Research Article
Volume 3 Issue 2 - 2019
Schistosomacidal Activity of Lannea Schimperi and Searsia Longipes against Cercariae, Schistosomula and Adult Stage of Schistosoma Mansoni
1Nelson Mandela African Institution of Science and Technology, Tanzania
2Kenya Medical Research Institute, Kenya
2Kenya Medical Research Institute, Kenya
*Corresponding Author: Musa Chacha, Nelson Mandela African Institution of Science and Technology, Tanzania.
Received: January 18, 2019; Published: January 30, 2019
Abstract
Purpose: To evaluate schistosomacidal activity of methanolic stem bark extracts of Lannea schimperi and Searsia longipes against cercariae, schistosomula and adult Schistosoma mansoni.
Methodology: Bioassays were conducted in vitro on 24 well plates for cercariae and schistosomula and 6 well plates for adult worms, whereby the aforementioned parasite stages were subjected to different concentrations of the extracts. Activity was assessed based on mortality, structural deformation and motility reduction.
Results: Both plants expressed significant activity against schistosomula at all tested concentrations and the highest activity at 0.25 to 2 mg/ml with the mortality rate of 100% after 6 hour. Searsia longipes and Lannea schimperi exhibited 100% activity against adult worms at the dose range of 0.5 to 2 mg/ml and 1 to 2 mg/ml respectively after 48 hours of exposure. Mortality of cercariae was divulged after 6 hours of exposure to extracts, whereby 100% mortality were observed at the concentration range of 1 to 2 mg/ml and 2 mg/ml for Searsia longipes and Lannea schimperi respectively.
Conclusion: To the best knowledge of the authors, this paper reports schistosomacidal activities of Lannea schimperi and Searsia longipes for the first time, however further studies to evaluate in vivo anti-schistosomal activity and phytochemical analysis are necessary.
Keywords: Lannea schimperi; Searsia longipes; Schistosomula; Cercariae; Schistosomiasis
Introduction
Schistosomiasis (Bilhazia) is one of the chronic threatening but neglected parasitic disease caused by worms under genus schistosoma. It is prevalent in tropical and subtropical regions and affects more than 74 countries globally [1]. Schistosomiasis infects more than 210 million peoples worldwide, whereby 91.4% occurs in African countries contributing to about 200,000 deaths annually [2, 3]. Furthermore it’s estimated that more than 800 million people are at risk of getting this disease globally [4]. Despite the seriousness of the disease in health and financial perspective, there are limited numbers of drugs for treatment of the aforementioned disease [5]. This is because most of people affected with schistosomiasis are poor, hence little attention and efforts are dedicated to treatment, prevention and control of this disease. According to WHO Model List of Essential Drugs [5], praziquantel and oxamniquine are outlined as drugs for the treatment of schistosomiasis. However, praziquantel remains the drug of choice for all forms of schistosomiasis occurring in human, because of its high efficacy, low toxicity, and ease of single oral administration [6, 7]. Oxamniquine is listed for use when praziquantel treatment fails and it is rarely used due to its toxicity [5, 8]. Despite being the only drug of choice, praziquantel is facing challenges of selective efficacy and resistance leading to the reduced cure rates and the failure of treatment [9- 11]. It is therefore of paramount importance to search for safe and reliable drugs to treat schistosomiasis, which will complement the available drugs [12]. Medicinal plants have been utilized for centuries in Africa for the treatment of many health conditions including helminthic infection such schistosomiasis [13,14]. It has however reported that 25% of the drugs have plant origin; henceforth plants can continuously save as the possible source for the new therapeutic agent. Lannea schimperi and Searsia longipes are plants which belong to the family Anacardiaceae and widely distributed in temperate region. Based on the ethno botanical survey conducted prior to the commencement of this study, it was revealed that, water decoctions from these plants are utilized ethno medically to manage schistosomiasis in Tanzania, particularly by people living in Manyara region. In present study, these plants are therefore investigated for their schistosomacidal activity.
Materials and Methods
Reagents
Fetal bovine serum (FBS) (SIGMA), RPMI 1640 media (SIGMA), analytical grade methanol, antibiotics (Penicillin & streptomycin) (SIGMA), sodium pentobarbital, distilled water and citrate saline (Sodium chloride and Sodium citrate).
Fetal bovine serum (FBS) (SIGMA), RPMI 1640 media (SIGMA), analytical grade methanol, antibiotics (Penicillin & streptomycin) (SIGMA), sodium pentobarbital, distilled water and citrate saline (Sodium chloride and Sodium citrate).
Sample collection
Lannea schimperi and Searsia longipes stem barks were collected from Endasaki ward in Manyara region with geographical coordinate of 4° 25' 0" S, 35° 31' 0" E. Prior to sample collection, the aforementioned plants were identified by botanists from TPRI and the voucher specimens were prepared and thereafter preserved in NHT (National Herbarium of Tanzania) with voucher specimen number NM 01 and NM 02 for Lannea schimperi and Searsia longipes respectively.
Lannea schimperi and Searsia longipes stem barks were collected from Endasaki ward in Manyara region with geographical coordinate of 4° 25' 0" S, 35° 31' 0" E. Prior to sample collection, the aforementioned plants were identified by botanists from TPRI and the voucher specimens were prepared and thereafter preserved in NHT (National Herbarium of Tanzania) with voucher specimen number NM 01 and NM 02 for Lannea schimperi and Searsia longipes respectively.
Sample preparation and extraction
Collected stem bark samples of Lannea schimperi and Searsia longipes were pulverized at Nelson Mandela African Institution of Science and Technology natural product laboratory and thereafter dried under shade for two weeks. Maceration method was used for extraction as described by Azwanida, (2015) [ 15]. Briefly, the dried plant materials were grinded by laboratory mill to get fine powder. Approximately, 1 kg of Lannea schimperi andSearsia longipes powder were measured by using weighing balance and soaked in 2.5 litters of methanol each and maintained for 48 hours. Afterward, extracts were filtered and concentrated under vacuum by using rotary evaporator. Following extraction process, 35 g and 45 g of Searsia longipes and Lannea schimperi crude extracts were obtained respectively. Moreover, in tradition settings people uses water for preparation of decoction from the aforementioned plants, therefore methanol were used as the extraction solvent in the present study since it has polarity close to water.
