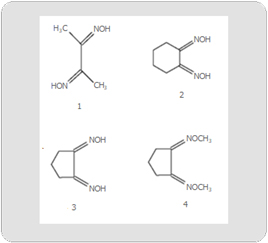
Although diverse origins pertain, reactive oxygen species (ROS) are frequently generated by redox cycling via electron transfer (ET) groups, such as imines or iminium species [1]. We believe it is not coincidental that these functionalities are often found in anticancer agent of their metabolites. Generally, the ET moieties display reduction potentials in the physiologically active range. Often ROS are also implicated in more traditional rationales, specifically enzyme inhibition, membrane or DNA insult, and interference with DNA or protein synthesis. The unifying theme of ET-OS also applies to other drug categories, as well as to toxins, carcinogens, hormones, and enzymes. Since this theoretical framework aids in our understanding of drug action, it can serve as a useful tool in drug design. This article deals with electron affinity (EA) of conjugated imines and iminiums in relation to anticancer and miscellaneous drugs. The unifying mechanism involving ROS-OS is also being applied in this case. The introductory article for the following imine series, including α –dimines, was published in 2002 [2]. The list also included related conjugated oximes (Figures 1-4). Reduction potential in relation to structures and bioactivity was also addressed in this previous article. All of the conjugated substrates gave reduction potentials conducive to ET in vivo.
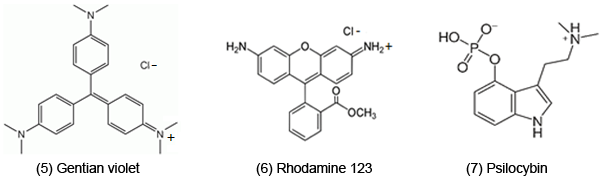
In 2010, an extensive review deals with mechanistic aspects of conjugated imines and iminiums, mainly in the biological and medicinal areas [1]. Mechanisms included reactive oxygen species (ROS), oxidative stress (OS) and electron transfer (ET). These previously addressed compounds included triarylmethane dyes like gentian violet [5] (DNA damage, amebicides). Rhodamine 123 [6] has a related structure (anticancer, inhibition of electron transfer).
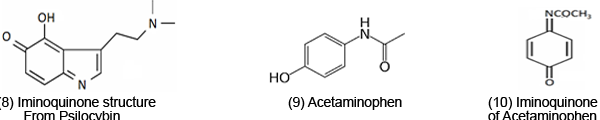
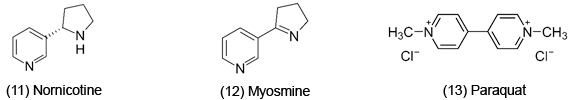
ET may be responsible for negative drug side effects. For instance, Psilocybin [7], a psychedelic drug, is oxidized to figure (8) and its toxicity is attributed to ET. In vivo, psilocybin is converted by dephosphorylation into the phenol psilocin, which also exhibits hallucinogenic properties. Acetaminophen [9], a painkiller, is metabolized to an iminoquinone [10]. Overdose can cause severe GSH depletion, which leads to a rise in toxic ROS. There is also evidence for induced lipid peroxidation [1]. Nornicotine [11], a tobacco alkaloid, is metabolized to myosmine [12]; its toxic properties of tobacco are well known.
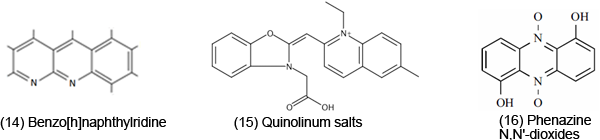
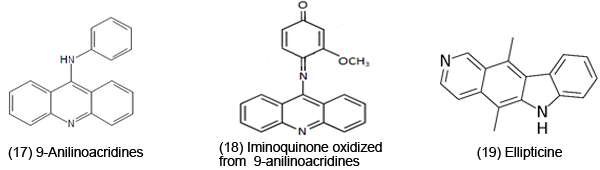
However, due to the toxicity of ROS, it is not surprising that it can be important in a variety of anti-cancer, anti-microbial, anti-fungal drugs. ET, ROS and OS have been increasingly implicated in the mode of action of drugs and toxins, e.g. anticancer drugs [3], carcinogens [4], cardiovascular toxins [5], toxins [6], ototoxins [7] and various other categories [8]. Paraquat [13], an herbicide, possesses a reduction potential favorable for in vivo ET. The toxicity appears to be related to ROS generation. The AOs are protective, such as amelioration of lipid and protein oxidation. Benzo[h]naphthylridine [14] exhibits antibacterial activity and when tested against several strains of gram positive bacteria, there was some correlation between activity and electrochemical characteristics. Quinolinium salts [15] display anticancer activity. Electron uptake is enhanced by the positive charge, as well as the phenyl and carboxyl substituents. Phenazine N,N'-dioxides [16] (e.g. dipolar analog of iminium) are anticancer, antiphage, antifungal, antibacterial and antiprotozoal; highest antimicrobial potency is associated with the most positive reduction potential. Iodinin, which has been rather extensively studied, is among the more potent of this class of antimicrobial agents. 9-Anilinoacridines [17] are oxidized to products, such as iminoquinone [18] (antitumor). These metabolites are more cytotoxic to leukemia cells than the parent. Metabolites of ellepticine [19] are antineoplastic. Electrochemical data demonstrate the ability of the hydroxylated metabolite of the parent to function as an ET agent.
