Research Article
Volume 4 Issue 1 - 2019
In vitro Antioxidant Preliminary Screening Profile of Methanolic Extract of Uvaria littoralis leaf.
Department of Pharmacy, Southeast University, Dhaka, Bangladesh
*Corresponding Author: Md. Shariful Islam, Department of Pharmacy, Southeast University, Banani, Dhaka, Bangladesh.
Received: June 17, 2019; Published: July 01, 2019
Abstract
To evaluate the antioxidant activity of Uvaria littoralis. Total phenolic content, Total Flavonols, and Total antioxidant activity were used to evaluate the antioxidant potential of Uvaria littoralis. Hydroxyl radical scavenging assay was used to find the scavenging activity of free hydroxyl radicals like hydrogen peroxide in the presence of different fractions of plant sample. Metal chelating activity was also observed. The crude methanol extract and its different fractions showed considerable total antioxidant activity.
In a phenolic test, comparing the phenolic content of different fractions with crude methanol extract it was observed that EAF and AQF contain more amounts of phenolic compounds than the crude extract. Total flavonoids content of different fractions with crude methanol extract has shown good flavonoids activity. In hydroxyl radical scavenging activity Petroleum ether fraction exhibited the highest scavenging activity with IC50 of 4.697 μg/ml, while Chloroform fraction exhibited the lowest activity with IC50 of 6.544 μg/ml. In the metal chelating activity, it has shown very well metal chelating activity.
Keywords: Uvaria littoralis; Antioxidant and Methanolic extract
Introduction
Oxidative persecution is the disequilibrium of the reactive oxygen species (ROS) generation and antioxidant defense capacity of the cell [1]. Spillover reactive oxygen species (ROS) causes oxidation of cellular proteins. DNA and lipids that ultimately dominance to the loss of functionality of the cell [2]. Oxidative persecution has also been coated to a comprehensive of pathological circumstance like kinds of cardiovascular functions [3], neurodegenerative diseases, cancer, diabetes, and arthritis as well as age-related deterioration of hepatic [4], and ROS is one of the major pathogenic events that come about in most of the long-standing liver disorders [5].
Functional vital organ of the liver is the largest organ of the body that engages crucially in metabolism, secretory, synthesis, excretory and detoxification function [6], the liver is a crucially important target of the toxicity of drugs, xenobiotics, and oxidative stress because of its unique metabolism and relationship to the gastrointestinal tract [7]. Liver diseases are caused by a variety of factors, but viral infection, carcinogenesis, alcohol, and drugs are known to be frequently associated with liver disease [1]. ROS plays a role in most long-lasting liver diseases like alcoholic liver disease, liver cirrhosis, hepatocellular carcinoma and hepatitis [8]. It would strongly believe that antioxidant supplement as an auspicious remedial intervention for the preclusion and dealings with hepatic disorders [9]. U. littoralis, belonging to the family Annonaceae. U. littoralis plant is a woody climber, 2-4 m long, and leaves up to 30 cm × 12 cm. Flowers are red to dark brown, and fruits are composed of 5-50 distinct carpels; fruitlet berry-like, globose to cylindrical, up to 2.5 cm long [10]. Therefore, U. Littoralis plant in lowlands in hedges, thickets and teak forests [11].
Methods and Materials
Chemical study on Uvaria littoralis
The plant material of leaves was collected during the month of January 2005 from Camilla, Hill track in fresh condition and identified by an expert taxonomist. A voucher specimen was submitted to the National Herbarium Bangladesh. Leaves were then washed properly to remove dirty materials and dried for several days with occasional sun drying. The dried leaves were ground into coarse powder by a grinding machine in the Department of Pharmacy, Southeast University, Dhaka, Bangladesh.
The plant material of leaves was collected during the month of January 2005 from Camilla, Hill track in fresh condition and identified by an expert taxonomist. A voucher specimen was submitted to the National Herbarium Bangladesh. Leaves were then washed properly to remove dirty materials and dried for several days with occasional sun drying. The dried leaves were ground into coarse powder by a grinding machine in the Department of Pharmacy, Southeast University, Dhaka, Bangladesh.
Cold extraction of the plant materials
Powdered plant materials (leaves)having of the weight of about 500gm were taken in an amber colored reagent bottle and soaked in 1.5 liters of methanol. The bottle with its contents was sealed and kept for a period of about 7 days with occasional shaking and stirring. The whole mixture was then filtered through cotton and then through what man No. 1 filters paper and was concentrated with a rotary evaporator under reduced pressure at 52°C temperature to afford crude extract (45.39 gm).
Powdered plant materials (leaves)having of the weight of about 500gm were taken in an amber colored reagent bottle and soaked in 1.5 liters of methanol. The bottle with its contents was sealed and kept for a period of about 7 days with occasional shaking and stirring. The whole mixture was then filtered through cotton and then through what man No. 1 filters paper and was concentrated with a rotary evaporator under reduced pressure at 52°C temperature to afford crude extract (45.39 gm).
Solvent-solvent partitioning of crude extract
An aliquot (20gm) of the concentrated methanolic extract was fractionated by modified Kupchan method (Van., et al. 1993) and the resultant fractions that are petroleum ether (PEF, 5.89gm), chloroform (CLF, 4.36gm), ethyl acetate (EAF, 2.86gm) and aqueous (AQF, 4.36gm) soluble fractions were obtained and used for the experiment purpose. Solvent-solvent partitioning of crude extract Solvent-solvent portioning was done using the protocol designed by kupchan and modified by Wagenen., et al., (1993).
