Research Article
Volume 3 Issue 2 - 2019
Synthesis, Characterization and 3d Molecular of Substituted Phenyl Thiourea Pyrimidine-2-Pyrazolines
Department of Chemistry, Vidya Vikas Art, Commerce & Science College, Samudrapur, Dist.-Wardha, 442305, INDIA
*Corresponding Author: Meghasham N. Narule, Department of Chemistry, Vidya Vikas Art, Commerce & Science College, Samudrapur, Dist.-Wardha, 442305, INDIA.
Received: March 18, 2019; Published: April 30, 2019
Abstract
The resent paper reports the synthesis and characterization of some new biologically active1-(3/-substituted phenyl-5/- amino pyrimidine)-3-(substituted phenyl)-5-(substituted phenyl)-2-pyrazolines 4(a-p) and phenyl isothiocyanate in presence of ethanol to get 1-(3/-substituted phenyl-5/-thiourea pyrimidine)-3-(substituted phenyl)-5-(substituted phenyl)-2- pyrazolines 8(a-p).The compounds so obtained were characterized by different chemical studies such as elemental analysis, infrared, H1-NMR, C13-NMR, Mass spectroscopy and 3D-molecular modeling and analysis for bond lengths and bond angles have been carried out for 8(a). Compounds synthesized have been screened for antimicrobial activity against staphylococcus aureus, E-coli, P. vulgaris, A. Niger, B. substillis, C. albicans for its antibacterial and antifungal.
Keywords: Phenyl isothiocyanate; Amino pyrimidine; 2-pyrazolines, etc
Introduction
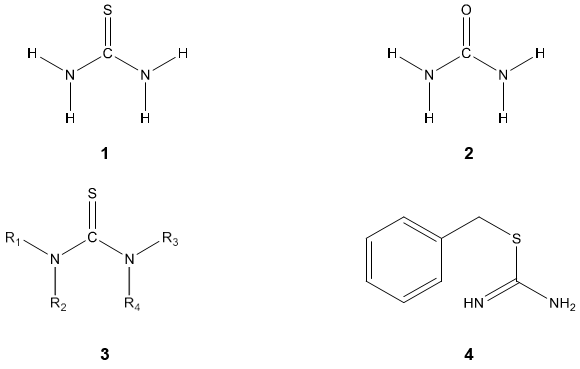
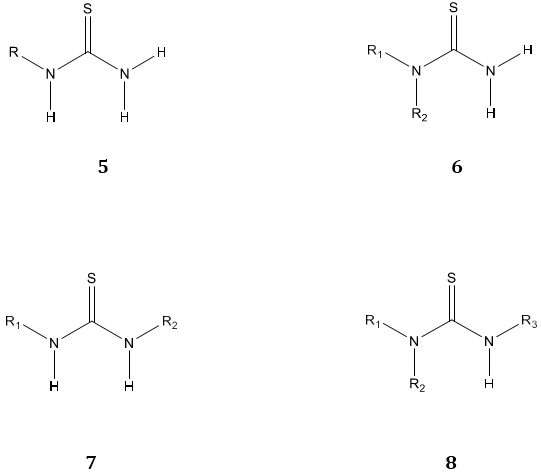
Thiourea 1 is the class of organic compounds containing sulfur, also known as thiocarbamide has a structural resemblance to Urea 2 in which oxygen atom is replaced by the sulfur atom [1]. They are generally represented as [NR1R2] CS [NR3R4] 3, where R1, R2, R3 & R4= hydrogen, phenyl, aryl, alkyl, cyclo alkyl, heterocycles, acyl etc or any substituent [2]. S-substituted thioureas are known as isothiourea, such as 2-benzyl isothiourea 4.
On the basis of the number of attached substituents on thiourea moiety they are categorized as mono N-substituted thiourea 5 obtained by replacing H of NH2 with R. Di-substituted thiourea obtained by replacing two hydrogen atoms of same NH2 group i.e. 1,1-disubstituted thiourea 6 or one H atom of each NH2 group i.e. 1,3-disubstituted thiourea 7 with R1 and R2. Trisubstituted thiourea is obtained by replacing two H atoms of the same NH2 group with R1, R2 and one hydrogen atom of another NH2 group with R3 i.e. 1,1,3-trisubstituted thiourea 8. [1]
Derivatives of thiourea play a vital role in all branches of chemistry. They are also useful in the field of dyes, photographic film, elastomers, plastics, and textiles. Certain thiourea derivatives are insecticides, preservatives and rodenticides. They show a broad spectrum of biological activities. Thioureas are also applicable in the characterization of organic compounds. For example, amines on reaction with isothiocyanate produce sharp melting point thiourea.
The derivatives of thiourea are valuable for numerous fields. They act as vulcanization accelerator in the process of vulcanization of rubber. It also shows catalytic property and acts as rust inhibitor of metal [3]. Thiourea derivatives are also applicable in the inhibition of corrosion of copper in the solution and the inhibiting efficiency increases with a decrease in temperature or an increase in the concentration of the thiourea derivatives [4].
The derivatives of thiourea act as important synthon for the synthesis of heterocyclic compounds. Thiourea derivatives play an important role in catalyst design and modification [5]. The phenomenon of using substituted thioureas derivatives as a catalyst in organic synthesis is known as thiourea organocatalysis [6]. Numerous asymmetric reactions, such as Mannich, Aldol, Henry, Michael and Biginelli reactions, have been accomplished by this catalysts [7]. Recently, bifunctional thiourea derivatives have been recognized as effective organocatalysts for asymmetric Michael addition reactions [8-10]. The development of simple and efficient bifunctional thiourea catalysts has great interest [5]. Carbohydrates are easily accessible chiral precursors of asymmetric catalysts [11].
Thioureas have various industrial applications. They have a potential application in the processing of gold [12]. Recently pyridine acyl thiourea 9 derivatives reported as Ionophore for the detection of Copper (II) in aqueous phase [13]. Polystyrene- supported N-methylthiourea reported as a convenient reagent for the hydrogenolysis of bicyclic endoperoxides [14]. Urea and thiourea substitute cyclo-tri phosphazene compounds reported as naked-eye sensors for F- and CN- anions [15]. Thiourea based hybrid materials used as a potential adsorbent for the removal of Hg(II) from aqueous solution [16].
Thiourea derivatives act as versatile ligands, able to coordinate with metal centers as neutral ligands, mono anions or dianions [17-22]. Both the ligands and their metal complexes display a wide variety of biological activity such as antifungal, herbicidal, antibacterial, antihelmintic, antitubercular, rodenticidal, insecticidal, antithyroid and plant-growth regulator properties [23-27].
Pharmacological Activities of Thiourea
Thiourea the earliest synthetic organic compound used, directly and indirectly, due to its easy availability. They are important sulfur and nitrogen containing compounds that have proved to be useful substances in drug research in recent years [28-33]. The presence of reactive group SC[NH2]2 or SC[NR1R2]2 or SC[NR1R2][NR3R4] is responsible for their pharmacological importance[34-35]. The number of thiourea units in the thiourea backbone derivatives contributed to the enhancement of antimicrobial activity [36]. Many studies reported compounds with a single thiourea moiety with antimicrobial properties [37, 38] whereas symmetrical and unsymmetrical bis thiourea were reported for their significant anticancer and antimycobacterial activities [39, 40]. Thioureas are well known precursors of nitrogen and sulfur containing heterocycles because of their reactive –CONHCSNH- group.
Thiourea the earliest synthetic organic compound used, directly and indirectly, due to its easy availability. They are important sulfur and nitrogen containing compounds that have proved to be useful substances in drug research in recent years [28-33]. The presence of reactive group SC[NH2]2 or SC[NR1R2]2 or SC[NR1R2][NR3R4] is responsible for their pharmacological importance[34-35]. The number of thiourea units in the thiourea backbone derivatives contributed to the enhancement of antimicrobial activity [36]. Many studies reported compounds with a single thiourea moiety with antimicrobial properties [37, 38] whereas symmetrical and unsymmetrical bis thiourea were reported for their significant anticancer and antimycobacterial activities [39, 40]. Thioureas are well known precursors of nitrogen and sulfur containing heterocycles because of their reactive –CONHCSNH- group.