Collected stem bark samples of Lannea schimperi and Searsia longipes were pulverized at Nelson Mandela African Institution of Science and Technology natural product laboratory and thereafter dried under shade for two weeks. Maceration method was used for extraction as described by Azwanida, (2015) [ 15]. Briefly, the dried plant materials were grinded by laboratory mill to get fine powder. Approximately, 1 kg of Lannea schimperi andSearsia longipes powder were measured by using weighing balance and soaked in 2.5 litters of methanol each and maintained for 48 hours. Afterward, extracts were filtered and concentrated under vacuum by using rotary evaporator. Following extraction process, 35 g and 45 g of Searsia longipes and Lannea schimperi crude extracts were obtained respectively. Moreover, in tradition settings people uses water for preparation of decoction from the aforementioned plants, therefore methanol were used as the extraction solvent in the present study since it has polarity close to water.
Snail’s collection and screening
Snails were collected from two spots namely Dunga and Car wash, located along the shores of Lake Victoria in Kisumu, Kenya (Figure 1(B)). Briefly, snail’s collection was done by using standard scoop and about 699 snails of different species were obtained [16]. Following collection, Biomphalaria pfeifferi snails were identified based on guideline described by PAHO, (1968) [17] and thereafter screened for cercariae shedding (Figure 1 (A)). Screening was done under inverted microscope (Olympus) after exposing snails plated on 24 well plates filled with snail’s water to the direct sun light for 1 hour. Positive snails which were shading Schistosoma mansoni cercariae were selected for use in bioassay [18].
Snails were collected from two spots namely Dunga and Car wash, located along the shores of Lake Victoria in Kisumu, Kenya (Figure 1(B)). Briefly, snail’s collection was done by using standard scoop and about 699 snails of different species were obtained [16]. Following collection, Biomphalaria pfeifferi snails were identified based on guideline described by PAHO, (1968) [17] and thereafter screened for cercariae shedding (Figure 1 (A)). Screening was done under inverted microscope (Olympus) after exposing snails plated on 24 well plates filled with snail’s water to the direct sun light for 1 hour. Positive snails which were shading Schistosoma mansoni cercariae were selected for use in bioassay [18].
Cercariae shedding and transformation
Shedding of cercariae was done by exposing Biomphalaria pfeifferi snails to light for one hour at KEMRI schistosome laboratory. Thereafter, portion of the obtained cercariae were transformed to schistosomula and the remaining portion were used for plants extract bioassay against cercariae stage. In a nutshell, transformation was done by using slightly modified mechanical method prescribed by Ramalho-Pinto., et al. (1974) [19]. Whereby, the obtained cercariaewere chilled on ice for 45 minutes, afterward cercariae suspension was centrifuged for 5 minutes at 1500 RPM. Supernatant was discarded and the pellet was suspended in RPMI 1640 (SIGMA) media. Following resumption, cercariae were mechanically transformed through vigorous agitation for two minutes by using vortex machine. After transformation, the obtained schistosomula were purified by using simple swilling method [20]. In this method of purification, transformed mixture was poured in the petri dish and the latter was gently swirled for about 15 rounds. Following gentle swirling, schistosomula accumulated at the center of the petri dish. Thereafter, schistosomula were transferred to a graduated eppendorf tube by using micropipette. This process was repeated three times to ensure no schistosomula has remained. The obtained schistosomula were enumerated and afterward maintained in RPMI 1640 (SIGMA) media supplemented with 10% FBS (SIGMA) and 1% penicillin and streptomycin (SIGMA) ready for bioassay.
Shedding of cercariae was done by exposing Biomphalaria pfeifferi snails to light for one hour at KEMRI schistosome laboratory. Thereafter, portion of the obtained cercariae were transformed to schistosomula and the remaining portion were used for plants extract bioassay against cercariae stage. In a nutshell, transformation was done by using slightly modified mechanical method prescribed by Ramalho-Pinto., et al. (1974) [19]. Whereby, the obtained cercariaewere chilled on ice for 45 minutes, afterward cercariae suspension was centrifuged for 5 minutes at 1500 RPM. Supernatant was discarded and the pellet was suspended in RPMI 1640 (SIGMA) media. Following resumption, cercariae were mechanically transformed through vigorous agitation for two minutes by using vortex machine. After transformation, the obtained schistosomula were purified by using simple swilling method [20]. In this method of purification, transformed mixture was poured in the petri dish and the latter was gently swirled for about 15 rounds. Following gentle swirling, schistosomula accumulated at the center of the petri dish. Thereafter, schistosomula were transferred to a graduated eppendorf tube by using micropipette. This process was repeated three times to ensure no schistosomula has remained. The obtained schistosomula were enumerated and afterward maintained in RPMI 1640 (SIGMA) media supplemented with 10% FBS (SIGMA) and 1% penicillin and streptomycin (SIGMA) ready for bioassay.
Preparation of the adult Schistosoma mansoni
Adult worms used in the present study were obtained from Swiss albino mice pre infected with Schistosoma mansoni cercariae through abdominal percutaneous exposure [21]. Briefly, 20 female Swiss albino mice weighing 18 ± 2g were anaesthetized with 0.2 ml of 5 mg/ml Sodium phenobarbital and shaved at the abdominal part. Thereafter, mice were infected with about 200 Schistosoma mansoni cercariae each. Infection was done by pouring the suspension containing the aforementioned number of the cercariae in special metal rings placed on shaved abdominal part of the mice as displayed in Figure 2. The suspension was maintained for 1 hour to allow penetration of the cercariae into the mice body. Following infection, mice were maintained for 8 weeks to allow the development of the schistosomula to an adult schistosome worms.
Adult worms used in the present study were obtained from Swiss albino mice pre infected with Schistosoma mansoni cercariae through abdominal percutaneous exposure [21]. Briefly, 20 female Swiss albino mice weighing 18 ± 2g were anaesthetized with 0.2 ml of 5 mg/ml Sodium phenobarbital and shaved at the abdominal part. Thereafter, mice were infected with about 200 Schistosoma mansoni cercariae each. Infection was done by pouring the suspension containing the aforementioned number of the cercariae in special metal rings placed on shaved abdominal part of the mice as displayed in Figure 2. The suspension was maintained for 1 hour to allow penetration of the cercariae into the mice body. Following infection, mice were maintained for 8 weeks to allow the development of the schistosomula to an adult schistosome worms.
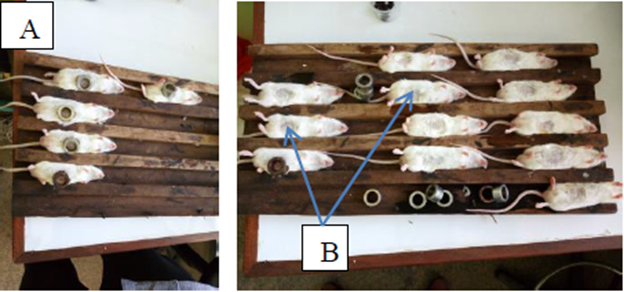
Figure 2: (A) Mice infection with aproximatelly 200 Schistosoma mansoni cercaria, (B) shaved abdomen that facillitate cercariae penetration.
