Review Article
Volume 2 Issue 5 - 2018
An Overview of Chemical Properties and Pharmacological Importance of Organobismuth Compounds
1Department of Pharmaceutical Chemistry, Integral University, Lucknow
2Department of Pharmaceutical Chemistry, Aryakul College of Pharmacy & Research, Lucknow
3Research Scholar Integral University, Lucknow
2Department of Pharmaceutical Chemistry, Aryakul College of Pharmacy & Research, Lucknow
3Research Scholar Integral University, Lucknow
*Corresponding Author: Shivbhadra Singh, Department of Pharmaceutical Chemistry, Integral University, Lucknow.
Received: June 14, 2018; Published: July 04, 2018
Abstract
There are several fields of chemistry, biochemistry and pharmaceutical chemistry where bismuth has been acting and potentially used in organic syntheses (phenylation or mild oxidizing agents), catalysts for carbon-carbon bond formation reaction and functional group transformations in many named and processed reaction, has industrial uses as precursors in advanced material science (superconductors, photorefractive systems, sol-gel processes and chemical vapor deposition techniques), bioactivity for the treatment of gastrointestinal disorders and as antitumor, antimicrobial, antibacterial, antispermatogenic and many more areas also. The present review article tends to highlight the chemical properties and potential applications of organobismuth compounds on human body both clinically and industrially.
Keywords: Organobismuth compounds; Superconductors; Photorefractive systems; Sol-gel processes; Chemical vapour deposition techniques
Introduction
Bismuth is a group 15 element of the periodic table, which also includes P, As and Sb. It has an atomic number of 83, an atomic mass of 209, electronic configuration of [Xe] 4f145d106s26p3 allowing bismuth to accept an electron pair readily as well as availability of unoccupied orbital elevate its affinity to extend coordination. Two main oxidation states (+3 example bismuth (IlI) halides, bismuth (lIl) subsalicylate etc. and +5 example bismuth (V) fluoride) and one naturally occurring isotope is (209Bi). Naturally occurring bismuth is found in small quantities throughout Earth's crust both as a pure metal and combined with other elements in various compounds. Bismuth has a relatively low crustal abundance (8ppb), it is 69th element in order of abundance, and is less common than silver, indium, cadmium, and only twice as common as gold [1]. The largest source of bismuth is found in the mineral bismuthinite [2], or bismuth glance (Bi2S3), bismite or bismuth ocher (Bi2O3) and bismutite [(BiO)2CO3H2O] [3]. Bismuth occurs naturally as the metal itself and is found as crystals in the sulphides ores of nickel, cobalt, silver and tin. Bismuth is typically obtained as a by-product in refining lead, copper [4], tin, silver, and gold ores found in Bolivia, Peru, Japan, Mexico, and Canada.
Bismuth is a soft, silvery metal with a bright, shiny surface and a yellowish or pinkish tinge. The metal breaks easily at room temperature. Its melting point is 271°C (520°F) and its boiling point is 1,560°C (2,480°F) [5]. Its density is 9.78 grams per cubic centimeter. The compounds of Bi (III) are the most important ones in analytical chemistry. Compounds of bismuth (IV) [6] may also exist. The compounds of bismuth (V) - alkali metal bismuthates - are known only in the solid state. The ions of bismuth (V) do not exist in solution [7].
There is also a single report of Bi (I) complex in solution obtained by dissolving metallic Bi in Conc. HCl however this solution is reported to be unsatable [8].
Reactions of bismuth metal with various reagent [9]
| S. No. | Reagents | Reactions with the Reagents |
| 1. | Nitrogen | No Reaction |
| 2. | Bromine | Reacts on heating to form Bi2Br3 |
| 3. | Water | No Reaction at room temperature, reacts slowly on calcination in a steam atmosphere with oxidation to Bi2O3 |
| 4. | Hydrogen | No Reaction |
| 5. | Air | No reaction either in dry or in wet air at room temperature; burns on heating to form ‘Bi203 |
| 6. | Iodine | Reacts on heating to form BiI3. |
| 7. | Nitric Acid | Reacts to form Bi(NO3)3 |
| 8. | Sulfuric Acid | Reacts on heating with liberation of S02. |
| 9. | Hydrochloric Acid | No Reaction |
| 10. | Lithium | Reacts on heating to form bismuthide |
| 11. | Selenium | Reacts on heating to form BiSe3 |
| 12. | Sulphur | React to form Bi2S3 |
| 13. | Tellurium | Reacts to form Bi2Te3 |
| 14. | Phosphorus | No Reaction |
| 15. | Chlorine | Reacts with ignition to form BiCl3 |
Table 1: Reaction of bismuth to reagents.