An aliquot (20gm) of the concentrated methanolic extract was fractionated by modified Kupchan method (Van., et al. 1993) and the resultant fractions that are petroleum ether (PEF, 5.89gm), chloroform (CLF, 4.36gm), ethyl acetate (EAF, 2.86gm) and aqueous (AQF, 4.36gm) soluble fractions were obtained and used for the experiment purpose. Solvent-solvent partitioning of crude extract Solvent-solvent portioning was done using the protocol designed by kupchan and modified by Wagenen., et al., (1993).
Drugs and Chemicals
Folin – ciocalteu reagent, Methanol, Aluminum Chloride, Ethanol, Ascorbic acid, EDTA, Sodium Phosphate, Sodium carbonate Ammonium Molybdate, FeCl3 (Sigma chemical company, USA), Gallic acid, Quercetin (Wako pure chemicals Ltd., Japan), Potassium Acetate, Sodium Acetate, Hydrogen peroxide, Sulphuric acid (Merck, Germany), 2-deoxy-D-ribose, Thiobarbituric acid, (+)-Catechin (Sigma-Aldrich, Japan), Phosphate buffer, Trichloro Acetic acid(Sigma-Aldrich, USA).
Folin – ciocalteu reagent, Methanol, Aluminum Chloride, Ethanol, Ascorbic acid, EDTA, Sodium Phosphate, Sodium carbonate Ammonium Molybdate, FeCl3 (Sigma chemical company, USA), Gallic acid, Quercetin (Wako pure chemicals Ltd., Japan), Potassium Acetate, Sodium Acetate, Hydrogen peroxide, Sulphuric acid (Merck, Germany), 2-deoxy-D-ribose, Thiobarbituric acid, (+)-Catechin (Sigma-Aldrich, Japan), Phosphate buffer, Trichloro Acetic acid(Sigma-Aldrich, USA).
Determination of total phenolics
Total phenolic content of extractives of U. Littoralis was determined to the method as described of [12] involving Folin-Ciocalteu reagent as an oxidizing agent and Gallic acid as standard. The total content of phenolic compounds in plant methanol extract and in different fractionates in Gallic acid equivalents (GAE) was calculated by the following formula
C = (c x V)/m
Total phenolic content of extractives of U. Littoralis was determined to the method as described of [12] involving Folin-Ciocalteu reagent as an oxidizing agent and Gallic acid as standard. The total content of phenolic compounds in plant methanol extract and in different fractionates in Gallic acid equivalents (GAE) was calculated by the following formula
C = (c x V)/m
Where, C = total content of phenolic compounds, mg/g plant extract, in GAE; c = the concentration of Gallic acid established from the calibration curve, mg/ml; V = the volume of extract, ml; m = the weight of different pure plant extracts, gm.
Determination of total Flavonols
Total Flavonols in the plant extracts were estimated using the method of Kumaran and Karunakaran [13]. Total content of Flavonols was expressed in terms of quercetin equivalent, QUE. (Standard curve equation: y= (c x V)/m,) mg of CAE/g of dry extract [13-14].
C = (c x V)/m
Total Flavonols in the plant extracts were estimated using the method of Kumaran and Karunakaran [13]. Total content of Flavonols was expressed in terms of quercetin equivalent, QUE. (Standard curve equation: y= (c x V)/m,) mg of CAE/g of dry extract [13-14].
C = (c x V)/m
Where, C = total content of flavonoid compounds, mg/g plant extract, in catechin equivalent, CAE. c = the concentration of quercetin established from the calibration curve, mg/ml; V = the volume of extract, ml; m = the weight of pure plant extracts, gm.
Determination of total antioxidant capacity
Total antioxidant capacity of different concentrations of U. Littoralis was determined by the method of [15] with some modifications.
Total antioxidant capacity of different concentrations of U. Littoralis was determined by the method of [15] with some modifications.
Hydroxyl radical scavenging assay
Hydroxyl radical scavenging activity of different concentrations of U. Littoralis was determined by the method as described by [16] with a slight modification. The percentage (%) inhibition activity was calculated from the following equation
% I = {(A0 – A1)/A0} X 100
Hydroxyl radical scavenging activity of different concentrations of U. Littoralis was determined by the method as described by [16] with a slight modification. The percentage (%) inhibition activity was calculated from the following equation
% I = {(A0 – A1)/A0} X 100
Where, A0 is the absorbance of the control, and A1 is the absorbance of the extract/standard. Then % inhibitions were plotted against concentration and from the graph IC50 was calculated.
Metal chelating activity assay
The chelating activity of fruits extracts of U. Littoralis for ferrous ions was measured according to [17].
The chelating activity of fruits extracts of U. Littoralis for ferrous ions was measured according to [17].
Results and Discussions
Determination of Total Phenolics
Phenolic content of the methanol extract of leaves of U. Littoralis and different fractions such as petroleum ether (PEF), chloroform (CLF), ethyl acetate (EAF) and aqueous fraction (AQF) were determined using Folin-Ciocalteu reagent. Phenolic content of the samples were calculated on the basis of the standard curve for gallic acid as shown in Table 1.1 and in Figure 1.1. The results were expressed as mg of gallic acid equivalent (GAE)/gm of dried extractives.
Phenolic content of the methanol extract of leaves of U. Littoralis and different fractions such as petroleum ether (PEF), chloroform (CLF), ethyl acetate (EAF) and aqueous fraction (AQF) were determined using Folin-Ciocalteu reagent. Phenolic content of the samples were calculated on the basis of the standard curve for gallic acid as shown in Table 1.1 and in Figure 1.1. The results were expressed as mg of gallic acid equivalent (GAE)/gm of dried extractives.