Eight weeks post infection, adult worms were obtained through perfusion of the mesenteric portal system by using appropriate perfusion solution made of 0.85% sodium chloride and 0.15% sodium citrate [22]. Following perfusion, obtained adult schistosomes were washed two times by using RPMI 1640 media to remove mice blood. Undamaged worms of both sexes were sorted out and afterward maintained in RPMI 1640 media supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin ready for bioassay.
Bioactivity assay of the plant extracts against different stages of the Schistosoma mansoni
In vitro plant extracts bioassay against cercariae stage
Bioassay was conducted on 24 multi well plates to validate the activity of the extracts against cercariae stage of the Schistosoma mansoni by using methods described by Aziz., et al. (2011) [23] and Simões., et al. (2015) [4] with modifications. In this case, cercariae were subjected to different concentrations of the methanolic plants extracts namely Lannea schimperi and Searsia longipes. Briefly, 4 mg/ml stock solutions of the aforementioned extracts were prepared by dissolving an appropriate amount of the extract in distilled water, and the dissolubility was facilitated by heating the suspension at 370C for 15 minutes.
In vitro plant extracts bioassay against cercariae stage
Bioassay was conducted on 24 multi well plates to validate the activity of the extracts against cercariae stage of the Schistosoma mansoni by using methods described by Aziz., et al. (2011) [23] and Simões., et al. (2015) [4] with modifications. In this case, cercariae were subjected to different concentrations of the methanolic plants extracts namely Lannea schimperi and Searsia longipes. Briefly, 4 mg/ml stock solutions of the aforementioned extracts were prepared by dissolving an appropriate amount of the extract in distilled water, and the dissolubility was facilitated by heating the suspension at 370C for 15 minutes.
Thereafter, from the stock solution different subsequent concentrations of 2 mg/ml, 1 mg/ml, 0.25 mg/ml, 0.05 mg/ml and 0.025 mg/ml were prepared on the plate following dilution with distilled water. Approximately 20 cercariae were subjected in each concentration and each well contained a final volume of 1 milliliter. Afterward, cercariae were monitored from 0 to 6 hours and bioactivities were assessed based on the loss of motility, mortality and structural deformation including tail detachment. All tests were done in duplicate and at least two tests were perfumed at different time in KEMRI Laboratory, Kenya.
In vitro plants extract bioassay against schistosomula stage
Following transformation of the cercariae stage of Schistosoma mansoni to schistosomula stage, bioassays were thereafter performed on 24 multi well plate to validate the activity of the plants extract against the latter stage. In this case, schistosomula were subjected to different concentrations of the methanolic plants extracts as previously described in cercariae bioassay. Briefly, 4 mg/ml stock solutions of the plant extracts were prepared after dissolving 20 mg of the extract powder in approximately 5 ml of the RPMI 1640 (SIGMA) media.
Following transformation of the cercariae stage of Schistosoma mansoni to schistosomula stage, bioassays were thereafter performed on 24 multi well plate to validate the activity of the plants extract against the latter stage. In this case, schistosomula were subjected to different concentrations of the methanolic plants extracts as previously described in cercariae bioassay. Briefly, 4 mg/ml stock solutions of the plant extracts were prepared after dissolving 20 mg of the extract powder in approximately 5 ml of the RPMI 1640 (SIGMA) media.
Thereafter, five different concentrations were prepared on the plate following further dilution of the stock solution with the RPMI 1640 (SIGMA) media supplemented with 10% fetal bovine serum (SIGMA) and 1% streptomycin and penicillin (SIGMA). Whereby, 2 mg/ml was the highest concentration used for each extract, followed by other four subsequent concentrations similar to above described in bioassay against cercariae. Following dilution, approximately 20 schistosomula were subjected to each concentration and each well contained a final volume of 1 milliliter. Afterward, schistosomula were monitored from 0 to 6 hours while incubated at 37°C and 5% CO2. Bioactivities were assessed based on the loss of motility, mortality, shortening of the body and increase in body opacity. All tests were done in duplicate and at least two tests were perfumed at different time.
Plants extract bioassay against adult worms.
This test was performed to evaluate the activity of Lannea schimperi and Searsia longipes methanolic extracts against adult stage of the Schistosoma mansoni. In this case, previously perfused adult worms of both sexes were subject to different concentrations of the Lannea schimperi and Searsia longipes methanolic extracts. In a nutshell, for each extract 8 mg/ml stock solution was prepared in a graduated eppendorf tube. Afterward, from the stock solutions 6 serial concentrations of 2 mg/ml, 1 mg/ml, 0.5 mg/ml, 0.25 mg/ml, 0.05 mg/ml and 0.025 mg/ml were prepared in the 6 well plates in duplicate. RPMI 1640 (SIGMA) media supplemented with 10% FBS, 1% Penicillin and streptomycin was used for dilution as well as culture media. Additionally, wells containing only RPMI 1640 and 0.01 mg/ml praziquantel were used as negative and positive control respectively. Thereafter, 10 adult worms (5 females and 5 males) were placed in each well and each well contained a final volume of 5 milliliter. Monitoring was done from 0 to 48 hour in 24 hours interval. Henceforth, activity of the plant extracts was assessed under dissection microscope based on loss motility, mortality and structural changes [24].
This test was performed to evaluate the activity of Lannea schimperi and Searsia longipes methanolic extracts against adult stage of the Schistosoma mansoni. In this case, previously perfused adult worms of both sexes were subject to different concentrations of the Lannea schimperi and Searsia longipes methanolic extracts. In a nutshell, for each extract 8 mg/ml stock solution was prepared in a graduated eppendorf tube. Afterward, from the stock solutions 6 serial concentrations of 2 mg/ml, 1 mg/ml, 0.5 mg/ml, 0.25 mg/ml, 0.05 mg/ml and 0.025 mg/ml were prepared in the 6 well plates in duplicate. RPMI 1640 (SIGMA) media supplemented with 10% FBS, 1% Penicillin and streptomycin was used for dilution as well as culture media. Additionally, wells containing only RPMI 1640 and 0.01 mg/ml praziquantel were used as negative and positive control respectively. Thereafter, 10 adult worms (5 females and 5 males) were placed in each well and each well contained a final volume of 5 milliliter. Monitoring was done from 0 to 48 hour in 24 hours interval. Henceforth, activity of the plant extracts was assessed under dissection microscope based on loss motility, mortality and structural changes [24].