Complexometric titration
Bismuth can be titrated by all of the three methods of complexometric titrations that is back titration, masking and demasking agent and residual titration method [10]. (Raroot S., et al.) developed a selective complexometric determination of bismuth with mercaptans as masking agents, and its estimation in alloys, an excess of EDTA is added and the surplus is back-titrated at pH 5–6 with lead nitrate (Xylenol Orange as indicator). Thioglycollic or mercaptopropionic acid is then added to decompose the bismuth-EDTA complex and the liberated EDTA is titrated with lead nitrate [11].
Bismuth can be titrated by all of the three methods of complexometric titrations that is back titration, masking and demasking agent and residual titration method [10]. (Raroot S., et al.) developed a selective complexometric determination of bismuth with mercaptans as masking agents, and its estimation in alloys, an excess of EDTA is added and the surplus is back-titrated at pH 5–6 with lead nitrate (Xylenol Orange as indicator). Thioglycollic or mercaptopropionic acid is then added to decompose the bismuth-EDTA complex and the liberated EDTA is titrated with lead nitrate [11].
Gravimetric Estimation
The sample is dissolved in HNO3 is precipitated after dilution by the addition of ammonium carbonate in excess and boiling, The precipitate is then filtered off, washed with hot water, dried, ignited and weighed. The ignition should performed carefully above a low red heat, the oxide (Bi2O3) formed has a colour of dark yellow or brown and becomes yellow on cooling [12].
The sample is dissolved in HNO3 is precipitated after dilution by the addition of ammonium carbonate in excess and boiling, The precipitate is then filtered off, washed with hot water, dried, ignited and weighed. The ignition should performed carefully above a low red heat, the oxide (Bi2O3) formed has a colour of dark yellow or brown and becomes yellow on cooling [12].
Volumetric Methods
Various methods of estimation of bismuth have been described volumetrically. A solution is made in HNO3 and from this basic bismuth oxalate is precipitated with ammonium oxalate. Then the precipitate is dissolved in HCl, the solution is neutralised with ammonium hydroxide, and any precipitated hydroxide is redissolved if H2SO4. This final solution is then heated to 700C and titrated with a standard solution of KMnO4 [13].
Various methods of estimation of bismuth have been described volumetrically. A solution is made in HNO3 and from this basic bismuth oxalate is precipitated with ammonium oxalate. Then the precipitate is dissolved in HCl, the solution is neutralised with ammonium hydroxide, and any precipitated hydroxide is redissolved if H2SO4. This final solution is then heated to 700C and titrated with a standard solution of KMnO4 [13].
Colorimetric and spectrophotometric methods
Thiourea, CS(NH2)2 reacts with bismuth ions in HNO3 or H2SO4 solutions to form an intense yellow coloration which is suitable for calorimetric determination of bismuth, especially when the amount of bismuth is larger than 1mg. The absorption maxima are at 322 nm and 470 nm, and fourfold sensitivity is obtained in the ultra violet region. When potassium iodide solution is added to a dilute H2SO4 solution containing a small amount of bismuth, a yellow to orange coloration, due to the formation of an IodobismuthateIon, is produced. The color intensity increases with Iodide concentration up to about 1% KI and then remains practically constant. Small amounts of bismuth in the range 0.05-0.5 mg can be determined by this method at 460 nm [14].
Thiourea, CS(NH2)2 reacts with bismuth ions in HNO3 or H2SO4 solutions to form an intense yellow coloration which is suitable for calorimetric determination of bismuth, especially when the amount of bismuth is larger than 1mg. The absorption maxima are at 322 nm and 470 nm, and fourfold sensitivity is obtained in the ultra violet region. When potassium iodide solution is added to a dilute H2SO4 solution containing a small amount of bismuth, a yellow to orange coloration, due to the formation of an IodobismuthateIon, is produced. The color intensity increases with Iodide concentration up to about 1% KI and then remains practically constant. Small amounts of bismuth in the range 0.05-0.5 mg can be determined by this method at 460 nm [14].