Some thiourea has been proved as a new class of potent non-nucleoside inhibitors of human viruses type 1 reveres Ariansscriptase (NNRTIS) [41, 42]. Thiourea derivatives have biological properties such as antioxidant [43], antibacterial [44, 45], antimicrobial [46], anti- HlV activity [47, 48], anti malarial [49] and anticancer [50]. Some urea and thiourea derivatives possess valuable antituberculosis, antibacterial and anticonvulsant properties [51-54].
Urea and thiourea compounds could be used for elimination or detoxification of super antigens from body fluids [55-57] and for the treatment of hemoglobinopathies in the cases of sickle cell anemia and β-thalassemia [58].
The combinations of urea and thiourea derivatives with benzothiazole have produced DNA topoisomerase [59, 60] or HIV reverse transcriptase inhibitors [61, 62]. A series of ureas and thioureas were synthesized, and their inhibitory activities against NO (free radical) production in lipopolysaccharide-activated macrophages were evaluated [63]. Substituted aryl thiourea and their derivative are shown diverse antimicrobial and antifungal effects [64].
Antimicrobial
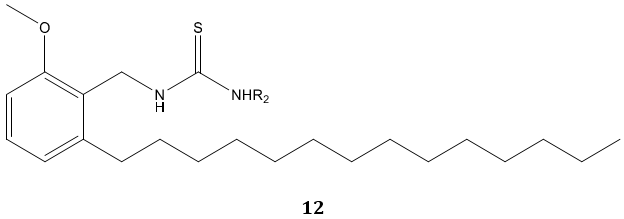
C. Naga Raju et al [65] reported urea and thiourea derivatives 10, 11 of diphenyl phosphoramidite as a good antimicrobial agent. In another study, N. S. Reddy et al [66] synthesized urea and thiourea derivatives 12 of anacardic acid mixture isolated from a natural product cashew nut shell liquid (CNSL) and screened for antibacterial activity. Most of the compounds were emerged active as compared with standard drug ampicillin.
C. Naga Raju et al [65] reported urea and thiourea derivatives 10, 11 of diphenyl phosphoramidite as a good antimicrobial agent. In another study, N. S. Reddy et al [66] synthesized urea and thiourea derivatives 12 of anacardic acid mixture isolated from a natural product cashew nut shell liquid (CNSL) and screened for antibacterial activity. Most of the compounds were emerged active as compared with standard drug ampicillin.
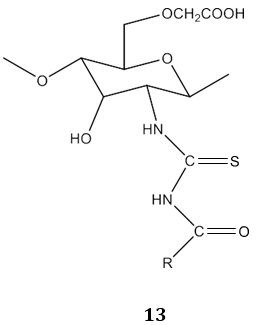
In another study, N. A. Mohamed & N. A. Abd El-Ghany [67] reported carboxymethyl chitosan acyl thiourea derivatives 13 as the antimicrobial agent.
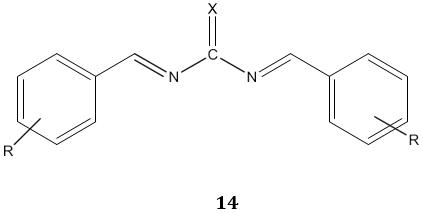
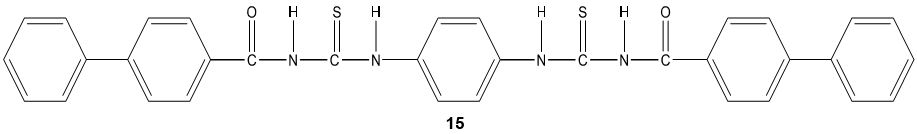
The acyl thiourea derivatives of CMCS have stronger activity against gram positive bacteria than gram negative bacteria. The CMCS derivatives also showed the significant inhibitory effect on the fungi. Bis-imine derivatives 14 possess significant antifungal activity [68]. N(4-substitution phenyl carbamothioyl)biphenyl- 4-carboxamide derivatives 15 possess a broad spectrum of antibacterial activity [69].
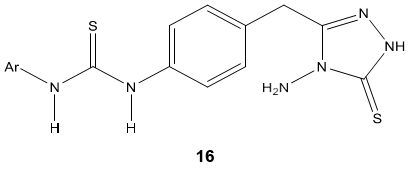
B. K. Kaymakcioglu & coworker [70] synthesized thiourea and urea derivatives 16 containing 1, 2, 4-triazole moieties and evaluated for their antifungal, larvicidal and anti-inflammatory activity against some human cell lines. Obtained results demonstrated no cytotoxic and anti-inflammatory activity but some of them showed good antifungal activity against Phomopsis species.
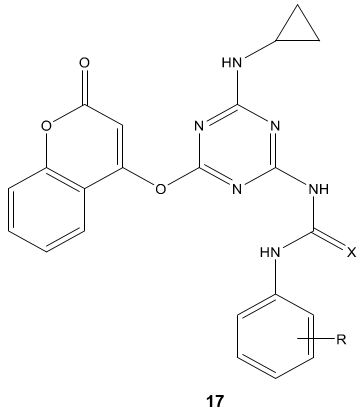
P. B. Kaswala et al [71] synthesized s-triazinyl urea and thiourea derivatives 17 and evaluated for their antibacterial activities against various Gram-positive and Gram-negative strains of bacteria. Some of them showed good to excellent in vitro antibacterial activity S. Aureus, B. Subtilis, E. Coli and P. Aeruginosa. It concluded that electron withdrawing groups increases the antibacterial activities compared to the electron donating group to the aromatic ring.
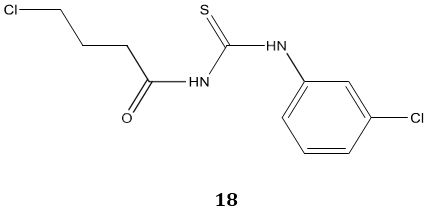
Carbonyl thiourea derivatives 18 were reported as an anti-amoebic agent against both Acanthamoeba species and their potent cytotoxic properties suggest them as a new anti-amoebic agent for the treatment of Acanthamoeba Keratitis [72].
Anticancer
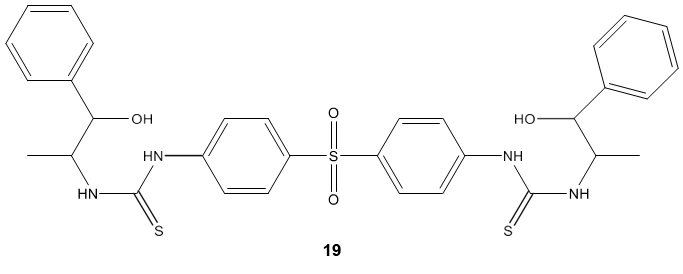
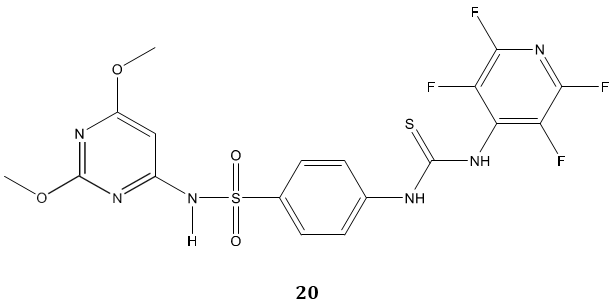
Sulfonamides carrying a biologically active thiourea, biphenyl sulfones bearing thiourea and oxazole thione were reported as a new class of anticancer agent. Bis- biphenyl sulfone 19 emerged active as doxorubicin as a reference drug against breast and liver cancer cell line and exhibited moderate activity against colon cancer cell line [73]. In another study, Ghorab et al [74] synthesized some novel fluorinated thiourea derivatives 20 carrying sulfonamide moieties and reported them as good anticancer and antibacterial agents. The molecular docking study suggested their nitrogen activated protein kinase-2 inhibitory activity.