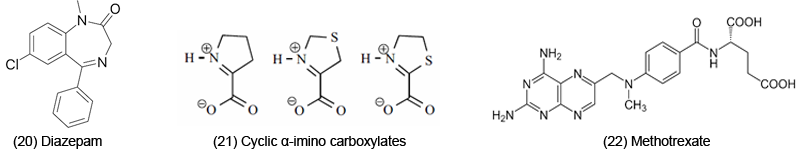
However, ET and ROS are complicated because they are involved in a variety of reactions, not all of which are harmful. Benzodiazepines, such as Diazepam [20], are CNS drugs; correlations involve reduction potentials, structure and drug activity. ET reactions may be a contributing factor to the physiological action. Cyclic α-imino carboxylates (e.g. Δ1-pyrroline- 2-carboxylate, Δ3-thiazoline-4-carboxylate, and Δ2- thiazoline-2-carboxylate (21) may exert various biological functions. ET has been suggested as a factor in some aspects of the bioactivity. In imine form, the substances can act as avid ligands for heavier metals. The resultant complexes might also be involved in ET.
In some cases, ET results in involvement with normal electrical effects (e.g. neurochemistry). Generally, active entities possessing ET groups display reduction potentials in the physiologically responsive range [9]. Hence, ET in vivo can occur resulting in production of ROS which can be beneficial in cell signaling at low concentrations, but produce toxic results at high levels. Electron donors consist of phenols, N-heterocycles or disulfides in proteins which produce relatively stable radical cations.
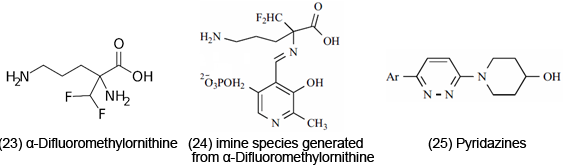
ET may have physiological effects through binding phenomenon. Methotrexate [22] treats arthritis and cancer; ET-OS may be involved as a possible mode of action for the anticancer drug. It is generally agreed that the drug functions by inactivation of dihydrofolate reductase. Tight binding to the enzyme occurs as a result of protonation at N-1 by acid residues, leading to the conjugated iminium core shown in [15], a potential ET agent. α-Difluoromethylornithine [23], believed to have an anticancer effect, on binding to pyridoxal phosphate yields figure [24]. ET may be involved in bioaction, due to ease of chelation by metal complexes.
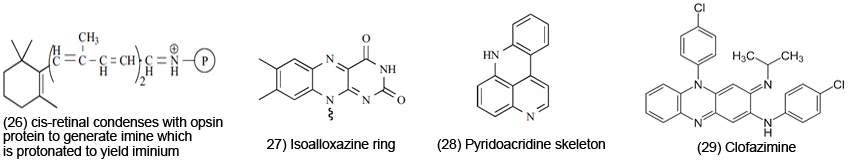
Conjugated Imines and Iminiums are involved in ET in the physiologically active range. For instance, Pyridazines [25] exhibit fungicidal, antiseptic and antibiotic activity. Their Iminium forms possess reduction potentials in the physiologically active range. Retinal iminium, rhodopsin-bound retinal, is involved in vision (ET events). The rhodopsin-bound retinal is a device for using light for stereochemical changes. In the initial chemical sequence of events in the vision process, cis-retinal condenses with the lysine amino group of opsin protein to generate imine, which is then protonated to yield iminium [26]. The isoalloxazine ring [27] is the active site of various flavins in which ET is involved in redox reactions in biosystems. Marine alkaloids are included with the pyridoacridine skeleton [28], a representative example. The class shows various bio and medicinal properties. Another previously examined compound, clofazimine [29] is effective against TB and leprosy. The p-quinoidal type system is believed responsible for activity, which may function in protonated (iminium) form.
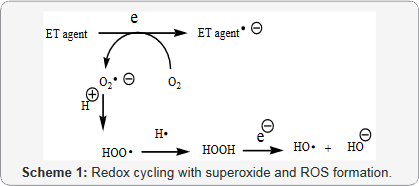
Conjugated imines and iminums fit into the unifying mechanism, which has been widely applied previously as set forth in Kovacic and Somanathan’s 2017 article involving electron transfer (ET), reactive oxygen species (ROS) and oxidative stress (OS) [10]. This unifying mechanism argues that the preponderance of bioactive substances, usually as the metabolites, incorporate ET functionalities. We believe these ET-metabolites play an important role in physiological responses. The main group includes quinones (or phenolic precursors), metal complexes (or complexors), aromatic nitro compounds (or reduced hydroxylamine and nitroso derivatives), and conjugated imines (or iminium species). Resultant redox cycling is illustrated in Scheme 1. In vivo redox cycling with oxygen can occur, giving rise to oxidative stress (OS) through generation of reactive oxygen species (ROS), such as hydrogen peroxide, hydroperoxides, alkyl peroxides, and diverse radicals (hydroxyl, alkoxyl, hydroperoxyl, and superoxide) (Scheme 1). Cellular and mitochondrial enzymes can also perform catalytically in the reduction of O2.