Chromatographic method
A forced flow liquid chromatographic method was reported in which bismuth (III) is retained on a cation exchange column from dilute acid and is then separated from other metal ions by elution with 0.5 M Hydrobromic acid. The separation method is selective and rapid for bismuth [15]. An another method by Reverse phase extraction chromatography using liquid anion exchanger N-n-octylaniline as a stationary phase on silica as solid support was reported. Bismuth (III) was eluted from column with acetate buffer and analyzed spectrophotometrically with potassium iodide metod [16].
A forced flow liquid chromatographic method was reported in which bismuth (III) is retained on a cation exchange column from dilute acid and is then separated from other metal ions by elution with 0.5 M Hydrobromic acid. The separation method is selective and rapid for bismuth [15]. An another method by Reverse phase extraction chromatography using liquid anion exchanger N-n-octylaniline as a stationary phase on silica as solid support was reported. Bismuth (III) was eluted from column with acetate buffer and analyzed spectrophotometrically with potassium iodide metod [16].
Nuclear Magnetic Resonance (NMR) and infrared (IR) spectroscopy
These are common techniques used to determine structure of organometallic compounds. Vibration spectroscopy such as Raman or infrared spectroscopy is used to determine the chemical composition of a material based on detection of vibration modes of constituent molecules. NMR is a research technique that exploits the magnetic properties of certain atomic nuclei. It determines the physical and chemical properties of atoms or the molecules in which they are contained. It relies on the phenomenon of nuclear magnetic resonance and can provide detailed information about the structure, dynamics, reaction state, and chemical environment of molecules [17].
These are common techniques used to determine structure of organometallic compounds. Vibration spectroscopy such as Raman or infrared spectroscopy is used to determine the chemical composition of a material based on detection of vibration modes of constituent molecules. NMR is a research technique that exploits the magnetic properties of certain atomic nuclei. It determines the physical and chemical properties of atoms or the molecules in which they are contained. It relies on the phenomenon of nuclear magnetic resonance and can provide detailed information about the structure, dynamics, reaction state, and chemical environment of molecules [17].
Structure and properties of organobismuth compounds
Bismuth being a radioactive element, it is stable because of it’s an extremely long half life (t1/2~2×1018 years) makes it practically stable, [18,19] it is a semimetal with interesting electronic properties such as high carrier mobility, low effective mass, low carrier density, long mean free path; moreover, it is highly anisotropic in its Fermi level, presenting a high magnetoresistance [20]. These properties are due to the spatial arrangement of its atoms [21]. Organobismuth compounds show great structural diversity which ranges from monomeric to polymeric supramolecular assemblies and due to lewis acid and eco-friendly nature [22] find extensive applications as biocides, catalysts, [23-29] additives for lubricants [30], and even in medicines etc [31-33]. Bi (III) catalysts are generally crystalline solids and are commercially available at low cost [34]. Decreasing availability and increasing diffuseness of the s electrons makes the +5 oxidation state less stable when compared with phosphorus, arsenic and antimony. Due to its more pronounced metallic character than antimony and arsenic, bismuth forms formal stoichiometric [35] compounds with other metals. Typical examples include M3Bi and MBi (M = Li, Na, K), M3 Bi2 (M = Mg, Ca), and MBi (M = La, Ce, Nd, Gd, Sm, Y, etc.).
Bismuth being a radioactive element, it is stable because of it’s an extremely long half life (t1/2~2×1018 years) makes it practically stable, [18,19] it is a semimetal with interesting electronic properties such as high carrier mobility, low effective mass, low carrier density, long mean free path; moreover, it is highly anisotropic in its Fermi level, presenting a high magnetoresistance [20]. These properties are due to the spatial arrangement of its atoms [21]. Organobismuth compounds show great structural diversity which ranges from monomeric to polymeric supramolecular assemblies and due to lewis acid and eco-friendly nature [22] find extensive applications as biocides, catalysts, [23-29] additives for lubricants [30], and even in medicines etc [31-33]. Bi (III) catalysts are generally crystalline solids and are commercially available at low cost [34]. Decreasing availability and increasing diffuseness of the s electrons makes the +5 oxidation state less stable when compared with phosphorus, arsenic and antimony. Due to its more pronounced metallic character than antimony and arsenic, bismuth forms formal stoichiometric [35] compounds with other metals. Typical examples include M3Bi and MBi (M = Li, Na, K), M3 Bi2 (M = Mg, Ca), and MBi (M = La, Ce, Nd, Gd, Sm, Y, etc.).