Sulfonamides carrying a biologically active thiourea, biphenyl sulfones bearing thiourea and oxazole thione were reported as a new class of anticancer agent. Bis- biphenyl sulfone 19 emerged active as doxorubicin as a reference drug against breast and liver cancer cell line and exhibited moderate activity against colon cancer cell line [73]. In another study, Ghorab et al [74] synthesized some novel fluorinated thiourea derivatives 20 carrying sulfonamide moieties and reported them as good anticancer and antibacterial agents. The molecular docking study suggested their nitrogen activated protein kinase-2 inhibitory activity.
ANTI HIV
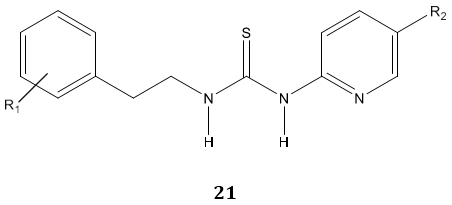
D. Cruz et al [75] examined anti-HIV and spermicidal activity of some novel thiourea derivatives 21 and reported them as dual function micro-biocides.
D. Cruz et al [75] examined anti-HIV and spermicidal activity of some novel thiourea derivatives 21 and reported them as dual function micro-biocides.
Antioxidant
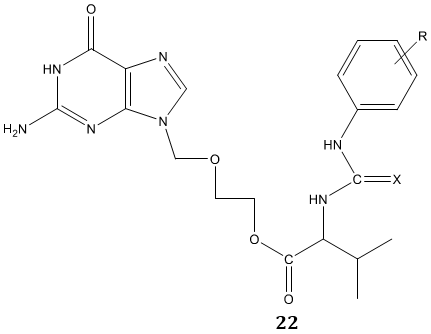
Katla et al [76] synthesized a series of novel urea and thiourea derivatives of valacyclovir 22 by reacting (S) -2-[(2-amino-6-oxo-6,9-dihydro-3H-purine-9- yl)methoxy]ethyl 2-amino-3-methyl butanoate (valacyclovir) with various aromatic isocyanates/thiocyanates in presence of N, N’-dimethyl piperazine as a base in THF: pyrimidine medium. Their screening against tobacco mosaic virus (TMV) and for antioxidant activity reported them as a potent antiviral and antioxidant agent.
Katla et al [76] synthesized a series of novel urea and thiourea derivatives of valacyclovir 22 by reacting (S) -2-[(2-amino-6-oxo-6,9-dihydro-3H-purine-9- yl)methoxy]ethyl 2-amino-3-methyl butanoate (valacyclovir) with various aromatic isocyanates/thiocyanates in presence of N, N’-dimethyl piperazine as a base in THF: pyrimidine medium. Their screening against tobacco mosaic virus (TMV) and for antioxidant activity reported them as a potent antiviral and antioxidant agent.
Antitubercular
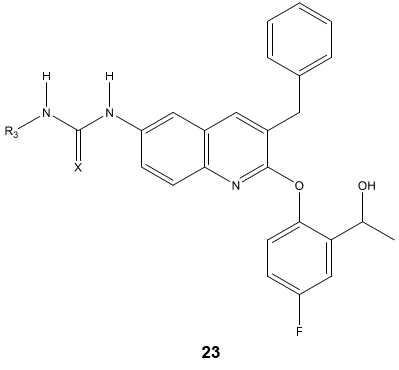
A novel series of quinoline derivatives 23 possessing triazolo, ureido and thioureido substituents have been evaluated for their antimycobacterium properties [77].
A novel series of quinoline derivatives 23 possessing triazolo, ureido and thioureido substituents have been evaluated for their antimycobacterium properties [77].
Carbonic Anhydrase Inhibitors
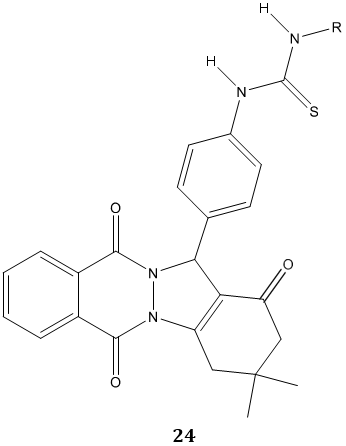
A phthalazine substituted urea and thiourea derivatives 24 were evaluated for inhibitory effects on the activity of purified human carbonic anhydrases (hCAs I and II) and reported as good inhibitory agents for carbonic anhydrase [78].
A phthalazine substituted urea and thiourea derivatives 24 were evaluated for inhibitory effects on the activity of purified human carbonic anhydrases (hCAs I and II) and reported as good inhibitory agents for carbonic anhydrase [78].
Neuronal and Inducible Nitric Oxide Synthase (nNOS and iNOS)
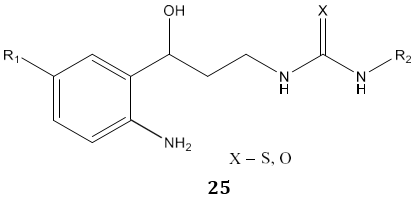
M. D. Carrión et al [79] synthesized N, N’-disubstituted thiourea and urea derivatives 25 as inhibitors of both Neuronal and Inducible Nitric Oxide Synthase.
M. D. Carrión et al [79] synthesized N, N’-disubstituted thiourea and urea derivatives 25 as inhibitors of both Neuronal and Inducible Nitric Oxide Synthase.
Tyrosinase Inhibitor
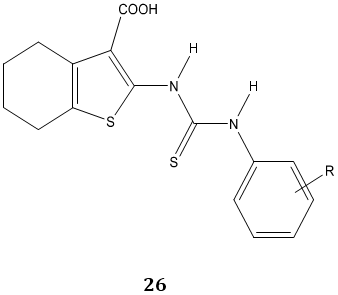
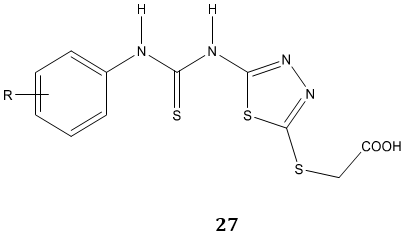
Tyrosinase is a key enzyme during the production of melanins in plants and animals. P. Liu et al [80] synthesized a novel series of N-aryl-N’-substituted phenylthiourea derivatives 26, 27 as potential tyrosinase inhibitors.
Tyrosinase is a key enzyme during the production of melanins in plants and animals. P. Liu et al [80] synthesized a novel series of N-aryl-N’-substituted phenylthiourea derivatives 26, 27 as potential tyrosinase inhibitors.
Synthetic Methods of Thiourea
A large number of methods have been reported in the literature for the synthesis of thiourea. Some of the common methods are described below.
A large number of methods have been reported in the literature for the synthesis of thiourea. Some of the common methods are described below.
From Isothiocyanate
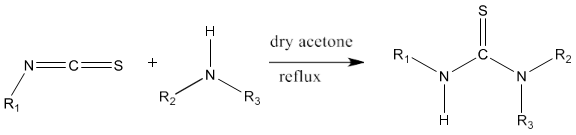
1) The standard method of synthesis of thiourea is based on the reaction of alkyl or aryl isothiocyanates with ammonia or substituted amines.
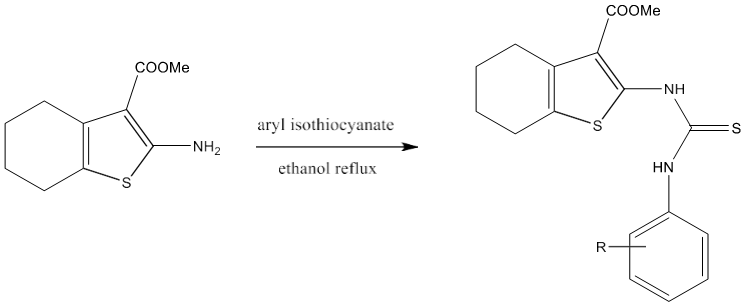
Thiourea can be prepared by refluxing a mixture of the compound containing a primary amino group with aryl isothiocyanate in ethanol under the atmosphere of nitrogen [80].