In addition to the above, there is a plethora of experimental evidence supporting the theoretical framework. This evidence includes generation of the common ROS, lipid peroxidation, degradation products of oxidation, depletion of AOs, effect of exogenous AOs, and DNA oxidation and cleavage products, as well as electrochemical data [11]. This comprehensive, unifying mechanism is consistent with the frequent observation that many ET substances display a variety of activities (e.g. multiple-drug properties), as well as toxic effects.
It is important to recognize that mode of action in the bio domain is often involved with many physiological actions and is multifaceted. In addition to the ET-ROS-OS in relation to mechanism, much attention in the literature is paid to AO action entailing physiological action.
Electron Affinity (EA) Calculations
EAs are related to electron uptake efficiency in electron transfer (ET) calculations, because the uptake efficiency is determined by ΔG, and ΔG = ΔH − TΔS = EA − TΔS. The entropy change can be approximated to be independent of electron acceptor, and therefore the free energy of electron uptake is almost completely controlled by electron affinity [12].
EAs are related to electron uptake efficiency in electron transfer (ET) calculations, because the uptake efficiency is determined by ΔG, and ΔG = ΔH − TΔS = EA − TΔS. The entropy change can be approximated to be independent of electron acceptor, and therefore the free energy of electron uptake is almost completely controlled by electron affinity [12].
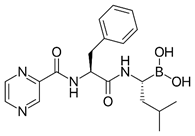
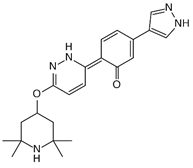
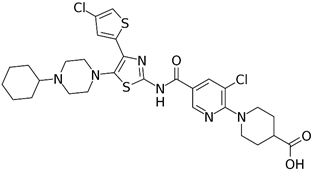
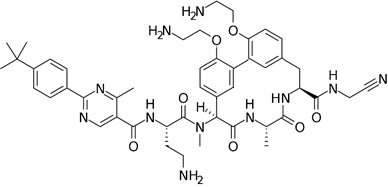
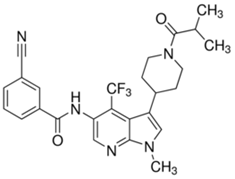
Appropriate computational methods were ascertained via benchmarking of a variety of imines against experimental data. All calculations were carried out in Gaussian-16 [13] on a Linux computer, with GaussView 6 as the visualizer [14]. Initial geometries were created from their associated structural formulas. We chose to compute adiabatic EA values for 14 imine type anti-cancer and miscellaneous drugs using Truhlar’s M06 functional and a correlation basis set with diffuse functions (aug-cc-pVDZ) [15][16] in the gas phase. All closed shell and open shell calculations are restricted and unrestricted, respectively. This method and basis set were chosen based on the speed of DFT methods, the necessity of diffuse functions, and the outcome of our benchmark calculations (benchmark details in supporting information). Many molecules (except for Binimetinib and Axitinib) were calculated as fragments that included the conjugated-imines, in the interest of reduced computational time. Imine, amide, and aromatic functionalities were included because they are assumed to affect the EA through resonance. Relevant cartesian coordinates are included in the supporting information. The results of these calculations are in Table 1. Adiabatic EAs were chosen as being more relevant to experimental measurements.
| Drugs** | EA(EV)* | Degrees of Conjugation | Purpose |
| Palbociclib | 1.35 | 6 | Anti-cancer |
| PF-06767711 | 1.16 | 9 | Misc. |
| Axitiniba | 1.06 | 13 | Anti-cancer |
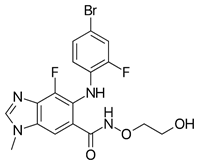
| G0775 | 0.94 | 7 | Misc. |
| Avatrombopag | 0.93 | 10 | Misc. |
| AWZ1066S frag | 0.86 | 5 | Misc. |
| Dacomitiniba | 0.8 | 7 | Anti-cancer |
| Binimetinib | 0.78 | 10 | Anti-cancer |
| Gleevec (or Imatinib)b | 0.76 | 13 | Anti-cancer |
| Bortezamibb | 0.69 | 4 | Anti-cancer |
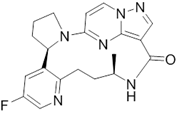
| LOXO-195 | 0.57 | 6 | Anti-cancer |
| Relugolixb | 0.53 | 12 | Anti-cancer |
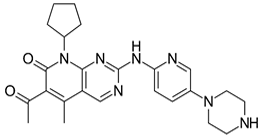
| Branaplam (NVS-SM1) | 0.4 | 9 | Misc |
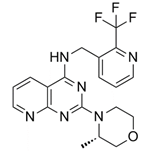
| Larotrectinib (Vitrakvi)b | 0.16 | 6 | Anti-cancer |
Computed EA’s at M06/Aug-cc-pVDZ
Table 1: EA and Conjugation of Drugs
*Calculation errors based on benchmarks range from +0.04 to +0.38 eV. Based on this error, the range of EAs is between -0.22 eV to 0.98 eV and 0.16 eV to 1.35 eV. Relative ordering between adjacent molecules on the table is uncertain (e.g. the relative ordering of Palbociclib and PF-06767711 EA values), but some trends are still discernable (e.g. Palbociclib is higher in EA than G0755).
- a: These molecules were simulated as fragments of a different size. Skeletal structures of all compounds and their fragments are in the supporting information.