Among the pentavalent organobismuth (V) compounds, only R3BiX2 compounds are well studied and have pentacoordination around the bismuth with a trigonal bipyramidal or square pyramidal configuration [36-38]. It is well known that group 15 elements characteristically exhibit structural changes on increasing or decreasing the content and the nature of organic group(s) bound to the central element as well as the anionic group [39]. As a matter of fact this fascinating aspect, apart from other consideration, makes their study rather more interesting. Unlike pentacoordinated covalent R3BiX2 compounds R4BiX are ionic in nature and R4Bi moiety has a charged tetrahedral configuration (R4Bi)+ [40], which is especially true in case of halide only. Further studies have shown that R4BiOR' (R= phenyl) to be covalent molecules with pentacoordination around bismuth, parallel to the observation made in case of R4SbOR compounds which also has a trigonal bipyramidal configuration. In both cases the oxygen atom occupies an apical position [39].
| S. No. | Compounds | Application | Ref. |
| 1. | Ph4BiF | Reagent | 41 |
| 2. | Organobismuth chloride and Triphenyl germylpropionate | Antiproliferative activity | 42 |
| 3. | Bu4N[PhBiX2Y] | Lewis acid | 43 |
| 4. | C6H11N(CH2C6H4)2BiBF4 | Catalyst | 44 |
| 5. | Water-soluble non-ionic triarylbismuthanes | X-ray contrast media | 45 |
| 6. | Cyclopropylbismuth | Reagent | 46 |
| 7. | Ar3Bi(OAc)2 and Ar3BiCl2 | Reagent | 47 |
| 8. | Dibismuthanes | Reagent | 48 |
| 9. | [S(CH2C6H4)2Bi(OH2)]+[ClO4]− | Catalyst | 49 |
| 10. | Ar3Bi=NCOR | Reagent | 50 |
| 11. | Tris[ortho-chloromethylphenyl] bismuthane | Reagent | 51 |
Table 2: Examples of organobismuth compounds with applications.
Examples of organobismuth compounds with application
Synthetic applicability of organobismuth compounds
The recent advances in Bismuth (III) chemistry have expanded the versatility and flexibility of modern green/eco-friendly catalysts for the reactions having carbon-carbon bond formation and functional group transformations along with in many named reactions like Michael Reaction, Friedel-Crafts Acylation, Hanztsch Reaction, Strecker Reaction, Diels Elder Reaction, Pechmann Reaction, Aldol Condensation, Knoevenagel Reaction, Reformatsky Reaction, Doebner condensation and in processed reaction like aliphatics, alicyclics, aromatics, amino acids, peptides, terpenes and steroids having pharmaceutical interest.
Synthetic applicability of organobismuth compounds
The recent advances in Bismuth (III) chemistry have expanded the versatility and flexibility of modern green/eco-friendly catalysts for the reactions having carbon-carbon bond formation and functional group transformations along with in many named reactions like Michael Reaction, Friedel-Crafts Acylation, Hanztsch Reaction, Strecker Reaction, Diels Elder Reaction, Pechmann Reaction, Aldol Condensation, Knoevenagel Reaction, Reformatsky Reaction, Doebner condensation and in processed reaction like aliphatics, alicyclics, aromatics, amino acids, peptides, terpenes and steroids having pharmaceutical interest.