Data Analysis
Data were organized by Microsoft excel and analyzed by using graph pad prism software, whereby overall statistically significant different between treatments mean were determined by using one way ANOVA at p value of < 0.05. Further, multiple comparison between treatments and control were done by using Tukey Kramer test at p value of < 0.05.
Data were organized by Microsoft excel and analyzed by using graph pad prism software, whereby overall statistically significant different between treatments mean were determined by using one way ANOVA at p value of < 0.05. Further, multiple comparison between treatments and control were done by using Tukey Kramer test at p value of < 0.05.
Results
In vitro test against schistosomula
Following In vitro bioassay on schistosomula stage of the Schistosoma mansoni, both plants exhibited significant anti-schistosomal activity at all tested concentrations. Additionally, both extracts were able to exhibit 100% mortality at concentration range of 0.25 mg/ml up to 2 mg/ml, whereby completely loss of motility was observed following 6 hours of schistosomula exposition. Regarding statistical analysis done using one way ANOVA at p value of < 0.05, overall significant difference between tested concentrations were observed. Further, statistically significant differences were also divulged when multiple comparison of the viability mean of each treatment were tested against that of the control by using Tukey Kramer test at p value of < 0.05. Moreover, time and concentration based reduction of schistosomula viability was also observed as depicted in Figure 3 and 4.
Following In vitro bioassay on schistosomula stage of the Schistosoma mansoni, both plants exhibited significant anti-schistosomal activity at all tested concentrations. Additionally, both extracts were able to exhibit 100% mortality at concentration range of 0.25 mg/ml up to 2 mg/ml, whereby completely loss of motility was observed following 6 hours of schistosomula exposition. Regarding statistical analysis done using one way ANOVA at p value of < 0.05, overall significant difference between tested concentrations were observed. Further, statistically significant differences were also divulged when multiple comparison of the viability mean of each treatment were tested against that of the control by using Tukey Kramer test at p value of < 0.05. Moreover, time and concentration based reduction of schistosomula viability was also observed as depicted in Figure 3 and 4.
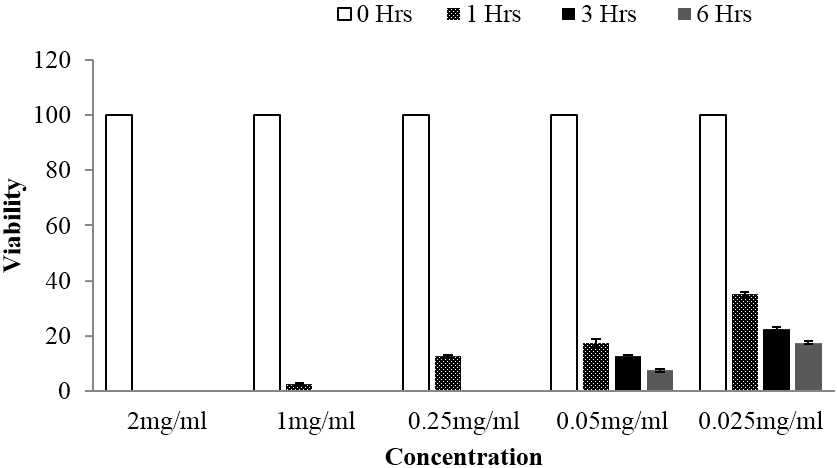
Figure 3: Viability of schistosomula stage of Schistosoma mansoni at different time of observation when subjected to different concentrations of Searsia longipes methanolic extract, whereby significant activity was observed at all tested concentrations.
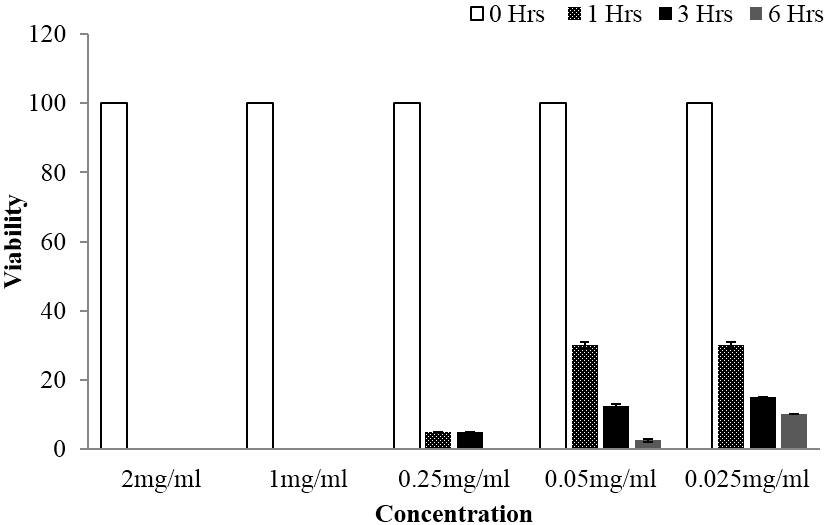
Figure 4: Viability of schistosomula stage of Schistosoma mansoni at different time of observation when subjected to different concentrations of Lannea schimperi methanolic extract, whereby significant activity was observed at all tested concentrations.
In vitro test against cercariae
For in vitro bioassay against cercariae stage, both extracts exhibited significant activity manifested through reduction of the cercariae viability. Whereby, overall significant different between viability means were observed following statistical analysis by using one way ANOVA at p value < 0.05. Further, multiple comparison between treatments and control were done by using Tukey Kramer test at p value < 0.05 and statistically significant differences on viability means were observed up to the lower concentration of 0.05 mg/ml for Searsia longipes extract. Whilst for Lannea schimperi extract, significant differences in viability means were observed up the lowest concentration used. Furthermore, in all tested plants extracts concentration based reduction of cercariae viability were observed, particularly after 6 hours of exposition as depicted in Figure 5 and 6. Meanwhile, concentration and time dependent cercariae motility reduction were also manifested as shown on Table 1. Nevertheless, at the concentrations less than 0.5 mg/ml Lannea schimperi was able to induce tail detachment.
For in vitro bioassay against cercariae stage, both extracts exhibited significant activity manifested through reduction of the cercariae viability. Whereby, overall significant different between viability means were observed following statistical analysis by using one way ANOVA at p value < 0.05. Further, multiple comparison between treatments and control were done by using Tukey Kramer test at p value < 0.05 and statistically significant differences on viability means were observed up to the lower concentration of 0.05 mg/ml for Searsia longipes extract. Whilst for Lannea schimperi extract, significant differences in viability means were observed up the lowest concentration used. Furthermore, in all tested plants extracts concentration based reduction of cercariae viability were observed, particularly after 6 hours of exposition as depicted in Figure 5 and 6. Meanwhile, concentration and time dependent cercariae motility reduction were also manifested as shown on Table 1. Nevertheless, at the concentrations less than 0.5 mg/ml Lannea schimperi was able to induce tail detachment.