- b: These molecules were simulated as fragments which included only the conjugate imines and the conjugated systems connected to them for reduced computational time.
Note that the range of EAs is near that of quinones (0.54-0.64 eV) and aromatic nitro compounds (0.59 eV for dinitrophenol) [17]. Anti-cancer drugs did not have systematically higher or lower EA values compared to miscellaneous compounds.
The EA increases with protonation from the increase in charge. Known pKa values for these anti-cancer drugs are sparse, and the likeliest protonation sites were found via Chemaxon’s Chemicalize website [18]. Chemicalize revealed that of all studied anti-cancer drugs, only Binimetinib is plausibly protonated on the conjugated imine.
There are many structural features that influence activity. In a study of study of structure-activity relationship (SAR), degree of conjugation appears to be contributing. A good correlation involves Axitinib with 13 conjugated units (high EA of 1.06) and Larotrectinib (or Vitrakvi) with 6 conjugated units (low EA of .16). A completely clear correlation is not shown, possibly because conjugation was discerned by counting the number of conjugated bonds on the larger conjugated system. Additionally, there may be other factors which could be contributing to variability, such as stereochemistry and polarity. Imine may be a prevalent feature in medicine and other areas due to the dual aspect of providing conjugation needed in ET and a site for protonation involved in ET. Future studies might examine activity from one drug to another.
Discussion
Anti-cancer Drugs
A recent review puts focus on Axitinib (30), a di-imine, in relation to pharmacological properties and its place in the treatment of patients with renal cell carcinoma (RCC) [21]. Use as second-line systems therapy resulted in improvement of the prognosis and quality of life in patients with RCC [22]. The drug provides new perspectives for its use to overcome the therapy resistance in gliomas [23]. There is induction of DNA damage and production of ROS, which are reversed by the AO N-acetylcysteine in pretreatment. Some production of oxidative stress was observed. There are therapeutic benefits for cancer patients with aberrant nuclear β-catenin activation whose mechanism is provided [24]. The drug was shown to be superior as second-line therapy for RCC. A related side effect of hypertension was observed. A discussion was presented of current indication for clinical use in the management of metastatic RCC.
A recent review puts focus on Axitinib (30), a di-imine, in relation to pharmacological properties and its place in the treatment of patients with renal cell carcinoma (RCC) [21]. Use as second-line systems therapy resulted in improvement of the prognosis and quality of life in patients with RCC [22]. The drug provides new perspectives for its use to overcome the therapy resistance in gliomas [23]. There is induction of DNA damage and production of ROS, which are reversed by the AO N-acetylcysteine in pretreatment. Some production of oxidative stress was observed. There are therapeutic benefits for cancer patients with aberrant nuclear β-catenin activation whose mechanism is provided [24]. The drug was shown to be superior as second-line therapy for RCC. A related side effect of hypertension was observed. A discussion was presented of current indication for clinical use in the management of metastatic RCC.
Relugolix [31], a di-imine, finds use in the treatment of fibroids, a smooth muscle tumor [25]. It demonstrated an effect in menstrual bleeding, and was generally well tolerated. The drug effectively lowered testosterone in men in a study involving prostate cancer [26]. The drug may provide useful therapeutic intervention in hormone-dependent diseases, such as prostate cancer, uterine fibroids and endometriosis.
Bortezomib (BTZ) [32], a di-imine, demonstrates clinical efficacy against cancers [27]. There is an increase of OS and activation of AO modules. Such overexpression of OS was prevented by concomitant treatment with the AO lipoic acid [28]. BTZ improves survival of patients with multiple myeloma [29]. Resistance to the drug parallels activation of OS. There is an indication of ROS. BTZ induces testicular toxicity during treatment of multiple myeloma [30]. Excessive OS is elicited. Use of AOs may represent an effective strategy for protection. Sensitivity to BTZ is dependent on oxidative status of the cells [31].
Up-regulation of CuZnSOD and glutathione peroxidase was associated with glutathione attenuated pro-oxidant production by BTZ [32]. BTZ induces OS in multiple myeloma cells [33]. Additionally, there was also enhancement of GSH levels. The drug triggers an OS response [34]. BTZ induced ROS generation [35]. The AO, N acetyl cysteine, attenuated the ROS generation, mitochondrial dysfunction, and apoptosis.
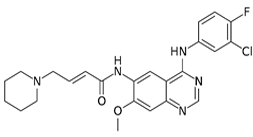
Dacomitinib [33], a di-imine, produces survival in patients with lung cancer [36]. OS plays a role. Another report deals with treatment of bladder cancer [37]. The drug provides a useful strategy against overexpressing cancer [38], as well as lung cancer [39]. A 2016 report deals with treatment of ovarian cancer [40].
Larotrectinib (or Vitrakvi) [34], a di-imine, shows broad clinical activity with multiple tumor types [41]. The drug is well tolerated and active against various tumors; representing a new treatment option [42]. Another recent article assessed the safety of young patients with tumors [43]; little CNS toxicity was observed. Lack of studies on Larotrectinib, involving ROS, OS, AO and redox is likely due to its relatively recent appearance.
Gleevec (or imatinib) [35] is a tri-imine. The drug enhances efficacy and selectivity toward cancer cells, presenting a promising tool to fight cancer [44]. It is a sustained drug for apoptosis in cancer cells [45].