1) The standard method of synthesis of thiourea is based on the reaction of alkyl or aryl isothiocyanates with ammonia or substituted amines.
Thiourea can be prepared by refluxing a mixture of the compound containing a primary amino group with aryl isothiocyanate in ethanol under the atmosphere of nitrogen [80].
2) Alkyl isothiocyanates on reaction with primary or secondary amines yield thiourea derivatives [81].
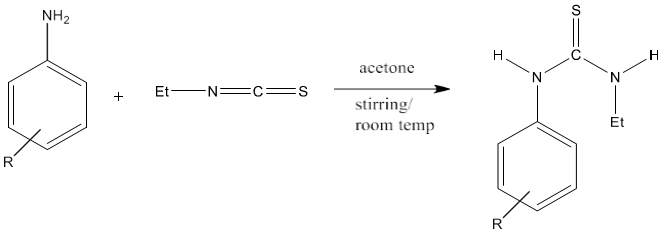
3) The equivalent amount of aromatic amine and ethyl isothiocyanate in acetone with constant stirring at room temperature produces thiourea with high yield. The yield of product increases by the addition of electron donor groups to aromatic amines [82].
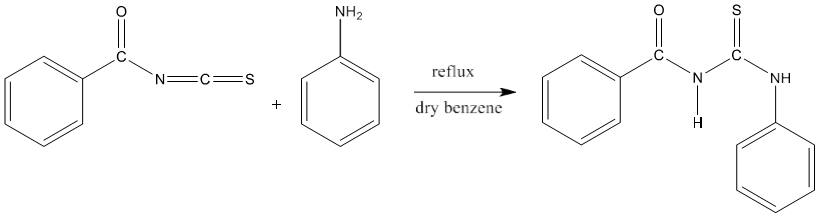
4) 1-phenyl -3-benzoyl-2-thiourea (PhBTU) synthesized by the reaction of benzoyl isothiocyanate and aniline in dry benzene [83].
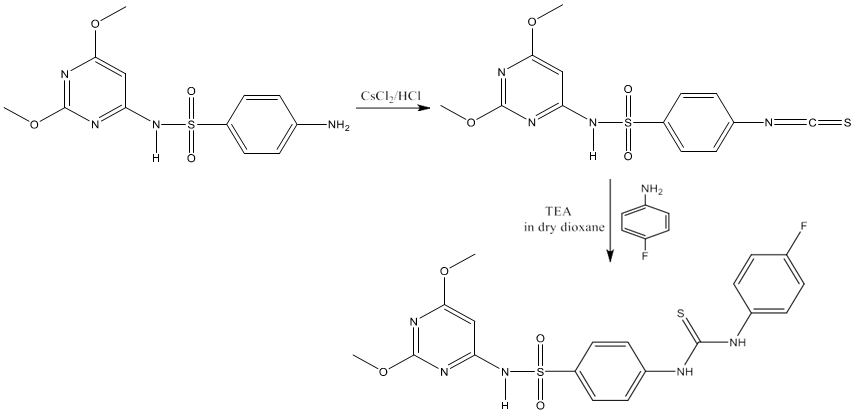
5) The treatment of isothiocyanatobenzene sulfonamide with a variety of fluorinated aromatic amines in dry dioxane at reflux temperature in the presence of a catalytic amount of triethyl amine furnished the novel fluorinated N, N-disubstituted thiourea in high yield [74].
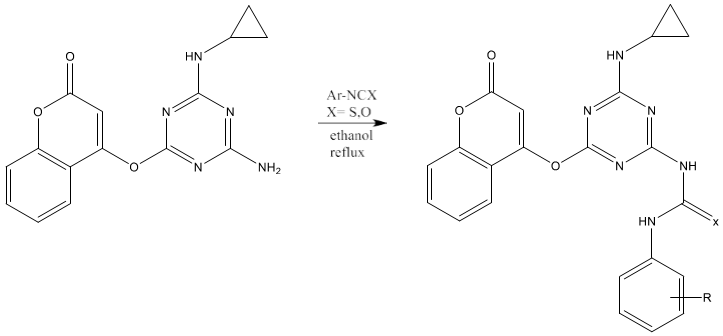
6) Thiourea can be synthesized by refluxing a mixture of 2-(Coumarinyl-4-oxy)- 4-(cyclopropylamino)-6-(amino)-s-triazine and aryl isothiocyanate in ethanol [71].
From Cyanamides
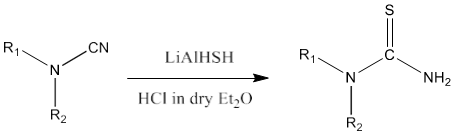
7) Lithium aluminium hydride on reaction with sulfur yields LiAlHSH. Cyanamide on reaction with LiAlHSH in presence of HCl in dry diethyl ether produces thiourea [84].
From Thiophosgene CSCl2
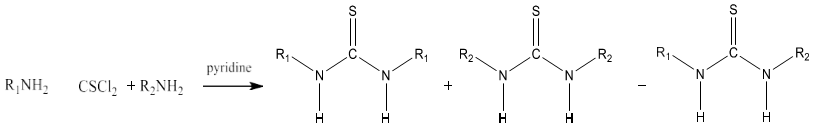
8) Thiourea can be produced by condensing primary and secondary amine with thiophosgene in presence of pyridine. This reaction produces a mixture of thiourea which can be separated by using chromatography. Symmetrical thiourea will be obtained by using a single amine [85].
8) Thiourea can be produced by condensing primary and secondary amine with thiophosgene in presence of pyridine. This reaction produces a mixture of thiourea which can be separated by using chromatography. Symmetrical thiourea will be obtained by using a single amine [85].
From Carbon Disulphide(CS2)
9) Primary amine or secondary amine on reaction with carbon disulfide produces symmetrical as well as unsymmetrical thioureas via the formation of amino dithiol as intermediate instead of isothiocyanate [86]. It works smoothly with aliphatic primary amines.
9) Primary amine or secondary amine on reaction with carbon disulfide produces symmetrical as well as unsymmetrical thioureas via the formation of amino dithiol as intermediate instead of isothiocyanate [86]. It works smoothly with aliphatic primary amines.
From Thiourea
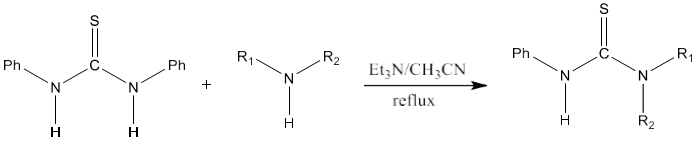
10) Di-substituted or tri-substituted thiourea derivatives can be synthesized from symmetrical thioureas as precursor [87].
10) Di-substituted or tri-substituted thiourea derivatives can be synthesized from symmetrical thioureas as precursor [87].
By Zinc Chloride Catalyzed Thermal Reaction
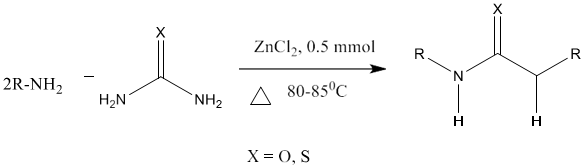
11) M. A. Pasha et al [88] reported the synthesis of N, N-disubstituted urea/thioureas by the reaction of amines or phenyl hydrazine with urea/thiourea in the presence of a catalytic amount of zinc chloride on a preheated hot plate at 82 - 85°C under solvent free conditions with increased reaction rate and high yield.
11) M. A. Pasha et al [88] reported the synthesis of N, N-disubstituted urea/thioureas by the reaction of amines or phenyl hydrazine with urea/thiourea in the presence of a catalytic amount of zinc chloride on a preheated hot plate at 82 - 85°C under solvent free conditions with increased reaction rate and high yield.