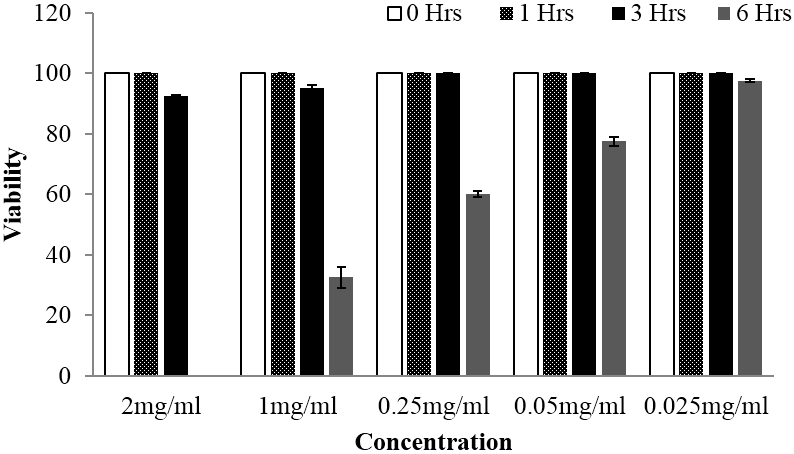
Figure 5: Viability of schistosomula stage of Schistosoma mansoni at different time of observation when subjected to different concentrations of Searsia longipes methanolic extract, whereby the activity was observed after 6 hours of exposure.
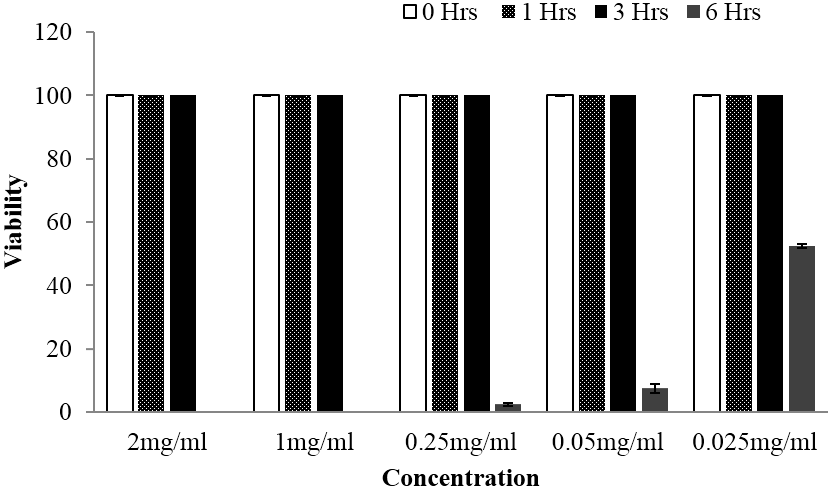
Figure 6: Viability of schistosomula stage of Schistosoma mansoni at different time of observation when subjected to different concentrations of Lannea schimperi methanolic extract, whereby the activity was observed after 6 hours of exposure.
| Concentration | 0 Hrs | 1 Hrs | 3 Hrs | 6 Hrs | ||||
| (mg/ml) | SLM | LSM | SLM | LSM | SLM | LSM | SLM | LSM |
| 2 | +++ | +++ | ++ | ++ | + | + | - | - |
| 1 | +++ | +++ | ++ | ++ | ++ | ++ | + | - |
| 0.25 | +++ | +++ | ++ | ++ | ++ | ++ | + | + |
| 0.05 | +++ | +++ | ++ | +++ | ++ | ++ | ++ | + |
| 0.025 | +++ | +++ | +++ | +++ | ++ | ++ | ++ | + |
| Control | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
+++ ¬¬= High motility, ++ = Moderate motility, + = Low motility and – = No motility
Table 1: Motility of the live cercariae following exposure to different concentrations of Lannea schimperi and Searsia longipes methanolic extracts.
Table 1: Motility of the live cercariae following exposure to different concentrations of Lannea schimperi and Searsia longipes methanolic extracts.
In vitro bioassay against adult worms
Searsia longipes methanolic extract (SLM) has divulged concentration based reduction of worm viability, where the viability reduction was observed to be high as concentration increased. At the three highest concentrations of 2 mg/ml, 1 mg/ml, and 0.5 mg/ml, worm viability was reduced to 0% after 48 hours of exposition as depicted in Table 3. Despite the effect on the viability of the worm, the extract has also demonstrated the concentration dependent reduction of worm motility especially at the concentrations distal to the lethal concentration. Meanwhile, Lannea schimperi methanolic extract (LSM)has exhibited 100% anti-schistosomal activity of all worms at two highest concentrations of 2 mg/ml and 1 mg/ml after 48 hours of exposition (Table 2). However at the concentration lower than 1 mg/ml the extract was observed to have no effect on the motility of the worms, hence the worms were highly motile even after 48 hours of exposure. Moreover, males worm were observed to be more susceptible to all tested concentrations of the extracts than female, whereby 100% mortality were observes at the concentration range of 0.025 to 2 mg/ml and 0.05 to 2 mg/ml for Searsia longipes and Lannea schimperi respectively. Additionally, dead male’s worm were also demonstrated fragmentation and tightly coiled characteristic as depicted on Figure 7. The viability of the control group subjected to RPMI 1640 only was 100% up to the end of the observation time.