Binimetinib (or Mektovi) [36], a mono-imine, represents a promising treatment for advanced melanoma [46]. It may also have a role for therapy involving melanoma patients. It is a selective inhibitor of MEK, a central kinase in the tumor-promoting pathway [47]. Binimetinib improved survival compared with Dacarbazine (or Imidazole Carboxamide) and may be a treatment option for patients with melanoma after failure of immunotherapy [48].
LOXO-195 [37], a tri-imine, has shown striking efficacy against different kinds of tumors [49]. The compound helped shrink tumors and exhibited very little toxicity.
Palbociclib, a di-imine, is a CDK4/6 inhibitor for the treatment of hormone receptor positive, metastatic breast cancer in combination with an aromatase inhibitor in postmenopausal women [50]. In a recent 2019 study, Palbociclib treatment after initial streptozotocin administration reduced OS and inflammation, thereby reducing cardiomyocyte death and preserving cardiac function in mice [51].
Miscellaneous Drugs
Filariasis is a tropical disease that affects millions of people, inflicting severe disability [52]. Currently, there is the lack of a safe drug therapy that can kill the adult filaria. The drug AWZ1066S [39], a tetra-imine, shows superior efficacy to existing therapies with minimal impact to the treated patients.
Filariasis is a tropical disease that affects millions of people, inflicting severe disability [52]. Currently, there is the lack of a safe drug therapy that can kill the adult filaria. The drug AWZ1066S [39], a tetra-imine, shows superior efficacy to existing therapies with minimal impact to the treated patients.
The drug Branaplam (or NVS-SM1 or LMI070) (40), a di-imine, is a potent and selective drug being developed to treat spinal muscular atrophy [53] [54]. It is believed to work by increasing survival motor neuron (SMN) proteins produced by the SMN2 gene [55]. Branaplam is potentially a better option because of its lower toxicity and pharmacokinetic profile, in particular an improved ability to enter the brain [56].
The drug Avatrombopag (41), a di-imine, is used for treatment of thrombocytopenia (TCP) which is a common problem in cancer patients [57] TCP is involved in a deficiency of platelets, which increases the risk of bleeding. TCP occurs when bone marrow makes too few platelets. TCP can result from chemotherapy, radiation treatment, or from the underlying disease itself. This recent article (2019) discusses TCP management and other aspects associated with cancer chemotherapy [57]. Avatrombopag is a TCP receptor agonist.
G0775 (42), a di-imine, kills some resistant bacterial strains by a proposed novel mechanism [58,59]. A beneficial property is the ability to penetrate the bacterial outer membrane. An important aspect is the ability to gum up the bacterial signal peptidase. It circumvents antibiotic resistance mechanism and retains activity against multidrug resistant bacterial strains. This may be a novel therapy.
The compound PF-06747711 (43), a mono-imine, targets a nuclear hormone receptor, which turns on gene expression in signaling pathways [60] [61]. It is of interest as a treatment of psoriasis. Psoriasis is an inflammatory disease with close relation to arthritis.
Sources of ROS
NADPH oxidase is an important producer of ROS which is involved in physiological responses in various organs. A study by Markovic., et al. 2017 dealt with the effect of maternal deprivation (MD) on NADPH oxidase expression [62]. The AO GSH was reduced in quantity along with changes in OS parameters. Results point to extensive changes in redox aspects.
NADPH oxidase is an important producer of ROS which is involved in physiological responses in various organs. A study by Markovic., et al. 2017 dealt with the effect of maternal deprivation (MD) on NADPH oxidase expression [62]. The AO GSH was reduced in quantity along with changes in OS parameters. Results point to extensive changes in redox aspects.
G72 is a gene which encodes a polypeptide. Activation of D-amino acid oxidase has been suggested as a means of reducing the neurotransmission of N-methyl-D-aspartate receptors [63]. G72 effectively increased ROS generation in cells. Evidence indicates participation of OS. The ROS result can be reversed by tempol, a superoxide dismutase mimetic, which is a scavenger of ROS.
An earlier report [64] deals with G72. Results point to G72 as a participant in reduction of mitochondrial and synoptic defects. Many of the other findings were similar to those of Wang et al. in 2015[64]. The ROS-OS aspects of mitochondrial action are treated elsewhere in this report. A more recent article by Akyol., et al. in 2017 on G72 protein levels also supports the suggestion that G72 is an activator of D-amino acid oxidase [65]. ROS generated by NO synthase has been implicated in an array of harmful behaviors [66]. Evidence demonstrates that the enzymes produce redox signaling cascades which interact in the brain with resultant influences on social behavior and cognitive function.
Mitochondria provide another source of ROS-OS which has been claimed as a contributor to aging [11]. Leakage of electrons occurs in the ET chain, which react with oxygen to produce superoxide, a precursor of other ROS. Various neurotransmitters can undergo autoxidation of which dopamine is an example [67]. The molecule incorporates a catechol entity which metabolizes to an ET o-quinone resulting in toxic ROS-OS. Other examples are cytochromes P450, ET metal complexes of protein (e.g. iron), monoamine oxidases, and microglia that are microphages of the nervous system capable of generating superoxide and H2O2 [10]. A frequent operator in generation of ROS-OS is ET which may play an important function.