Simple One Pot Synthesis
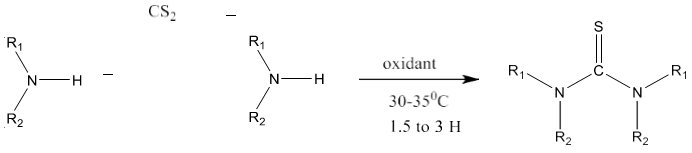
12) M. M. Milosavljević et al [89] introduced simple one pot synthesis of thiourea from required amines, carbon disulfide and an oxidant i.e. hydrogen peroxide, Ethylene Diamine Tetra Acetic acid (EDTA) / sodium percarbonate or air in water. High conversion of the starting material into the product was achieved using EDTA/ sodium percarbonate as an oxidant. Symmetrical as well as unsymmetrical thioureas can be synthesized
12) M. M. Milosavljević et al [89] introduced simple one pot synthesis of thiourea from required amines, carbon disulfide and an oxidant i.e. hydrogen peroxide, Ethylene Diamine Tetra Acetic acid (EDTA) / sodium percarbonate or air in water. High conversion of the starting material into the product was achieved using EDTA/ sodium percarbonate as an oxidant. Symmetrical as well as unsymmetrical thioureas can be synthesized
Microwave Assisted Synthesis
13) Nucleophilic addition of either alkyl or phenyl isothiocyanate into primary amino derivatives in situ produces thiourea [79].
13) Nucleophilic addition of either alkyl or phenyl isothiocyanate into primary amino derivatives in situ produces thiourea [79].
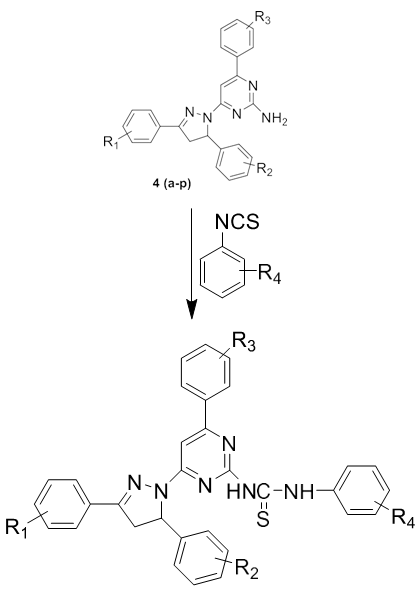
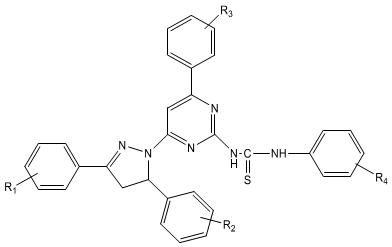
On the basis of the survey of the literature, thiourea derivatives possess a broad spectrum of pharmacological activities. This prompted us to synthesized various novel thiourea derivatives and evaluate their antimicrobial activities against selected bacterial and fungal strains. In the present study a series of novel thiourea derivatives 1-(3/-substituted phenyl-5/-thiourea pyrimidine)-3-(substituted phenyl)-5-(substituted phenyl)-2-pyrazolines 8(a-p) have been synthesized from 1-(3/-substituted phenyl-5/-amino pyrimidine)-3-(substituted phenyl)-5- (substituted phenyl) -2-pyrazolines 4(a-p) by refluxing with phenyl isothiocyanate in ethanol solvent. Scheme 5.1 The structures of all synthesized compounds were assigned on the basis of Elemental Analysis, IR, 1H NMR, 13C NMR and Mass Spectral data.
| R1 | R2 | R3 | R4 | |
| 8 a | 4-NO2 | 4-N(CH3)2 | 4-OH | H |
| 8 b | 4-NO2 | 4-N(CH3)2 | 4-OCH3 | H |
| 8 c | 4-NO2 | 4-N(CH3)2 | 3-NO2 | H |
| 8 d | 4-NO2 | 4-N(CH3)2 | 4-N(CH3)2 | H |
| 8 e | 4-NO2 | 3-NO2 | 4-OH | H |
| 8 f | 4-NO2 | 3-NO2 | 4-OCH3 | H |
| 8 g | 4-NO2 | 3-NO2 | 3-NO2 | H |
| 8 h | 4-Cl | 3-NO2 | 4-OH | H |
| 8 i | 4-Cl | 3-NO2 | 4-OCH3 | H |
| 8 j | 4-Cl | 3-NO2 | 3-NO2 | H |
| 8 k | 4-Cl | 3-NO2 | 4-N(CH3)2 | H |
| 8 l | 4-NO2 | 4-OH | 2,4-(Cl)2 | H |
| 8 m | 4-OH | H | 4-N(CH3)2 | H |
| 8 n | 4-OH | 4-OCH3 | 4-OH | H |
| 8 o | 4-Cl | 4-N(CH3)2 | 4-N(CH3)2 | H |
| 8 p | 4-Cl | 4-N(CH3)2 | 4-OCH3 | H |
Biological Studies
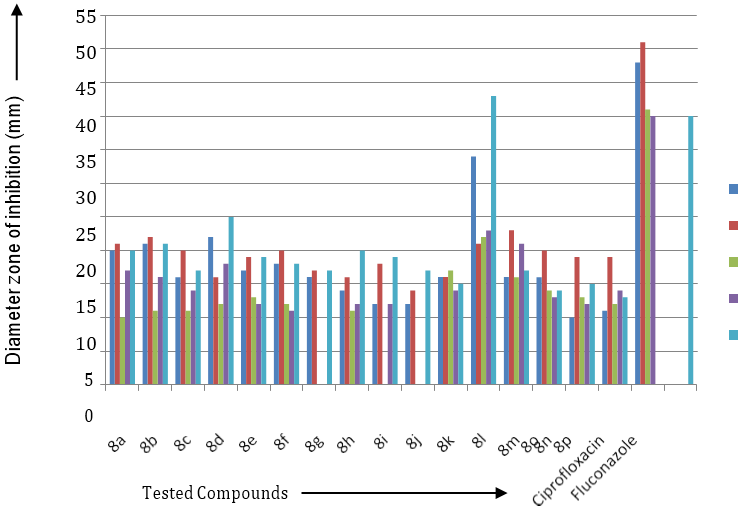
Biological Study of 1-(3/-substituted phenyl-5/- amino pyrimidine)-3-(substituted phenyl)-5-(substituted phenyl)-2-pyrazolines 4(a-p) and phenyl isothiocyanate in presence of ethanol to get 1-(3/-substituted phenyl-5/-thiourea pyrimidine)-3-(substituted phenyl)-5-(substituted phenyl)-2- pyrazolines 8(a-p). The synthesized compounds were tested at 100ml concentration against staphylococcus aureus, E-coli, P. vulgaris, A. niger, B. substillis, C. albicans for its antibacterial and antifungal screening as shown in Table-I.