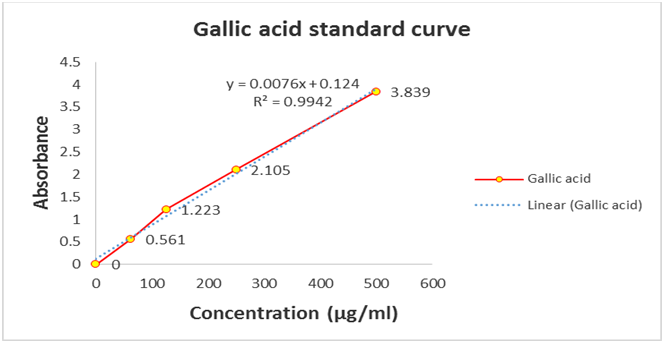
Figure 1.1: Standard curve of Gallic acid for the determination of total phenolics The results of phenolic
content of methanol extract (CME) and its fractions are shown in Table 1.2 and in Figure 1.2.
The phenolic content of methanol extract was 331.39mg of GAE/gm of dried extract. Among the fractions the highest phenolic content was found in EAF (694.30 mg of GAE/gm of dried extract) followed by AQF (408.67 mg of GAE/gm of dried extract), CLF (399.91 mg of GAE/gm of dried extract) and PEF (103.33mg of GAE/gm of dried extract). Comparing the phenolic content of different fractions with crude methanol extract it was observed that EAF and AQF contain more amounts of phenolic compounds than the crude extract.
| Concentration (µg/ml) | Absorbance | Absorbance Mean ± STD | ||
| 62.5 | 0.561 | 0.556 | 0.567 | 0.561± 0.0045 |
| 125 | 1.223 | 1.213 | 1.228 | 1.221± 0.0062 |
| 250 | 2.105 | 2.12 | 2.06 | 2.095± 0.025 |
| 500 | 3.839 | 3.81 | 3.842 | 3.830± 0.014 |
Table 1.1: Absorbance of Gallic acid at different concentrations after treatment with Folin-Ciocalteu reagent.
Determination of total Flavonols
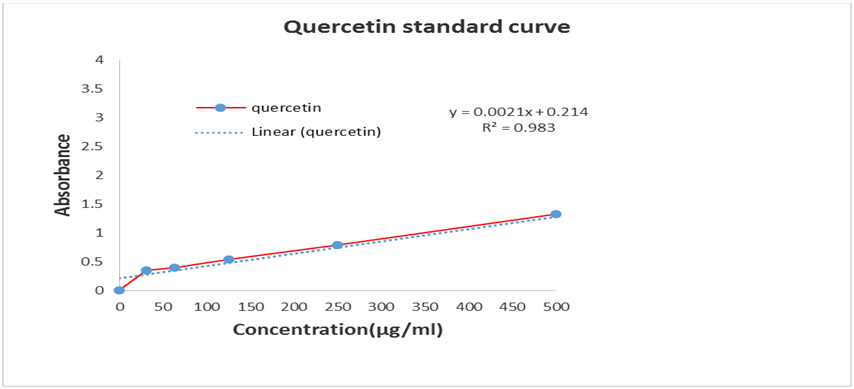
Total Flavonols content of methanol extract (CME) and four different fractions such as petroleum ether (PEF), chloroform (CLF), ethyl acetate (EAF) and aqueous fraction (AQF) were determined using much known Kumaran and Karunakaran. Flavonols content of the samples was calculated on the basis of the standard curve for quercetin as shown in Table 1.3 and in Figure 1.3. The results were expressed as µg of quercetin equivalent (QUE)/mg of extractives.
Total Flavonols content of methanol extract (CME) and four different fractions such as petroleum ether (PEF), chloroform (CLF), ethyl acetate (EAF) and aqueous fraction (AQF) were determined using much known Kumaran and Karunakaran. Flavonols content of the samples was calculated on the basis of the standard curve for quercetin as shown in Table 1.3 and in Figure 1.3. The results were expressed as µg of quercetin equivalent (QUE)/mg of extractives.
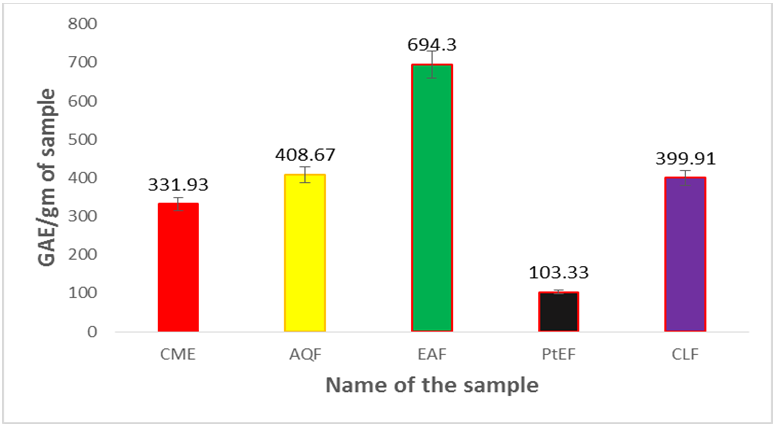
Figure 1.2: Total phenol content (mg/gm plant extract in Gallic acid equivalent)
of leaves of Uvaria littoralis.