It is necessary to recognize the importance of the multifaceted nature of physiological action. In addition to ET-ROS-OS-AO, other factors are at play as indicated in this discussion: cell signaling, mitochondria, receptor binding and enzyme inhibition [16]. The literature addresses the basic aspects of these items: cell signaling [68], mitochondria [8], and receptor binding [69].
Apoptosis
Apoptosis, also known as programmed call death, is essential for functioning of most organisms. Reports show that ROS and related OS play important roles. The protean Bcl-2 prevents cellular death apparently by an AO mechanism. AOs, such as protean Bcl-2, and SODs, are known to prevent cells from dying. Redox change can be part of signal transduction during apoptosis. Mitochondria are an important part of apoptosis. OS and apoptosis, which are clearly linked, are implicated in various diseases, such as AIDS, cancer, autoimmunity, diabetes, ischemia, and many brain illnesses (e.g. Alzheimer’s and Parkinsons).
References
- Kovacic P and Somanathan R. “Mechanism of Conjugated Imine and Iminium Species, including Marine Alkaloids: Electron Transfer, Reactive Oxygen Species, Therapeutics and Toxicity”. Current Bioactive Compounds 6.1 (2010): 46-59.
- Niufar NN., et al. “Reduction Potentials of Conjugated Aliphatic Ketones, Oximes, and Imines: Correlation with Structure and Bioactivity”. Revista de la Sociedad Química de México 46.4 (2002): 307-312.
- Kovacic P., et al. “Mechanisms of anticancer agents: Emphasis on oxidative stress and electron transfer”. Current Pharmaceutical Design: Ingenta Connect Publication 6 (2000): 277-309.
- Kovacic P and Jacintho, JD. “Reproductive Toxins: Pervasive Theme of Oxidative Stress and Electron Transfer”. Current Medicinal Chemistry 8 (2001): 863-892.
- Kovacic P and Thurn LA. “Cardiovascular Toxicity From the Perspective of Oxidative Stress, Electron Transfer, and Prevention by Antioxidants”. Current Vascular Pharmacology 3.2 (2005): 107-117.
- Kovacic P., et al. “Mechanism of Mitochondrial Uncouplers, Inhibitors, and Toxins: Focus on Electron Transfer, Free Radicals, and Structure-Activity Relationships”. Current Medicinal Chemistry 12.22 (2005): 2601-2623.
- Kovacic P and Somanathan, R. “Ototoxicity and noise trauma: Electron transfer, reactive oxygen species, cell signaling, electrical effects, and protection by antioxidants: Practical medical aspects”. Medical Hypotheses 70.5 (2008): 914-923.
- Halliwell B and Gutteridge, J. Free radicals in biology and medicine (3rd ed., Oxford science publications). Oxford: New York: Clarendon Press ; Oxford University Press (1999): 192-194.
- Peter Kovacic and Wil Weston. “Cause and Treatment of Schizophrenia: Electron Transfer, Reactive Oxygen Species, Oxidative Stress, Antioxidants, and Unifying Mechanism”. Chronicles of Pharmaceutical Science 1.6 (2017): 332-340.
- Peter Kovacic and Ratnasamy Somanathan. “Various Factors Involving Aging: Electron Transfer, Reactive Oxygen Species And Oxidative Stress”. International Journal of Current Research 9.7 (2017): 53518-53528.
- Kovacic P and Somanathan R. “Unifying Mechanism for Nutrients as Anticancer Agents: Electron Transfer, Reactive Oxygen Species and Oxidative Stress”. Global Journal of Health Science 9.8 (2017): 66-83.
- Kovacic P and Cooksy A. “Novel, unifying mechanism for amphotericin B and other polyenedrugs: electron affinity, radicals, electron transfer, autoxidation, toxicity, and antifungal action”. RSC Medicinal Chemistry 3 (2012): 274-280.
- Frisch M J., et al. Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT (2016).
- Dennington, R., et al. GaussView, Version 6.1, Semichem Inc., Shawnee Mission, KS (2016).
- Zhao Y and Truhlar DG. “The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals”. Theoretical Chemistry Accounts 120 (2008): 215-241.
- Dunning TH. “Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen”. The Journal of Chemical Physics 90.2 (1989): 1007-1023.
- Briegleb DG. “Electron Affinity of Organic Molecules”. Angewandte Chemie 3.9 (1964): 617-632.
- http://www.chemicalize.org/
- Bellesoeur A., et al. “Axitinib in the Treatment of Renal Cell Carcinoma: Design, Development, and Place in Therapy”. Drug Design, Development and Therapy 11 (2017): 2801-2811.
- Umeyama Y., et al. “Axitinib in Metastatic Renal Cell Carcinoma: Beyond the Second-Line Setting”. Future Oncology 13.21 (2017): 1839-1852.
- Morelli MB., et al. “Axitinib Induces Senescence-Associated Cell Death and Necrosis in Glioma Cell Lines: The Proteasome Inhibitor, Bortezomib, Potentiates Axitinib-Induced Cytotoxicity in a p21(Waf/Cip1) Dependent Manner”. Oncotarget 8.2 (2017): 3380-3395.
- Qu Y., et al. “Axitinib Blocks Wnt/β-catenin Signaling and Directs Asymmetric Cell Division in Cancer”. Proceedings of the National Academy of Sciences of the United States of America 113.33 (2016): 9339-9344.