Biological Study of 1-(3/-substituted phenyl-5/- amino pyrimidine)-3-(substituted phenyl)-5-(substituted phenyl)-2-pyrazolines 4(a-p) and phenyl isothiocyanate in presence of ethanol to get 1-(3/-substituted phenyl-5/-thiourea pyrimidine)-3-(substituted phenyl)-5-(substituted phenyl)-2- pyrazolines 8(a-p). The synthesized compounds were tested at 100ml concentration against staphylococcus aureus, E-coli, P. vulgaris, A. niger, B. substillis, C. albicans for its antibacterial and antifungal screening as shown in Table-I.
| S. No. | Derivative | Diameter of zone of inhibition (mm) for organism | ||||
| Bacterial Strains | Fungal strains |
|||||
| Gram negative | Gram positive | |||||
| E.Coli | P.Aeruginosa | S. Aureus | B.Subtilis | A.Niger | ||
| 1 | 8a | 20 | 21 | 10 | 17 | 20 |
| 2 | 8b | 21 | 22 | 11 | 16 | 21 |
| 3 | 8c | 16 | 20 | 11 | 14 | 17 |
| 4 | 8d | 22 | 16 | 12 | 18 | 25 |
| 5 | 8e | 17 | 19 | 13 | 12 | 19 |
| 6 | 8f | 18 | 20 | 12 | 11 | 18 |
| 7 | 8g | 16 | 17 | -- | -- | 17 |
| 8 | 8h | 14 | 16 | 11 | 12 | 20 |
| 9 | 8i | 12 | 18 | -- | 12 | 19 |
| 10 | 8j | 12 | 14 | -- | -- | 17 |
| 11 | 8k | 16 | 16 | 17 | 14 | 15 |
| 12 | 8l | 34 | 21 | 22 | 23 | 43 |
| 13 | 8m | 16 | 23 | 16 | 21 | 17 |
| 14 | 8n | 16 | 20 | 14 | 13 | 14 |
| 15 | 8o | 10 | 19 | 13 | 12 | 15 |
| 16 | 8p | 11 | 19 | 12 | 14 | 13 |
| 17 | Ciprofloxacin | 48 | 51 | 41 | 40 | -- |
| 18 | Fluconazole | -- | -- | -- | -- | 40 |
Table 1: The in Vitro antimicrobial activity of compound 8(a-p).
 E-Coli (Standard) |
 E-Coli |
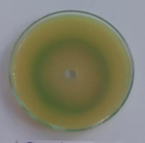 P. Aeruginosa(Standard) |
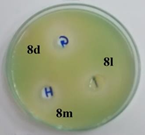 P. Aeruginosa |
 S. Aureus (Standard) |
 S. Aureus |
 B. Subtilis (Standard) |
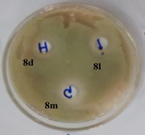 B. Subtilis |
 A. Niger (Standard) |
 A. Niger |
Figure 1: Photographs showing zone of inhibition of 8d, 8l, 8m & standards.
Experimental
Method of Synthesis of 1-(3/-substituted phenyl-5/-thiourea pyrimidine)-3-(substituted phenyl)-5-(substituted phenyl)-2- pyrazolines 8(a-p).
The method number 6 was adopted for the synthesis of thiourea.
A mixture of 1-(3/-substituted phenyl-5/- amino pyrimidine)-3-(substituted phenyl)-5-(substituted phenyl)-2-pyrazolines 4(a-p) (0.01 mole) and phenyl isothiocyanate (0.01 mole) in ethanol was refluxed for 5-6 hours. The progress of the reaction was monitored by TLC. After completion of the reaction the solvent was removed by distillation and the resulting solid was recrystallized from the suitable solvent Scheme 5.1. Physicochemical characterization data of synthesized compounds 8(a-p) were shown in Table 5.1.
| ID | R1 | R2 | R3 | Molecular Formula | MP°C | Yield % | Analysis | ||
| C% | H% | N% | |||||||
| 8a | 4-NO2 | 4N(CH3)2 | 4-OH | C34H30N8O3S | 169 | 71 | 64.76 | 4.76 | 17.78 |
| 64.80 | 4.80 | 17.80 | |||||||
| 8b | 4-NO2 | 4N(CH3)2 | 4-OCH3 | C35H32N8O3S | 171 | 57 | 65.22 | 4.97 | 17.89 |
| 65.18 | 5.03 | 17.92 | |||||||
| 8c | 4-NO2 | 4N(CH3)2 | 3- NO2 | C34H29N9O4S | 167 | 63 | 61.91 | 4.40 | 19.12 |
| 61.88 | 4.48 | 19.16 | |||||||
| 8d | 4-NO2 | 4N(CH3)2 | 4N(CH3)2 | C36H35N9O2S | 192 | 58 | 65.75 | 5.33 | 19.18 |
| 65.71 | 5.38 | 18.15 | |||||||
| 8e | 4-NO2 | 3-NO2 | 4-OH | C32H24N8O5S | 184 | 62 | 60.76 | 3.80 | 17.72 |
| 60.80 | 3.86 | 17.67 | |||||||
| 8f | 4-NO2 | 3-NO2 | 4-OCH3 | C33H26N8O5S | 179 | 69 | 61.3 | 4.03 | 17.34 |
| 61.28 | 4.09 | 17.30 | |||||||
| 8g | 4-NO2 | 3-NO2 | 3- NO2 | C32H23N9O6S | 201 | 63 | 58.09 | 3.48 | 19.06 |
| 58.04 | 3.51 | 19.12 | |||||||
| 8h | 4-Cl | 3-NO2 | 4-OH | C32H24N7O3SCl | 156 | 71 | 61.84 | 3.86 | 15.78 |
| 61.83 | 3.90 | 15.72 | |||||||
| 8i | 4-Cl | 3-NO2 | 4-OCH3 | C33H26N7O3SCl | 174 | 75 | 62.36 | 4.09 | 15.43 |
| 62.34 | 4.05 | 15.38 | |||||||
| 8j | 4-Cl | 3-NO2 | 3- NO2 | C32H23N8O4SCl | 166 | 64 | 59.08 | 3.54 | 17.23 |
| 59.06 | 3.58 | 17.25 | |||||||
| 8k | 4-Cl | 3-NO2 | 4N-(CH3)2 | C34H29N8O2SCl | 148 | 67 | 62.96 | 4.48 | 17.28 |
| 62.94 | 4.42 | 17.32 | |||||||
| 8l | 4-NO2 | 4-OH | 2,4-(Cl)2 | C32H23N7O3SCl2 | 189 | 69 | 58.62 | 3.51 | 14.96 |
| 58.58 | 3.48 | 14.99 | |||||||
| 8m | 4-OH | H | 4N(CH3)2 | C34H31N7OS | 190 | 70 | 69.74 | 5.30 | 16.75 |
| 69.76 | 5.26 | 16.82 | |||||||
| 8n | 4-OH | 4-OCH3 | 4-OH | C33H28N6O3S | 211 | 71 | 67.35 | 4.76 | 14.29 |
| 67.40 | 4.74 | 14.32 | |||||||
| 8o | 4-Cl | 4N(CH3)2 | 4N(CH3)2 | C36H35N8SCl | 168 | 71 | 66.87 | 5.42 | 17.34 |
| 66.90 | 5.38 | 17.38 | |||||||
| 8p | 4-Cl | 4N(CH3)2 | 4-OCH3 | C35H32N7OSCl | 179 | 59 | 66.35 | 5.06 | 15.48 |
| 66.27 | 5.01 | 15.42 | |||||||
Table 5.1: Physicochemical characterization data of synthesized compounds 8(a-p)
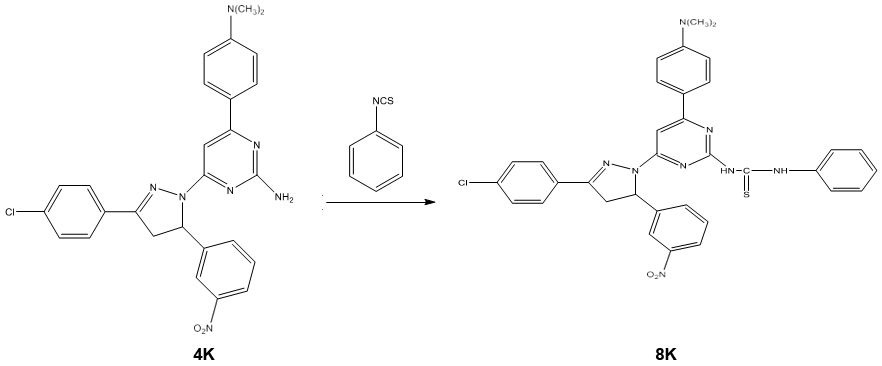
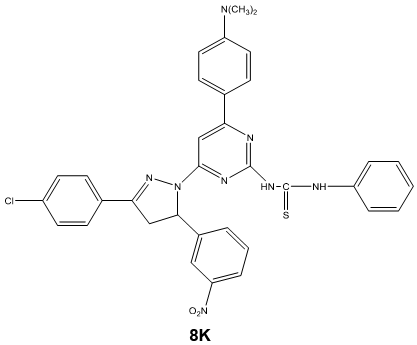
Synthesis of 1-[3/-(4//-dimethyl amino phenyl)-5/-(phenyl thiourea) pyrimidine]-3-(4/- chloro phenyl)-5-(3/-nitro phenyl)-2-pyrazoline 8k
A mixture of 1-[3/-(4//-dimethyl amino phenyl)-5/-amino pyrimidine]-3-(4/- chlorophenyl)-5-(3/-nitro phenyl)-2-pyrazoline 4k (0.01 mole) and phenyl isothiocyanate (0.01 mole) in ethanol was refluxed for 4 hours. The progress of the reaction was monitored by TLC. After completion of the reaction, the solvent was removed by distillation and the resulting solid was recrystallized from ethanol. Scheme 5.1
Characterization of 1-[3/-(4//-dimethyl amino phenyl)-5/-(phenyl thiourea) pyrimidine]-3-(4/- chloro phenyl)-5-(3/-nitro phenyl)-2- pyrazoline 8k
- Solubility: The product is off white crystalline solid, M.P. 148 0C, soluble in ethanol.