Here, CME=Crude methanol extract, PEF= Petroleum ether fraction, CLF = Chloroform fraction, EAF = Ethyl acetate fraction, AQF = Aqueous fraction. Values are mean of triplicate experiments and represented as mean ± STD.
| Sample | No. of sample | Concentration (µg/ml) | Absorbance | GAE /gm of dried sample | GAE/gm of dried sample mean ± STD |
| CME | 1 | 500 | 1.389 | 332.9 | 331.93±0.762 |
| 2 | 500 | 1.382 | 331.04 | ||
| 3 | 500 | 1.385 | 331.84 | ||
| AQF | 1 | 500 | 1.679 | 409.20 | 408.67±0.433 |
| 2 | 500 | 1.675 | 408.14 | ||
| 3 | 500 | 1.677 | 408.68 | ||
| EAF | 1 | 500 | 2.761 | 693.94 | 694.30±0.332 |
| 2 | 500 | 2.764 | 694.74 | ||
| 3 | 500 | 2.762 | 694.21 | ||
| PEF | 1 | 500 | 0.517 | 103.42 | 103.33±0.333 |
| 2 | 500 | 0.515 | 102.88 | ||
| 3 | 500 | 0.518 | 103.68 | ||
| CLF | 1 | 500 | 1.644 | 400 | 399.91±0.542 |
| 2 | 500 | 1.641 | 399.21 | ||
| 3 | 500 | 1.646 | 400.53 |
Table 1.2: Determination of total phenolic content of the crude methanol extract and its different fractions of Uvaria littoralis.
| Concentration (µg/ml) | Absorbance | Absorbance Mean ± STD | ||
| 31.25 | 0.356 | 0.350 | 0.351 | 0.352±0.0026 |
| 62.50 | 0.393 | 0.391 | 0.0.396 | 0.393±0.0020 |
| 125 | 0.530 | 0.535 | 0.537 | 0.534±0.0029 |
| 250 | 0.793 | 0.797 | 0.795 | 0.795±0.0016 |
| 500 | 1.319 | 1.321 | 1.318 | 1.319±0.0012 |
| 1000 | 2.301 | 2.304 | 2.309 | 2.304±0.0033 |
Table 1.3: Absorbance of Quercetin at different concentrations for quantitative determination of total Flavonols.
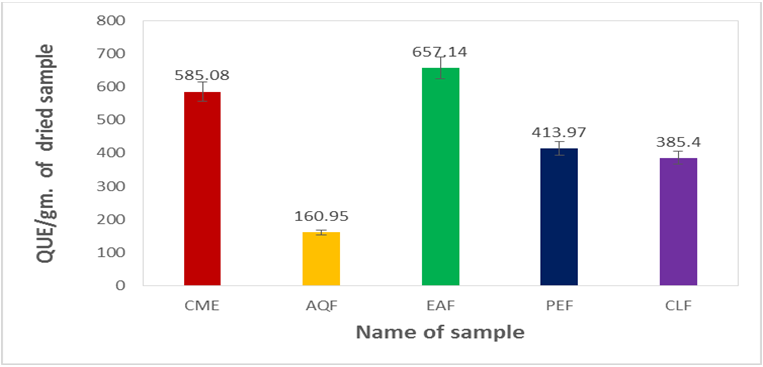
The flavonol content of methanol extract and its different fractions were shown in Table 1.4 and Figure 1.4.
The Flavonols content of methanol extract was 585.08gm of QUE/mg of dried extract. Among the fractions the highest flavonoid content was found in EAF (657.14 mg of QUE/gm. Of dried extract) followed by PEF (413.97 mg of QUE/gm. Of dried extract), CLF (385.40 mg of QUE/gm. Of dried extract) and AQF (160.95mg of QUE/gm. Of dried extract). Comparing the amount of total flavonoids content of different fractions with crude methanol extract it was observed that EAF contains a higher amount of while the others contain a similar amount of Flavonols as that of the crude extract.
| Sample | No. of sample | Concentration (µg/ml) | Absorbance | QUE/gm. of dried sample |
QUE/gm. of dried sample mean ± STD |
| CME | 1 | 500 | 0.825 | 581.90 | 585.08 ± 2.37 |
| 2 | 500 | 0.829 | 585.71 | ||
| 3 | 500 | 0.831 | 587.20 | ||
| AQF | 1 | 500 | 0.381 | 159.05 | 160.95 ± 2.06 |
| 2 | 500 | 0.382 | 160.00 | ||
| 3 | 500 | 0.386 | 163.81 | ||
| EAF | 1 | 500 | 0.902 | 655.24 | 657.14 ± 2.057 |
| 2 | 500 | 0.907 | 660.00 | ||
| 3 | 500 | 0.903 | 656.19 | ||
| PEF | 1 | 500 | 0.646 | 411.43 | 413.97 ± 1.96 |
| 2 | 500 | 0.649 | 414.28 | ||
| 3 | 500 | 0.651 | 416.19 | ||
| CLF | 1 | 500 | 0.615 | 381.90 | 385.40 ± 2.73 |
| 2 | 500 | 0.619 | 385.71 | ||
| 3 | 500 | 0.622 | 388.57 |
Table 1.4: Determination of total Flavonols content of the crude methanol extract and its different fractions of Uvaria littoralis.