- Osuga Y., et al. “Oral Gonadotropin-Releasing Hormone Antagonist Relugolix Compared With Leuprorelin Injections for Uterine Leiomyomas: A Randomized Controlled Trial”. Obstetrics & Gynecology 133.3 (2019): 423-433.
- MacLean DB., et al. “Medical Castration Using the Investigational Oral GnRH Antagonist TAK-385 (Relugolix): Phase 1 Study in Healthy Males”. The Journal of Clinical Endocrinology and Metabolism 100.12 (2015): 4579-4587.
- Tsakiri EN., et al. “Milder Degenerative Effects of Carfilzomib vs. Bortezomib in the Drosophila Model: A Link to Clinical Adverse Events”. Scientific Reports 7.1 (2017): 17802.
- Tibullo D., et al. “Effect of Lipoic Acid on the Biochemical Mechanisms of Resistance to Bortezomib in SH-SY5Y Neuroblastoma Cells”. Molecular Neurobiology 55.4 (2018): 3344-3350.
- Frassanito MA., et al. “Halting Pro-Survival Autophagy by TGFβ Inhibition in Bone Marrow Fibroblasts Overcomes Bortezomib Resistance in Multiple Myeloma Patients”. Leukemia 30.3 (2016): 640-648.
- Li W., et al. “The Proteasome Inhibitor Bortezomib Induces Testicular Toxicity by Upregulation of Oxidative Stress, AMP-activated Protein Kinase (AMPK) Activation and Deregulation of Germ Cell Development in Adult Murine Testis”. Toxicology and Applied Pharmacology 285.2 (2015): 98-109.
- Muñoz-Galván S., et al. “MAP17 (PDZKIP1) Expression Determines Sensitivity to the Proteasomal Inhibitor Bortezomib by Preventing Cytoprotective Autophagy and NFκB Activation in Breast Cancer”. Molecular Cancer Therapeutics 14.6 (2015): 1454-1465.
- Salem, K., et al. “Copper-zinc Superoxide Dismutase-Mediated Redox Regulation of Bortezomib Resistance in Multiple Myeloma”. Redox Biology 4 (2015): 23-33.
- Yin, L., et al. “Targeting MUC1-C Is Synergistic With Bortezomib in Downregulating TIGAR and Inducing ROS-mediated Myeloma Cell Death”. Blood 123.19 (2014): 2997-3006.
- Weniger MA., et al. “Treatment-induced Oxidative Stress and Cellular Antioxidant Capacity Determine Response to Bortezomib in Mantle Cell Lymphoma”. Clinical Cancer Research 17.15 (2011): 5101-5112.
- Yu C., et al. “The Hierarchical Relationship Between MAPK Signaling and ROS Generation in Human Leukemia Cells Undergoing Apoptosis in Response to the Proteasome Inhibitor Bortezomib”. Experimental Cell Research 295.2 (2004): 555-566.
- Mok TS., et al. “Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib With Gefitinib in Patients With Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations”. Journal of Clinical Oncology 36.22 (2018): 2244-2250.
- Tamura, S., et al. “Molecular Correlates of In Vitro Responses to Dacomitinib and Afatinib in Bladder Cancer”. Bladder Cancer 4.1 (2018): 77-90.
- Fan YF., et al. “Dacomitinib Antagonizes Multidrug Resistance (MDR) in Cancer Cells by Inhibiting the Efflux Activity of ABCB1 and ABCG2 Transporters”. Cancer Letters 421 (2018): 186-198.
- Tang, Z.H., et al. “Identification of a Novel Autophagic Inhibitor Cepharanthine to Enhance the Anti-Cancer Property of Dacomitinib in Non-Small Cell Lung Cancer”. Cancer Letters 412 (2018): 1-9.
- Xu, L., et al. “Dacomitinib, a New pan-EGFR Inhibitor, Is Effective in Killing Ovarian Cancer Cells”. Discovery Medicine 22.122 (2016): 297-309.
- Bhangoo MS and Sigal D. “TRK Inhibitors: Clinical Development of Larotrectinib”. Current Oncology Reports 21.2 (2019): 14.
- Hong, D.S., et al. “Larotrectinib in Adult Patients With Solid Tumours: A Multi-Centre, Open-Label, Phase I Dose-Escalation Study”. Annals of Oncology 30.2 (2019): 325-331.
- Laetsch, T.W., et al. “Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: a multicentre, open-label, phase 1 study”. The Lancet Oncology 19.5 (2018): 705-714.
- Kassem, M.A., et al. “Maximizing the Therapeutic Efficacy of Imatinib Mesylate-Loaded Niosomes on Human Colon Adenocarcinoma Using Box-Behnken Design”. Journal of Pharmaceutical Sciences 106.1 (2017): 111-122.
- Sadat Shandiz, S.A., et al. “Novel Imatinib-Loaded Silver Nanoparticles for Enhanced Apoptosis of Human Breast Cancer MCF-7 Cells”. Artificial Cells, Nanomedicine and Biotechnology 45.6 (2017): 1-10.
- Queirolo P and Spagnolo, F. “Binimetinib for the Treatment of NRAS-mutant Melanoma”. Expert Review of Anticancer Therapy 17.11 (2017): 985-990.
- Koelblinger P., et al. “A Review of Binimetinib for the Treatment of Mutant Cutaneous Melanoma”. Future Oncology 13.20 (2017): 1755-1766.