- Elemental Test: Lassaigne’s test is used to detect the presence of nitrogen, sulfur and chlorine. It comprises two steps; in the first step sodium fusion extract is prepared by fusing a small quantity of titled compound with sodium metal in a fusion tube. Then heated to red hot and plunged into distilled water. The obtained solution is boiled for few minutes, cooled and filtered. The filtrate is known as sodium fusion extract.
- Test for Nitrogen: The sodium fusion extract of titled compound gave Prussian blue coloration on heating with 1-2 drops of NaOH solution, freshly prepared FeSO4 solution and 2-3 drops of FeCl3 solution.
- Test for Sulfur: The sodium fusion extract of titled compound gave black precipitate with lead acetate acidified with acetic acid. It gives a positive test for Sulfur.
- Test for Chlorine: The sodium fusion extract of titled compound gave curdy white precipitate with AgNO3 solution which is soluble in NH4OH.
- Test for Nitro group: It gave positive Mulliken’s test. Ethanolic solution of compound gave black precipitate with NH4Cl, Zn dust and tollen’s reagent (ammonical AgNO3 solution).
- Test for NH2 group: It does not give a positive dye test.
- Elemental Analysis: From the analytical data the molecular formula of the compound 8k was found to be C34H29N8O2SCl. Calculated : %C- 62.96, %H – 4.48, %N – 17.28; Found %C – 62.94, %H – 4.42, %N -17.32.
- Spectral Analysis [90-95] :
- FTIR (KBr, λ max, cm-1): IR spectrum of compound 8k characteristics absorption band which are correlated as follows : 3697.68, 3318.77 (-HN-CS-NH- str), 3050.71, 2906.51 (Ar-H str), 2822.51, 2732.54 (N(CH3)2 str), 1662.29 (C=S str), 1604.23 (C=N str), 1552.37 (C=C str), 1537.40, 1374.32 (Ar-NO2 str), 1314.51 (C-N str),729.67 (C-Cl str).
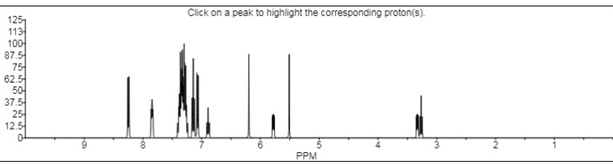
- 1H NMR (δ ppm): 1H NMR spectra was recorded on DMSO shown in Fig 5.2 showed some characteristics absorption peaks. 9.68, 7.96 (s, 2H, N-H), 6.65-8.30 (m, 18H, Ar-H), 5.40-5.46 (dd, 1H, C5-pyrazoline), 3.72-3.76 (dd, 1H, C4cis-pyrazoline), 3.16-3.22 (dd, 1H, C4trans-pyrazoline), 3.06 (s, 6H, N(CH3)2).
- 13C NMR (δ ppm): 13C NMR at 173.08, 110.98-151.89, 166.52, 157.68, 155.89, 152.18, 94.66, 58.99, 43.82, 40.78.
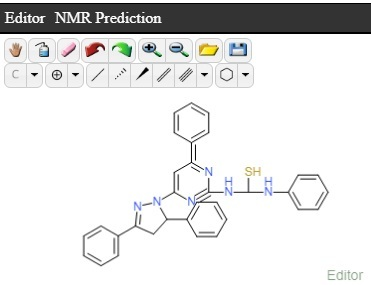
- Mass spectra: m/z (M+): The mass spectrum of titled compound 8k molecular ion peaks at m/z 649.12. It showed several other peaks at m/z 497.32, m/z 300.22, m/z 198.21 m/z 151.03 etc. The IR spectra of compound 8k showed strong absorption band at 1662.29 cm-1 attributed to C=S stretching. The presence of two bands at 3697.68 & 3318.77 cm-1 indicates the presence of two N-H stretching. The 1H NMR spectrum of compound 8k showed singlet at δ 9.68 ppm and δ 7.96 ppm due to the proton of thiourea group (-HN-CS-NH). The absence of singlet in the range of δ 4-5 ppm indicated the absence of primary amino group. The 13C NMR spectrum was consistent with the 1H NMR spectrum. The result of elemental analysis and mass spectrum was in agreement with those of calculated values. Based on above spectral data, the structure of compound 8k was confirmed as 1-[3/-(4//-dimethyl amino phenyl)-5/- (phenyl thiourea) pyrimidine]-3-(4/- chloro phenyl)-5-(3/-nitro phenyl)-2- pyrazoline 8k.
Conclusion
Efficient methods for synthesis of 1-(3/-substituted phenyl-5/-thiourea pyrimidine)-3-(substituted phenyl)-5-(substituted phenyl)-2- pyrazolines 8(a-p) with excellent yield have been developed. The result of this study indicate that present synthetic method is a simple efficient in expensive and easy synthesis of biologically active compound 8(a-p)these compound showing good result tested at 100 mg Conc. against E-coli, S-aureus, P-Vulgaris, A-niger , C-albicans.
Acknowledgement
The authors are thankful to the Head, Department of Pharmaceutical Science Nagpur University for screening anti-microbial activities, Head RSIC, CDRI, Lucknow for providing the spectral data of the compounds.
The authors are thankful to the Head, Department of Pharmaceutical Science Nagpur University for screening anti-microbial activities, Head RSIC, CDRI, Lucknow for providing the spectral data of the compounds.
References
- Shakeel A., et al. J Drug Design and Med Chem 2. 1 (2016): 10-20
- Rigaudy J and Klesney S. Nomenclature of Organic Chemistry: Sections A, B, C, D, E, F and H. Pergamon Press Oxford (1979).
- Tripath R., et al. Res J Chem Sci 2 (2012): 18-27.
- Mostafa H A, Zaghloul E I & Moussa M N, Portugaliae Electrochimica Acta 20. 2 (2002): 63-75.
- Nagy S., et al. Studia UBB CHEMIA LXII, 1 (2017): 183-194.
- Schriner P. Chem Soc Rev 32 (2003): 289-296.
- Taylor M S and Jacobsen E N. Angewandte Chemie Int Ed 45. 10 (2006) 1520-43.
- Connon S J. Chemical Communications (2008): 2499.
- Takemoto Y. Chemical and Pharmaceutical Bulletin 58 (2010): 593.
- Reddy B V S., et al. Royal Society of Chemistry Advances 4 (2014): 9107.
- Irmak M., et al. Chemical Communications (2007): 177.
- Groenewaldt T. J of the South African Institute of Mining and Metallurgy 77 (1977): 217-223.
- Khairul W M., et al. Procedia Chemistry 20 (2016): 105-114.
- Spivey A C., et al. Chem Commun (2005): 4426–4428.
- Ozay H., et al. Turk J Chem 39 (2015): 777 – 788.