Determination of total antioxidant capacity
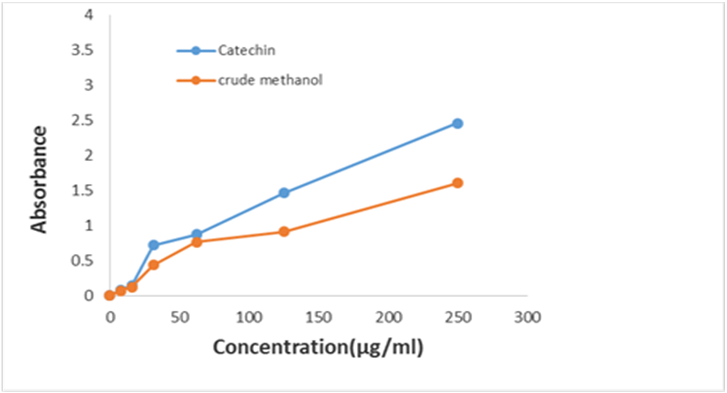
The antioxidant activity of the different extractives was assessed by phosphomolybdenum method. The phosphomolybdenum method was based on the reduction of Mo (V1) to Mo (v) by the antioxidant compound and the formation of green phosphate/Mo (v) complex with a maximal absorption at 695 nm. Total antioxidant activity of plant extracts and (+)-catechin (standard) were depicted in Table 1.5 and 1.6 and in Figure 1.5 and 1.6. As shown in Figure 1.5, crude methanol extract showed considerable antioxidant activity compared to (+)-catechin (Standard). The activity was less than that of catechin. At the concentration 62.50 µg/ml, the absorbance of crude methanol extract and (+)-catechin were 0.4363 and 0.875 respectively. The extract was found to increase the total antioxidant activity with the increasing concentration of the extract. At 250 µg/ml, the absorbance of crude methanol extract and (+)-catechin were 1.6037 and 2.457 respectively.
The antioxidant activity of the different extractives was assessed by phosphomolybdenum method. The phosphomolybdenum method was based on the reduction of Mo (V1) to Mo (v) by the antioxidant compound and the formation of green phosphate/Mo (v) complex with a maximal absorption at 695 nm. Total antioxidant activity of plant extracts and (+)-catechin (standard) were depicted in Table 1.5 and 1.6 and in Figure 1.5 and 1.6. As shown in Figure 1.5, crude methanol extract showed considerable antioxidant activity compared to (+)-catechin (Standard). The activity was less than that of catechin. At the concentration 62.50 µg/ml, the absorbance of crude methanol extract and (+)-catechin were 0.4363 and 0.875 respectively. The extract was found to increase the total antioxidant activity with the increasing concentration of the extract. At 250 µg/ml, the absorbance of crude methanol extract and (+)-catechin were 1.6037 and 2.457 respectively.
Further, the total antioxidant activity of four different fractions of crude methanol extract such as petroleum ether (PEF), chloroform (CLF), ethyl acetate (EAF) and aqueous fraction (AQF) was investigated. Among the four different fractions, EAF showed the highest total antioxidant activity with absorbance 1.883 at 250 µg/ml concentration followed by PEF (absorbance of 1.838 at 250 µg/ml), CLF (absorbance of 1.368 at 250µg/ml) and AQF (absorbance of 0.798 at 250 µg/ml). Our result demonstrates that all the extractives of Uvaria littoralis have appreciable total antioxidant activity.
| Name of sample | Conc. (µg/ml) | Absorbance | Absorbance Mean ± STD | ||
| A | B | c | |||
| (+)-Catechin | 7.825 | 0.082 | 0.083 | 0.085 | 0.083 ± 0.0012 |
| 15.625 | 0.145 | 0.148 | 0.141 | 0.145 ± 0.0029 | |
| 31.25 | 0.723 | 0.720 | 0.718 | 0.720 ± 0.0020 | |
| 62.50 | 0.876 | 0.874 | 0.877 | 0.875 ± 0.0012 | |
| 125 | 1.461 | 1.463 | 1.469 | 1.464 ± 0.0033 | |
| 250 | 2.457 | 2.459 | 2.456 | 2.457 ± 0.0012 | |
| Crude methanol extract | 7.825 | 0.071 | 0.075 | 0.078 | 0.0747 ± 0.0029 |
| 15.625 | 0.123 | 0.126 | 0.121 | 0.1233 ± 0.0021 | |
| 31.25 | 0.434 | 0.436 | 0.439 | 0.4363 ± 0.0020 | |
| 62.5 | 0.763 | 0.765 | 0.768 | 0.7653 ± 0.0021 | |
| 125 | 0.912 | 0.915 | 0.918 | 0.915 ± 0.0024 | |
| 250 | 1.606 | 1.604 | 1.601 | 1.6037 ± 0.0020 | |
Table 1.5: Total antioxidant activity of the crude methanol extract of Uvaria littoralis and (+)-catechin (standard) at different concentrations.
Total antioxidant activity
Total antioxidant activity of four different fractions of crude methanol extract such as petroleum ether (PEF), chloroform (CLF), ethyl acetate (EAF) and aqueous fraction (AQF) was investigated. Among the four different fractions, EAF showed the highest total antioxidant activity with absorbance 1.883 at 250 µg/ml concentration followed by PEF (absorbance of 1.838 at 250 µg/ml), CLF (absorbance of 1.368 at 250µg/ml) and AQF (absorbance of 0.798 at 250 µg/ml). Our result demonstrates that all the extractives of U. Littoralis have appreciable total antioxidant activity.