Searsia longipes methanolic extract (SLM) has divulged concentration based reduction of worm viability, where the viability reduction was observed to be high as concentration increased. At the three highest concentrations of 2 mg/ml, 1 mg/ml, and 0.5 mg/ml, worm viability was reduced to 0% after 48 hours of exposition as depicted in Table 3. Despite the effect on the viability of the worm, the extract has also demonstrated the concentration dependent reduction of worm motility especially at the concentrations distal to the lethal concentration. Meanwhile, Lannea schimperi methanolic extract (LSM)has exhibited 100% anti-schistosomal activity of all worms at two highest concentrations of 2 mg/ml and 1 mg/ml after 48 hours of exposition (Table 2). However at the concentration lower than 1 mg/ml the extract was observed to have no effect on the motility of the worms, hence the worms were highly motile even after 48 hours of exposure. Moreover, males worm were observed to be more susceptible to all tested concentrations of the extracts than female, whereby 100% mortality were observes at the concentration range of 0.025 to 2 mg/ml and 0.05 to 2 mg/ml for Searsia longipes and Lannea schimperi respectively. Additionally, dead male’s worm were also demonstrated fragmentation and tightly coiled characteristic as depicted on Figure 7. The viability of the control group subjected to RPMI 1640 only was 100% up to the end of the observation time.
| Concentration | 0 Hrs | 24 Hrs | 48 Hrs | |||
| (mg/ml) | M | F | M | F | M | F |
| 2 | 100 | 100 | 0 | 80 | 0 | 0 |
| 1 | 100 | 100 | 20 | 100 | 0 | 0 |
| 0.5 | 100 | 100 | 20 | 100 | 0 | 40 |
| 0.25 | 100 | 100 | 20 | 100 | 0 | 40 |
| 0.05 | 100 | 100 | 40 | 100 | 0 | 60 |
| 0.025 | 100 | 100 | 40 | 100 | 20 | 80 |
| RPMI 1640a | 100 | 100 | 100 | 100 | 100 | 100 |
| PZQb | 100 | 100 | 0 | 0 | 0 | 0 |
aNegative control
bPositive control (0.01 mg/ml of Praziquantel)
Table 2: Percentage viability of the adult schistosome worms from 0 to 48 hours of exposure to different concentrations of Lannea schimperi methanolic extract.
bPositive control (0.01 mg/ml of Praziquantel)
Table 2: Percentage viability of the adult schistosome worms from 0 to 48 hours of exposure to different concentrations of Lannea schimperi methanolic extract.
| Concentration | 0 Hrs | 24 Hrs | 48 Hrs | |||
| (mg/ml) | M | F | M | F | M | F |
| 2 | 100 | 100 | 0 | 20 | 0 | 0 |
| 1 | 100 | 100 | 0 | 20 | 0 | 0 |
| 0.5 | 100 | 100 | 0 | 60 | 0 | 0 |
| 0.25 | 100 | 100 | 0 | 100 | 0 | 20 |
| 0.05 | 100 | 100 | 0 | 100 | 0 | 40 |
| 0.025 | 100 | 100 | 40 | 100 | 0 | 40 |
| RPMI 1640a | 100 | 100 | 100 | 100 | 100 | 100 |
| PZQb | 100 | 100 | 0 | 0 | 0 | 0 |
aNegative control
bPositive control (0.01 mg/ml of Praziquantel)
Table 3: Percentage viability of the adult schistosome worms from 0 to 48 hours of exposure to different concentrations of Searsia longipes methanolic extract.
bPositive control (0.01 mg/ml of Praziquantel)
Table 3: Percentage viability of the adult schistosome worms from 0 to 48 hours of exposure to different concentrations of Searsia longipes methanolic extract.
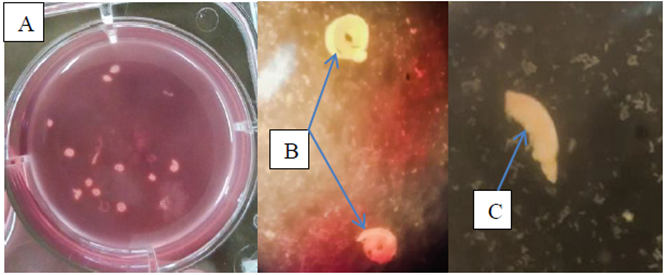
Figure 7: (A, B) Plate contain the pooled dead adult worms displaying tight coiling characteristics after being exposed to Searsia longipes (SLM) and Lannea schimperi (LSM) methanolic extracts. (C) Worms fragmentation after been exposed to Lannea schimperi methanolic extract (LSM).
Discussion
Schistosomiasis is termed as the neglected tropical disease of public health imperative, and it has ranked as second parasitic disease leading for causing death following malaria [25-27]. Moreover, there are only two drugs namely praziquantel and oxamniquine recommended by WHO (World Health Organization) for management of schistosomiasis. Despite being the only drugs available for the treatment of schistosomiasis, the aforementioned drugs are facing the challenge of resistance, whereby field resistance to these drugs were reported in Kenya, Senegal and Egypt [27-29]. Due to these reasons, searching for the new drug to combat this disease is therefore mandatory [27]. Study on medicinal plants is an emerging field which has demonstrated the potential of solving this problem [30]. In this aspect, medicinal plants are investigated as the possible source of new therapeutic agents based on their ethno botanical uses [31].
Lannea schimperi and Searsia longipes are plants in the family Anacardiaceae. These plants are utilized ethno medically for management of an array of disease conditions including helminthic infections [14]. Further, in some bioactivity studies Lannea schimperi has demonstrated the broad spectrum anti-microbial, antifungal and anti-inflammatory activity [32-34]. Aziz and coworkers (2011) [23] have reported that, plants possessing the aforementioned activity are more likely to have anti-schistosomal activity. Therefore, these activities and ethno medicinal information cumulatively have made these plants to be the possible candidates for evaluation of anti-schistosomal activity [23]. Thus, in present study the Lannea schimperi and Searsia longipes were investigated for the in vitro schistosomacidal activity against three life stages of Schistosoma mansoni namely cercariae, schistosomula and adult worm. Since current drugs lack efficacy on the first two stages and considering their importance in pathophysiology of the disease, it is therefore necessary to test the extracts against these stages [29,23].
In the current study, in vitro evaluations of both Lannea schimperi and Searsia longipes methanolic extract against cercariae stage have exhibited significant anti-schistosomal activity. Whereby, Searsia longipes methanolic extract divulged 100% mortality of cercariae at the highest concentration of 2 mg/ml, meanwhile Lannea schimperi has demonstrated 100% reduction of cercariae viability at two higher concentrations of 2 mg/ml and 1 mg/ml. Further, concentration based reduction of cercariae viability were observed in both extracts, however Lannea schimperi was more efficacious compared to Searsia longipes extract. Similar results were observed by Mohamed., et al. (2005) and Tekwu., et al. (2017) [35, 30] and reported the cercariacidal activity of Nigella sativa seeds and Rauwolfia vomitoria extracts respectively to be concentration dependent. The same observation were also reported by Kiros., et al. (2014) [36], whereas the cercariacidal activity of aqueous extract of Glinus lotoides fruits were reported to increase as concentration increased. Additionally, activity of both extract tested in the present study were observed to be time specific and in all tested concentration activity occurred after six hours of exposure.