- Mureseanu M., et al. Journal of Hazardous Materials 182. 1 (2010): 197-203.
- Mansuroglu D S., et al. J Coord Chem 61 (2008): 3134- 3146.
- Ozer C K., et al. J Coord Chem 62 (2009): 266- 276.
- Binzet G., et al. J Coord Chem 59 (2006): 1395-1406
- Ugur D., et al. Russ J Coord Chem 32 (2006): 669-675.
- Emen M F., et al. Polish J Chem 79 (2005): 1615-1626.
- Arslan H., et al. J Coord Chem 59 (2006): 223-228.
- Yuan Y F., et al. Inorg Chim Acta 324 (2001): 309- 317.
- Zhang Y M., et al. Phosphorus Sulfur Silicon Related Element 179 (2004): 2007-2013.
- Zhang Y M., et al. Indian J Chem Sect B 37 (1998): 604-606.
- Zhou W Q., et al. J Mol Struct 690 (2004): 145-150.
- Eweis M, Elkholy S S & Elsabee M Z, Int J Biol Macromol, 38 (2006): 1-8.
- Vega-Pérez J M., et al. Eur J Med Chem 58 (2012): 591–612.
- Yao J, Chen J, He Z, Sun W & Xu W, Bioorg Med Chem,20 (2012): 2923–2929.
- Shantharam C S., et al. Eur J Med Chem 60 (2013): 325–332.
- Yang W., et al. Bioorg Med Chem 21, (2013): 1050–1063.
- Keche A P., et al. Bioorg Med Chem 22 (2012): 6611–6615.
- Burgeson J R., et al. Bioorg Med Chem Lett 22 (2012) 4263–4272.
- Madan V K., et al. J Ind Chem Soc 68 (1991): 471-472.
- Mohanta P K., et al. Tetrahedron 56 (1999): 629-637.
- Fernandez E R., et al. J Inorg Biochem 99 (2005): 1559-1572
- Saeed A., et al. Chemistry 18. 5 (2009): 152-158.
- Manjula S N., et al. Euro J Med Chem 44 (2009): 2923-2929
- Phetsuksiri B., et al. J Biological Chem 278 (2003): 53123–53130.
- Sharma S K., et al. Med Chem 53 (2005): 5197- 5212.
- Ren J, Diprose J., et al. J Biol Chem 275 (2000): 5633-39.
- Heinisch G., et al. Antiviral Chemistry & Chemotherapy 8. 5 (1997): 443-446
- Shakir Z. International J. of Applied Chemistry 8 (2012): 63-69.
- Halim N., et al. IPCBEE 14 (2011): 53-59.
- Rauf M., et al. J of Inorganic Biochemistry (2009): 1135-1144.
- Osmond J and Taraced K. Biology of Reproduction 639 (2000): 196-205
- Al-Masaudi N., et al. Chem Biodiversity 3 (2006): 515- 518
- Al-Masoudi N A., et al. Antiviral Chemistry & Chemotherapy 18 (2007): 191-200
- Solomon V., et al. Eur J Med Chem (2010): 4990-4996
- Saeed S., et al. Eur J Med Chem 45. 4 (2010): 1323 - 1331.
- Upadhayaya R S., et al. Bioorg Med Chem 17 (2009): 4681–4692
- Khan S A., et al. Eur J Med Chem 43 (2008): 2272–2277.
- Sett P P., et al. Bioorg Med Chem Lett 14 (2004): 5569–5572.
- Kaymakçıoğlu B K., et al. Eur J Pharm Sci 26, (2005): 97–103.
- Polacco J C. Plant Physiol 58 (1976): 350.
- Smith J., et al. J Org Chem 125 (1996): 8811.
- Carlsson J., et al. Biochem J 139 (1974): 221.
- Longo L D. Environ Health Perspect 74 (1987): 93.
- Knolker H J., et al. Synlett 6 (1996): 502.
- Esteves-Souza A., et al. Bioorg Med Chem 14 (2006): 492.
- Choi S J., et al. Bioorg Med Chem 14 (2006): 1229.
- Venkatachalam T K., et al. Bioorg Med Chem 12 (2004): 4275.
- Majer P and Randad R S. J Org Chem 59. 7 (1994): 1937-38.
- Jadhav S., et al. Rasayan J Chem 3. 1 (2010): 27-31.
- Madhavaa G., et al. Der Pharmacia Lettre 4. 4 (2012): 1194-1201.
- Reddy N S., et al. Int J Org Chem 2 (2012): 267-275.
- Mohamed N A and El-Ghany N A A. International Journal of Biological Macromolecules 50 (2012): 1280–1285.
- Sonnekar V S., et al. Res J Pharm Biol Che Sci RJPBCS 4. 2 (2013):1411-18
- AL-hazam H A., et al. Der Pharma Chemica 5. 4 (2013): 51-57
- Kaymakcioglu B K., et al. Molecules 18 (2013): 3562-3576
- Kaswala P B., et al. ARKIVOC 11 (2009): 326-335
- Ibrahim M A., et al. Molecules 19 (2014): 5191-5204
- Alqasoumi S I., et al. Acta Poloniae Pharmaceutica Drug Research 72. 6 (2015): 1183-1191.
- Ghorab M M., et al. Chemistry Central Journal, 11. 32 (2017): 1-14
- D’Cruz O J., et al. Biology of Reproduction 63 (2000): 196–205.
- Katla V R., et al. J Serb Chem Soc 79. 3 (2014): 283–289
- Upadhayaya R S., et al. Bioorg & Med Chem 17 (2009): 4681–4692
- Berber N., et al. Journal of Chemistry (2013): 1-8
- Chayah M., et al. Med chem DOI: 10.1039/C5MD00477B
- Liu P., et al. Bioorg Med Chem 24. 8 (2016): 1866-71
- Miyabe H and Takemoto Y. Bulletin of the Chemical Society 81 (2008): 785-795
- Yahyazadeh A and Ghasemi Z Eur Chem Bull 2. 8 (2013): 573-575.
- Alkherraz A M., et al. International Journal of Chemical, Molecular, Nuclear, Materials and Metallurgical Engineering 8. 2 (2014)
- Koketsu M., et al. Heteroatom Chemistry 14 (2003): 374- 378
- Huang Y B., et al. Springer (2012): 191-212
- Maddani M R and Prabhu K R J Org Chem 75 (2010): 2327–2332.
- Ramadas K., et al. Tetrahedron letters 34 (1993): 6447-50
- Pasha M A and Reddy M B M Synthetic Communications 39 (2009): 2928–2934.
- Milosavljević M M., et al. J Serb Chem Soc, 81. 3 (2016): 219–231.
- Silverstien R M, Bassler G C & Morrill T C, ‘Spectroscopy identification of organic compounds’ 4th Ed (1981): John Wiley & Sons, Inc, New York.
- Clothup N B., et al. “Introduction to infrared and Raman spectroscopy”. Academic Press, (1964) New York
- Kemp W ‘Organic Spectroscopy’, 3rd Ed, (1996)
- Dyer J R, ‘Application of absorption spectroscopy of organic compounds’, Prentice Hall, (1974) Creswell C G, Runquist O A & Cambell M M, ‘Spectral analysis of organic compounds’, 2nd Ed (1972) Longman, Great Britain
- Fulmer G R., et al. Organometallics, 29 (2010): 2176-2179.
Citation:
Meghasham N. Narule. “Synthesis, Characterization and 3d Molecular of Substituted Phenyl Thiourea Pyrimidine-2-Pyrazolines”. Chronicles of Pharmaceutical Science 3.2 (2019): 805-827.
Copyright: © 2019 Meghasham N. Narule. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































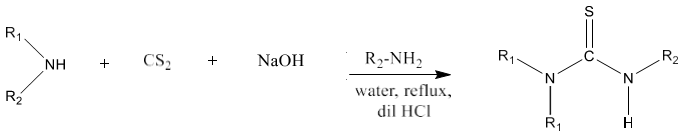
 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.