Total antioxidant activity of four different fractions of crude methanol extract such as petroleum ether (PEF), chloroform (CLF), ethyl acetate (EAF) and aqueous fraction (AQF) was investigated. Among the four different fractions, EAF showed the highest total antioxidant activity with absorbance 1.883 at 250 µg/ml concentration followed by PEF (absorbance of 1.838 at 250 µg/ml), CLF (absorbance of 1.368 at 250µg/ml) and AQF (absorbance of 0.798 at 250 µg/ml). Our result demonstrates that all the extractives of U. Littoralis have appreciable total antioxidant activity.
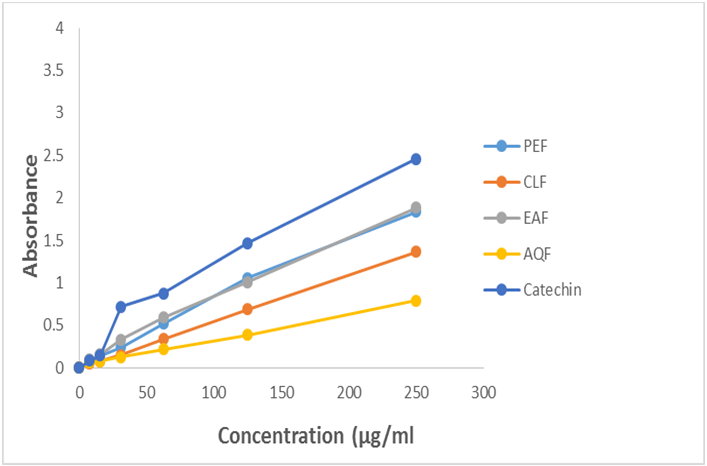
Figure 1.6: Total antioxidant activity of different fractions of crude methanol extract of Uvaria littoralis.
Here, CLF =Chloroform fraction, EAF =Ethyl acetate fraction, PEF = Petroleum ether fraction, AQF = Aqueous fraction, (+)-catechin= Standard
| Name of sample | Concentration (µg/ml) | Absorbance | Absorbance Mean ± STD | ||
| a | b | c | |||
| Petroleum ether fraction | 7.8125 | 0.081 | 0.083 | 0.082 | 0.082 ± 0.008 |
| 15.625 | 0.134 | 0.137 | 0.139 | 0.137 ± 0.002 | |
| 31.25 | 0.235 | 0.239 | 0.241 | 0.238 ± 0.025 | |
| 62.5 | 0.517 | 0.519 | 0.522 | 0.519 ± 0.002 | |
| 125 | 1.050 | 1.057 | 1.061 | 1.056 ± 0.004 | |
| 250 | 1.791 | 1.852 | 1.870 | 1.838 ± 0.0338 | |
| Chloroform fraction | 7.8125 | 0.052 | 0.057 | 0.053 | 0.054 ±0.0022 |
| 15.625 | 0.075 | 0.077 | 0.081 | 0.078 ± 0.025 | |
| 31.25 | 0.147 | 0.145 | 0.153 | 0.148 ± 0.003 | |
| 62.5 | 0.332 | 0.337 | 0.344 | 0.338 ± 0.005 | |
| 125 | 0.685 | 0.689 | 0.684 | 0.686 ± 0.0022 | |
| 250 | 1.360 | 1.367 | 1.368 | 1.365 ±0.0035 | |
| Ethyl acetate fraction | 7.8125 | 0.095 | 0.093 | 0.097 | 0.095 ±0.0016 |
| 15.625 | 0.151 | 0.156 | 0.158 | 0.155 ± 0.003 | |
| 31.25 | 0.328 | 0.331 | 0.336 | 0.332 ±0.0033 | |
| 62.5 | 0.590 | 0.593 | 0.595 | 0.593 ± 0.002 | |
| 125 | 1.005 | 1.009 | 1.012 | 1.009 ± 0.003 | |
| 250 | 1.872 | 1.910 | 1.867 | 1.883 ± 0.019 | |
| Aqueous fraction | 7.8125 | 0.059 | 0.062 | 0.058 | 0.06 ± 0.0017 |
| 15.625 | 0.072 | 0.079 | 0.076 | 0.076 ± 0.003 | |
| 31.25 | 0.123 | 0.128 | 0.133 | 0.128 ±0.0041 | |
| 62.5 | 0.211 | 0.219 | 0.221 | 0.217 ±0.0043 | |
| 125 | 0.380 | 0.386 | 0.389 | 0.385 ± 0.004 | |
| 250 | 0.785 | 0.791 | 0.798 | 0.791± 0.0053 | |
Table 1.6: Total antioxidant activity of different fractions of crude methanol extract of Uvaria littoralis at different concentrations.
Determination of Metal chelating activity assay
The results for the metal chelating activity of crude methanol extract of U. Littoralis and it fractionates are given in Table 1.7 and 1.8 and in Figure 1.7 and 1.8. The inhibitory activity of crude extract not increased with increasing concentration and the highest activity was obtained at 250 µg/ml concentration.
The results for the metal chelating activity of crude methanol extract of U. Littoralis and it fractionates are given in Table 1.7 and 1.8 and in Figure 1.7 and 1.8. The inhibitory activity of crude extract not increased with increasing concentration and the highest activity was obtained at 250 µg/ml concentration.