Interestingly, Lannea schimperi methanolic extract has observed to induce tail detachment particularly at concentration below 0.5 mg/ml. The same observation was reported by Tekwu., et al. (2017) [30] during an in vitro evaluation of cercaricidal activity of Rauwolfia vomitoria. Ability to induce tail detachment might be of medical implication because tails play a vital role during infection particularly in identification of the susceptible host. Perrett and coworkers (1995) [37] reported the cercariacidal activity of Millettia thonningii and isoflavonoid and alpinumisoflavone were anticipated to induce that activity. Since Lannea schimperi and plant from Searsia genus have also reported to possess flavonoids, therefore these compounds together with other related compounds may be responsible for the cercariacidal activity exhibited in present study.
Moreover, the in vitro evaluations of Lannea schimperi and Searsia longipes methanolic extract against schistosomula stage have also demonstrated significant activity. Whereby, a concentration and time based reduction of the schistosomula viability were observed. Similar findings were reported by Aziz and coworkers (2011) [23] during an in vitro evaluation of Plectranthus tenuiflorus extract on different life stages of Schistosoma mansoni, where the viability reduction were proportional to the increase of concentration. Further, extracts seemed to have an impact on the muscular function which in turn lead to the reduced and completely loss of motor activity. Contrary to Tekwu., et al. (2017) [30] and in harmony with Aziz., et al. (2011) [23], in the current study, schistosomula were observed to be more susceptible to the extract than other life stages.
Nonetheless, in vitro evaluation of the extracts on the adult worms has also exhibited significant activity. Whereby, Searsia longipes extract was more efficacious, since it caused 100% mortality of the males worms up to the lowest concentration used in the present study and 100% mortality of all worms at the three higher concentrations after 48 hours of exposition. Whilst, Lannea schimperi extract induced 100% mortality of the male worms up to the second lower concentration of 0.05 mg/ml and 100% mortality of all worms at two highest concentrations. These observations indicated that, extracts are more potent to male’s worms than female’s worms. Henceforth, these observations agree with the Cioli and corkers (1995) [28] report on the anti-schistosomal activity of oxamniquine, whereby it was reported to have more potency to male’s worms than females. Additionally, both extracts were able to induce dose dependent reduction of motility of the worms; similar result was reported by De Oliveira and cowekers (2014) [31] when evaluating the activity of Baccharis trimera on adult Schistosoma mansoni. Motility reduction induced by these plant extract may be related to the ability to cause effect on neural transmitters such as dopamine, acetylcholine etc. Meanwhile, both extracts were observed to induce worm coiling characteristics particularly on males; this may be related to what referred as contraction by De Oliveira., et al. (2014) [31]. Worms subjected to positive control (Praziquantel) have also demonstrated coiling characteristic, however was not tight as that of the extracts. Tightly coiling/ contraction characteristic exhibited by treated worm may be due to induced calcium ion influx, resulted from the extract impact on the receptors and channels such as FMRFamide-related peptide and serotonin that modulate Ca2+ ion level [38,39]. Further, Lannea schimperi extract at concentration ranging from 2 mg/ml to 0.025 mg/ml was able to induce worm fragmentation. Since integument wall was reported to be the main target for many anti-schistosomal agents as reported by Dias and coworkers (2017) [40], thus worm fragmentation may be related to the extract effect on the integument wall. Henceforth, these observations signifies that Lannea schimperi and Searsia longipes which both belong to the family Anacardiaceae, have activity against different life stages of Schistosoma mansoni namely cercariae, schistosomula and adult stage. Hence the extract from this plant can be used to treat schistosomiasis as well as to prevent its transition.
Conclusion
Lannea schimperi and Rhus longipes have demonstrated broad spectrum activity against Schistosoma mansoni, whereby were able to exhibit significant activity against different life stages of Schistosoma mansoni. Lannea schimperi methanolic extract seemed to be more efficacious to schistosomula since it induced 100% mortality of the schistosomula at the lower concentration than Searsia longipes extract. Meanwhile, Searsia longipes were more effective to adult worms and cercariae stage, whereby 100% activity were observed at the concentration lower than that of the Lannea schimperi. Therefore, the present study is the first to evaluate the schistosomacidal activity of Lannea schimperi and Searsia longipes extracts. However, this study is not enough, hence further studies should be conducted to evaluate in vivo schistosomacidal activity, phytochemical compounds and toxicity profile of the aforementioned extracts.
Ethical Approval
This study piggy back rode on a Kenya Medical Research Institute approved protocol, KEMRI/SERU/CBRD/PROP164/3406.
This study piggy back rode on a Kenya Medical Research Institute approved protocol, KEMRI/SERU/CBRD/PROP164/3406.
Conflict of interest
The authors declare to have no conflict of interest.
The authors declare to have no conflict of interest.
Acknowledgement
This research was funded by Nelson Mandela African Institution of Science and Technology through African Development Bank scholarship project. Moreover, heartfelt thanks are extended to KEMRI for the provision of the laboratory space, consumables and all necessary technical assistance.
This research was funded by Nelson Mandela African Institution of Science and Technology through African Development Bank scholarship project. Moreover, heartfelt thanks are extended to KEMRI for the provision of the laboratory space, consumables and all necessary technical assistance.
References
- Ross AGP., et al. Schistosomiasis. New Engl. J. Med. Rev. 346.16 (2002): 1212–1220.
- Rollinson D., et al. “Time to Set the Agenda for Schistosomiasis Elimination”. Acta Trop 128.2 (2013): 423–440.
- WHO. Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis. World Health Organization technical report series. (2002).
- Simões LF., et al. Effect of Piper Tuberculatum Extract on Adult Schistosoma mansoni : In vitro and In vivo Tests. Journal of Tropical Pathology. 44.1 (2015): 56–66.
- World-Health-Organization. WHO Model List of Essential Medicines. (2013): 5.
- World Health Orgnization (WHO). The Control of Schistosomiasis, Technical Report Series. (1993): pp 1–86.
- Winstanley P. Handbook of Drugs for Tropical Parasitic Infections (2nd Edition). 1996.
- Katz N., et al. Therapy of Schistosomiasis Mansoni : The Brazilian Contribution. Acta Trop.108 (2008): 72–78.
- Fallon PG. "Schistosome Resistance to Praziquantel.1". Drug Resistance Updates (1998): 236-241.
- Botros SS and Bennett JL. “Praziquantel Resistance”. Expert Opinion on Drug Discovery 2.1 (2007): S35–S40.
- Aly IB., et al. “Immunological and Parasitological Parameters after Treatment with Dexamethasone in Murine Schistosoma Mansoni”. Memórias do Instituto Oswaldo Cruz. 105.6 (2010): 729–735.