- Dummer R., et al. “Binimetinib Versus Dacarbazine in Patients With Advanced NRAS-mutant Melanoma (NEMO): A Multicentre, Open-Label, Randomised, Phase 3 Trial”. The Lancet Oncology 18.4 (2017): 435-445.
- Dolgin E. “Loxo TRK Inhibitor Data Wows Oncologists”. Nature Biotechnology 35.8 (2017): 694-695.
- Chavan BB., et al. “In Vitro and in Vivo Metabolic Investigation of the Palbociclib by UHPLC-Q-TOF/MS/MS and in Silico Toxicity Studies of Its Metabolites”. Journal of Pharmaceutical and Biomedical Analysis 157: 59-74.
- Wang Z., et al. “Palbociclib Improves Cardiac Dysfunction in Diabetic Cardiomyopathy by Regulating Rb Phosphorylation”. American Journal of Translational Research 11.6 (2019): 3481-3489.
- Hong, W.D., et al. “AWZ1066S, a Highly Specific Anti- Wolbachia Drug Candidate for a Short-Course Treatment of Filariasis”. Proceedings of the National Academy of Sciences of the United States of America 116.4 (2019): 1414-1419.
- Calder AN., et al. “Small Molecules in Development for the Treatment of Spinal Muscular Atrophy”. Journal of Medicinal Chemistry 59.22 (2016): 10067-10083.
- Cheung AK., et al. “Discovery of Small Molecule Splicing Modulators of Survival Motor Neuron-2 (SMN2) for the Treatment of Spinal Muscular Atrophy (SMA)”. Journal of Medicinal Chemistry 61.24 (2018): 11021-11036.
- Peña A. “LMI070 oral candidate For SMA type 1 holds promise, preclinical study shows”. SMA news today Nov 16 (News) (2018).
- Kuter DJ. “Managing Thrombocytopenia Associated With Cancer Chemotherapy”. Oncology (Williston Park) 29.4 (2015): 282-294.
- Długosz-Danecka M., et al. “Avatrombopag for the treatment of immune thrombocytopenia”. Journal Expert Review of Clinical Immunology 15.4 (2019): 327-339.
- Satyanarayana M. “Aryl compound attacks Gram-negative bacteria in a new way”. C&E news 96.37 (2018).
- Smith PA., et al. “Optimized Arylomycins Are a New Class of Gram-negative Antibiotics”. Nature 561.7722 (2018): 189-194.
- Satyanarayana M. “Small molecule advances the hunt for a psoriasis pill”. C&E News Sept. 11 (2018) online.
- Schnute ME., et al. “Discovery of 3-Cyano- N-(3-(1-isobutyrylpiperidin-4-yl)-1-methyl-4-(trifluoromethyl)-1 H-pyrrolo[2,3- b]pyridin-5-yl)benzamide: A Potent, Selective, and Orally Bioavailable Retinoic Acid Receptor-Related Orphan Receptor C2 Inverse Agonist”. Journal of Medicinal Chemistry 61.23 (2018): 10415-10439.
- Markovic B., et al. “Long-Term Effects of Maternal Deprivation on Redox Regulation in Rat Brain: Involvement of NADPH Oxidase”. Oxidative Medicine and Cellular Longevity 2017 (2017): 7390516.
- Wang M., et al. “Identification of pLG72-Induced Oxidative Stress Using Systemic Approaches”. BioMed Research International (2015): 429253.
- Drews E., et al. “Involvement of the Primate Specific Gene G72 in Schizophrenia: From Genetic Studies to Pathomechanisms’. Neuroscience & Biobehavioral Reviews 37.10 (2012): 2410-2417.
- Akyol, E.S., et al. “Increased Serum G72 Protein Levels in Patients With Schizophrenia: A Potential Candidate Biomarker”. Acta Neuropsychiatrica 29.2 (2017): 80-86.
- Walton JC., et al. “Neuronal Nitric Oxide Synthase and NADPH Oxidase Interact to Affect Cognitive, Affective, and Social Behaviors in Mice”. Behavioural Brain Research 213 (2013): 320-326.
- Peter Kovacic and Wil Weston. “Treatment of Parkinson’s Disease with Phenolic Antioxidant Drugs: Oxidative Stress, Reactive Oxygen Species and Selectivity.” Chronicles of Pharmaceutical Science 1.4 (2017): 193-198.
- Kovacic, P and Somanathan, R. “Cell Signaling and Cancer: Integrated, Fundamental Approach Involving Electron Transfer, Reactive Oxygen Species, and Antioxidants”. Cell Signaling & Molecular Targets in Cancer, New York, Chapter 12 (2012): 273-297.
- Kovacic P., et al. “Unifying Electrostatic Mechanism for Phosphates and Sulfates in Cell Signaling”. Journal of Receptors and Signal Transduction 27.5-6 (2007): 433-443.
Citation: Peter Kovacic., et al. “Unifying Mechanism Involving Electron Affinity of Conjugated Imine as Anti-Cancer Agents and Miscellaneous Drugs: Reactive Oxygen Species, Oxidative Stress, and Electron Transfer”. Chronicles of Pharmaceutical Science 5.1 (2020): 38-51.
Copyright: © 2020 Peter Kovacic., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution,
and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.