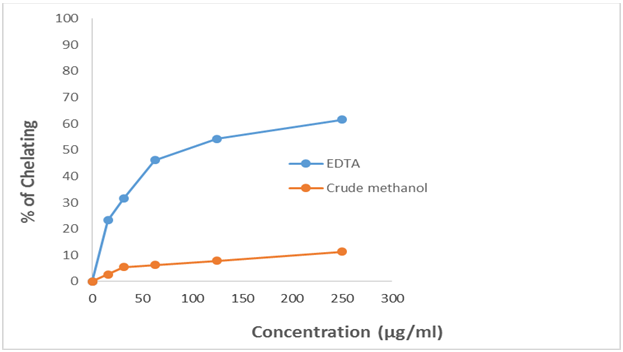
Figure 1.7: Metal Chelating Activity of crude methanol extract (CME) of Uvaria littoralis and EDTA (Standard).
| Name of sample | Conc. (µg/ml) | % of Chelating | % of Chelating Mean ± STD | IC50 (µg/ml) | ||
| a | b | C | ||||
| EDTA | 15.625 | 23.18 | 23.36 | 23.32 | 23.28 ± 0.077 | 67.787 |
| 31.25 | 31.48 | 31.43 | 31.49 | 31.46 ± 0.026 | ||
| 62.5 | 46.01 | 46.21 | 46.08 | 46.1 ± 0.082 | ||
| 125 | 54.17 | 54.19 | 54.34 | 54.23 ± 0.076 | ||
| 250 | 61.50 | 61.69 | 61.31 | 61.5 ± 0.155 | ||
| Crude methanol extract | 15.625 | 2.86 | 2.67 | 2.76 | 2.76 ± 0.078 | 1099.384 |
| 31.25 | 5.42 | 5.48 | 5.44 | 5.44 ± 0.029 | ||
| 62.5 | 6.31 | 6.37 | 6.29 | 6.32 ± 0.034 | ||
| 125 | 7.89 | 7.93 | 7.97 | 7.93 ± 0.033 | ||
| 250 | 11.34 | 11.27 | 11.51 | 11.37 ± 0.101 | ||
Table 1.7: Metal chelating activity of the crude methanol extract of Uvaria littoralis and EDTA (standard) at different concentrations.
The results with the IC50 value of the samples of lipid metal chelating activity assay of plant extracts and EDTA (standard) are given in Table 1.9, 1.10 and in Figure1.9. Our results demonstrate that the crude methanol extract has low inhibitory activity against metal chelating activity in comparison with the standard EDTA. TheIC50 of EDTA (standard) and crude methanol extract (CME) were 67.787μg/ml and 1099.384μg/ml respectively.
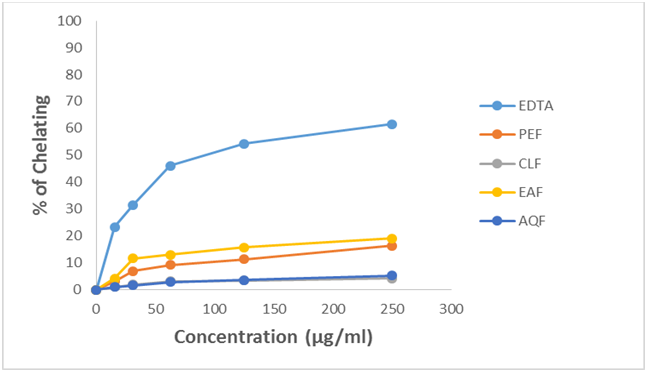
Figure 1.8: Metal chelating activity of different fractions of crude methanol extract of Uvaria littoralis at different concentrations.
| Name of sample | Conc. (µg/ml) | % of Chelating | % of Chelating Mean ± STD |
IC50 (µg/ml) | ||
| a | b | C | ||||
| Petroleum ether fraction | 15.625 | 3.08 | 3.05 | 3.12 | 3.083 ± 0.029 | 763.592 |
| 31.25 | 6.96 | 6.99 | 6.92 | 6.956 ± 0.029 | ||
| 62.5 | 9.14 | 9.18 | 9.21 | 9.176 ± 0.028 | ||
| 125 | 11.23 | 11.28 | 11.33 | 11.28 ± 0.041 | ||
| 250 | 16.41 | 16.41 | 16.29 | 16.37 ± 0.056 | ||
| Chloroform fraction | 15.625 | 1.02 | 1.07 | 1.01 | 1.033 ± 0.026 | 2981.159 |
| 31.25 | 1.97 | 1.93 | 1.99 | 1.963 ± 0.025 | ||
| 62.5 | 3.12 | 3.08 | 3.17 | 3.123 ± 0.037 | ||
| 125 | 3.48 | 3.44 | 3.51 | 3.48 ± 0.027 | ||
| 250 | 4.16 | 4.19 | 4.23 | 4.193 ± 0.029 | ||
| Ethyl acetate fraction | 15.625 | 4.18 | 4.23 | 4.17 | 4.121 ± 0.025 | 655.4798 |
| 31.25 | 11.63 | 11.61 | 11.68 | 11.64 ± 0.041 | ||
| 62.5 | 13.03 | 13.07 | 13.09 | 13.06 ± 0.045 | ||
| 125 | 15.77 | 15.67 | 15.72 | 15.72 ± 0.074 | ||
| 250 | 19.02 | 19.06 | 19.13 | 19.07 ± 0.029 | ||
| Aqueous fraction | 15.625 | 1.01 | 1.15 | 0.98 | 1.047 ± 0.031 | 2413.127 |
| 31.25 | 1.55 | 1.58 | 1.51 | 1.546 ± 0.029 | ||
| 62.5 | 2.88 | 2.83 | 2.89 | 2.867 ± 0.026 | ||
| 125 | 3.69 | 3.63 | 3.62 | 3.647 ± 0.030 | ||
| 250 | 5.17 | 5.22 | 5.15 | 5.18 ±0.029 | ||
Table 1.8: Metal chelating activity of different fractions of crude methanol extract of Uvaria littoralis at different concentrations.