- Gouveia MJ., et al. “Drug Repurposing for Schistosomiasis: Combinations of Drugs or Biomolecules”. Pharmaceuticals. 11.1 (2018): 1–34.
- Maroyi A. “An Ethnobotanical Survey of Medicinal Plants Used by the People in Nhema Communal Area, Zimbabwe”. Journal of Ethnopharmacology 136.2 (2011): 347–354.
- Okoth, D.A. “Phytochemistry and bioactive natural products from Lannea alata , Lannea rivae, Lannea schimperi and Lannea schweinfurthii”. University of Kwazulu-Natal (2014).
- Azwanida NN. “A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation”. Medicinal & Aromatic Plants. 4.3 (2015): 3–8.
- Odiere MR., et al. “Geographical Distribution of Schistosomiasis and Soil-Transmitted Helminths among School Children in Informal Settlements in Kisumu City, Western Kenya”. Parasitology.138.12 (2011): 1569–1577.
- Pan American Health Organization (PAHO). Identification of the Snail Intermediate Hosts of Schistosomiasis in the Americas. World Heal. Organ. 3.168 (1968): 132.
- Frandsen F and Christensen NO. “An Introductory Guide to the Identification of Cercariae from African Freshwater Snails with Special Reference to Cercariae of Trematode Species of Medical and Veterinary Importance”. Acta Troica 41.2 (1984): 181–202.
- Ramalho-Pinto FJ., et al. Schistosoma Mansoni: Defined System for Stepwise Transformation of Cercaria to Schistosomule in Vitro. Experimental Parasitology 36.3 (1974): 360–372.
- Marxer M., et al. Development of an in Vitro Drug Screening Assay Using Schistosoma Haematobium Schistosomula. Parasites &. Vectors 5.1 (2012): 165.
- Lewis F. Unit 19.1 - Schistosoma Life Cycle Maintenance. J. clincal Immunol. 1998: 1–28.
- Duvall RH and DeWitti WB. “An Improved Perfusion Technique for Recovering Adult Schistosomes from Laboratory Animals”. The American Journal of Tropical Medicine and Hygiene 16.4 (1967): 483-486.
- Aziz IZA., et al. “In Vitro Anti-Schistosomal Activity of “Plectranthus Tenuiflorus” on Miracidium, Cercaria and Schistosomula Stages of Schistosoma Mansoni”. Research Journal of Parasitology 6.2 (2011): 74-82.
- Xiao S., et al. “In Vitro and In Vivo Activities of Synthetic Trioxolanes against Major Human Schistosome Species”. Antimicrobial Agents Chemotherapy 51.4 (2007): 1440–1445.
- Van Bogaert LJ. “Biopsy-Diagnosed Female Genital Schistosomiasis in Rural Limpopo, South Africa”. International Journal of Gynecology and Obstetrics. 115.1 (2011): 75-76.
- Riveau G., et al. “Safety and Immunogenicity of RSh28GST Antigen in Humans: Phase 1 Randomized Clinical Study of a Vaccine Candidate against Urinary Schistosomiasis”. PLOS Neglected Tropical Diseases 6.7(2012): e1704.
- Cioli D., et al. “Schistosomiasis Control: Praziquantel Forever?” Molecular and Biochemical Parasitology195.1 (2014): 23–29.
- Pica-mattoccia DCL., et al. Antischistosomal Drugs : Pharmacol. Ther. 68(1995): 35–85.
- Vale N., et al.” Praziquantel for Schistosomiasis: Single-Drug Metabolism Revisited, Mode of Action, and Resistance”. Antimicrobial Agents and Chemotherapy. 61.5 (2017): AAC-02582.
- Tekwu EM., et al. “In Vitro Assessment of Anthelmintic Activities of Rauwolfia Vomitoria ( Apocynaceae ) Stem Bark and Roots against Parasitic Stages of Schistosoma Mansoni and Cytotoxic Study”. Journal of Parasitology Research (2017): 1-11.
- De Oliveira RN., et al. “Anthelmintic Activity in Vitro and in Vivo of Baccharis Trimera ( Less ) DC against Immature and Adult Worms of Schistosoma Mansoni”. Experimental Parasitology. 139(2014): 63–72.
- Egbe EO., et al. “Phytochemistry, Antinociceptive and Anti-Inflammatory Actvities of Methanolic Leaves Extract of Lannea Schimperi (Hoschst. Ex Rich) ENG”. Recent Patents on Biotechnology9.2 (2016): 145-152.
- Kisangau DP., et al. "Use of Traditional Medicines in the Management of HIV/AIDS Opportunistic Infections in Tanzania: A Case in the Bukoba Rural District". Journal of Ethnobiology and Ethnomedicine 3(2007): 1–8.
- Haule EE., et al. "A Study of Antimicrobial Activity, Acute Toxicity and Cytoprotective Effect of a Polyherbal Extract in a Rat Ethanol-HCl Gastric Ulcer Model". BMC Research Notes5(2012): 546.
- Mohamed AM., et al. "Sativa Seeds against Schistosoma Mansoni Different Stages". Memórias do Instituto Oswaldo Cruz100.2 (2005): 205–211.
- Kiros G., et al. "Laboratory Assessment of Molluscicidal and Cercariacidal Effects of Glinus Lotoides Fruits". BMC Research Notes 7.1(2014): 220.
- Perrett S., et al. "The Plant Molluscicide Millettia Thonningii (Leguminosae) as a Topical Antischistosomal Agent". Journal of Ethnopharmacology47.1(1995): 49-54.
- Fetterer RH., et al. "Praziquantel, Potassium and 2,4-Dinitrophenol: Analysis of Their Action on the Musculature of Schistosoma Mansoni". European Journal of Pharmacology 64.1(1980): 31-38.
- Greenberg RM. "Ca2+ Signalling, Voltage-Gated Ca2+ Channels and Praziquantel in Flatworm Neuromusculature". Parasitology 131. l (2005): S97-S108.
- Dias MM., et al."In Vitro Schistosomicidal and Antiviral Activities of Arctium Lappa L. (Asteraceae) against Schistosoma Mansoni and Herpes Simplex Virus-1". Biomedicine & Pharmacotherapy 94(2017): 489-498.
Citation:
Musa Chacha., et al. “Schistosomacidal Activity of Lannea Schimperi and Searsia Longipes against Cercariae, Schistosomula and Adult Stage of Schistosoma Mansoni”.
Chronicles of Pharmaceutical Science 3.2 (2019): 787-799.
Copyright: © 2019 Musa Chacha., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.