Further, the metal chelating activity of all the fractions of crude methanol extract such as petroleum ether (PEF), chloroform (CLF), ethyl acetate (EAF) and aqueous fraction (AQF) have been investigated at 250 µg/ml concentration. Among the fractions the highest activity was found in EAF (19.07% inhibition), followed by PEF (16.29% inhibition), AQF (5.15% inhibition) and CLF (4.23% inhibition).
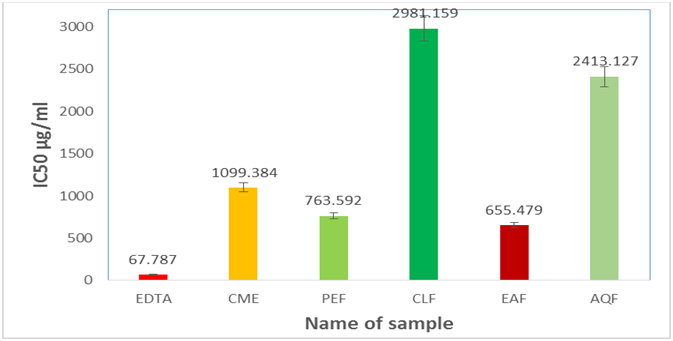
Figure 1.9: IC50 (µg/ml) values of different extractives of Uvaria littoralis for metal chelating activity assay.
Conclusion
Our in vitro study suggests that U. Littoralis inhibits acetyl cholinesterase activity and multiple components of oxidative stress pathway that can contribute to Alzheimer’s pathology. The crude methanol extract and its different fractions showed considerable antioxidant activity. Although the in vivo effectiveness of Uvaria littoralis and its components remain to be investigated, the results indicate that U. Littoralis may be of value for an effective treatment for Alzheimer’s disease.
Acknowledgements
The authors would like to thank the Department of Pharmacy, Southeast University, Bangladesh for their support during the research.
The authors would like to thank the Department of Pharmacy, Southeast University, Bangladesh for their support during the research.
Consent
It is not applicable.
It is not applicable.
Competing interests
Authors have no declared that no competing interests exist.
Authors have no declared that no competing interests exist.
References
- Hossain S., et al. “Leucaszeylanica (L.) R. Br. Protects ethanol and hydrogen peroxide-induced oxidative stress on hepatic tissue of rats”. International Current Pharmaceutical Journal 4.2.9 (2013): 148-151.
- Rahman M., et al. “Antioxidant activity of Centellaasiatica (Linn.) urban: Impact of extraction solvent polarity”. Journal of Pharmacognosy and Phytochemistry 1.6 (2013).
- Heistad DD., et al. “Novel aspects of oxidative stress in cardiovascular diseases”. Circulation Journal 73.2 (2009): 201-207.
- G Ignazio., et al. “Biochemical mechanisms in drug-induced liver injury: Certainties and doubts”. World Journal of Gastroenterology 15.39 (2009): 4865-4876.
- Cesaratto L., et al. “The importance of redox state in liver damage”. Annals of Hepatology 3.3 (2004): 86-92.
- Saladin KS. Anatomy & Physiology: The Unity of Form and Function (6th ed., pp), (2011): 887-925.
- Jaeschke H., et al. “Mechanisms of hepatotoxicity”. Toxicological Sciences 65.2 (2002): 166-176.
- Ha HL., et al. “Oxidative stress and antioxidants in hepatic pathogenesis”. World Journal of Gastroenterology 16.48 (2010): 6035.
- Medina J and Moreno-Otero R. “Pathophysiological basis for antioxidant therapy in chronic liver disease”. Drugs 65.17 (2005): 2445-2461.
- Backer, C.A. &Bakhuizen van den Brink, R.C., 1963 1968. Flora of Java. 3 Volumes. Noordhoff, Groningen, the Netherlands.
- Heyne, K. De nuttigeplanten van NederlandschIndië [The useful plants of the Dutch East Indies]. 2nd ed. 3 Volumes. Departement van Landbouw, Nijverheiden Handel in NederlandschIndië. 1953 pp; 1927.
- Singleton V L and Rossi JA. “Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents”. American Journal of Enology and Viticulture 16 (1965): 144-158.
- Kumaran A and Karunakaran RJ. “In vitro antioxidant activities of methanol extracts of Phyllanthus species from India”. LWT- Food Science and Technology 40.2 (2007): 344-352.
- Prieto P., et al. “Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E”. Annals of Biochemistry 269.2 (1999): 337-341.
- Islam S., et al. “Estimation of phytochemical, antioxidant screening profile and thrombolytic activities of methanolic extract of Antidesmabunius L. Leaf”. Horticulture International Journal 2.6 (2018): 358-363.
- MS Ahammed and Islam MS. “In vitro Antioxidant and Cholinesterase Inhibitory Activities of Methanolic Extract of Grewia nervosa L. (Family: Tiliaceae) Leaf”. European Journal of Medicinal Plants 25.2 (2018): 1-33.
- J Sabate. “The contribution of vegetarian diets to health and disease: A paradiagram Shift.” The American Journal of Clinical Nutrition 78.3 (2003): 502-507.
Citation:
Md. Shariful Islam., et al. “In vitro Antioxidant Preliminary Screening Profile of Methanolic Extract of Uvaria littoralis leaf.”. Chronicles of Pharmaceutical Science 4.1 (2019): 10-21.
Copyright: © 2019 Md. Shariful Islam., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.