| S. No. | Organobismuth compounds used | Uses in Name/Processed reaction | Ref. |
| 1. | BiCl3 | Michael Reaction | 52 |
| 2. | Bi(NO3)3 | Oxidation of alcohols | 53 |
| 3. | Bi-ZnF2 | Aldol Condensation | 54 |
| 4. | Bi(0)/O2 | Epoxides to α-diketones | 55 |
| 5. | BiBr3 | Benzylation of alcohols | 56 |
| 6. | Bismuth Acetate | Protodeboration of Di/Triborylated Indoles | 57 |
| 7. | Bi (OTf)3.xH2O | Doebner condensation | 58 |
| 8. | BiI3 and Bi2(SO4)3 | Synthesis of Thioacetals | 59 |
| 9. | Bi (TFA) | Biginelli Reaction | 60 |
| 10. | Bi(OTf)3·xH2O | Friedel-Crafts alkylation of phenol | 61 |
| 11. | Ac2O/Bi(OTf)3·xH2O | Acetylation of geraniol | 62 |
| 12. | Bi(OAc)3 | cholesterol and cholesterol formate into the corresponding 3β-acetoxy derivative | 63 |
| 13. | Bi(NO3)3·5H2O | oxidation of the allylic alcohol moiety of carveol to Carvone | 64 |
| 14. | BiCl3 | Strecker Reaction, Pechmann Reaction | 65,66 |
| 15. | bismuth(III) mandelate | Oxidatiojn of epoxides | 67,68 |
Table 3: Organobismuthcompounds and their application in organic synthesis.
Industrial applications of Bismuth as precursors in advanced material science
Bismuth is potentially used as superconductors, photorefractive systems, sol-gel processes, and chemical vapor deposition techniques. Bi High temperature superconductors draw attention owing to extraordinary correlation between superconducting characteristics and special features of crystal lattices [41-45].
Bismuth is potentially used as superconductors, photorefractive systems, sol-gel processes, and chemical vapor deposition techniques. Bi High temperature superconductors draw attention owing to extraordinary correlation between superconducting characteristics and special features of crystal lattices [41-45].
It was found that the crystal structure of Bi HTSCs represent spaces with confined dimensionality, the layered and quasi2D character of these structures determines the sharp anisotropy of their physical properties. Therefore some specific features of high temperature superconductors, namely, prominent dependence of critical current on temperature, broadening of the temperature range of the superconducting transition in a magnetic field, and creep effect of magnetic vortexes at temperatures far lower than the temperature of transition into the superconducting state appear in them most distinctly. The anisotropic character of Bi HTSCs should be taken into account in the technology to form microstructures of superconducting composites [46-69]. The bismuth tellurite, generally photorefractive material for holographic data storage offering unique fixing capabilities due to orthorhombic structure and optically biaxial without centre of symmetry [70].
The synthesis of bismuth nanoparticles has been recently reported by using sol-gel method like bismuth oxide nanoparticles, due to its optical and electrical properties such as refractive index, large energy band gap, dielectric permittivity as well as remarkable photoluminescence and photoconductivity. These properties make bismuth oxide an interesting candidate for applications in the fields such as optoelectronics, optical coatings, and gas sensors [71]. The bismuth Ferrite nano particles, because of their simultaneous coexistence of ferroelectric and anti-ferromagnetic order parameters in perovskite structure, are useful for applications is non linear optics, thin film capacitors, photo electrochemical cells, non volatile memories etc [72]. It was reported that the bismuth vanadate (BiVO4) nano particles, one of the photocatalysts, has been recently recognized as high potential application for the degradation of organic pollutants in wastewater [73-75]. The bismuth titanate electroceramic thin films as reported are strongly anisotropic in terms of ferroelectric properties such as polarization and coercive field [76].
The chemical vapour deposition (CVD) is bottom-up approach to make micro or nanoscale materials. It has applications in processing low dimension thermoelectric materials. There are many types of CVDs including ambient pressure, photo-thermal, metal-organic, plasma enhanced and ion beam CVD etc. The simplest ambient pressure deposition has been used to make Bi2Se3 nanowires on graphite papers without using catalysts [77]. Metal-organic chemical vapor deposition (MOCVD) has caught attention and been used for depositing the Bi-Te and Sb-Te thermoelectric films [78-79]. The molded rubber compositions have been widely used in a variety of applications particularly in automotive field as gaskets, seals, hoses, grommets, tubing, rubstrips and bumpers. The bismuth dimethyldithiocarbamate Bi[(CH3)2NC(S)S]3 are still potentially applicable as an vulcanization accelerator for natural rubber and SBR, especially in cable covers and mechanical goods [80].
The extensive application of plastics in biomedical science and engineering makes radiopacities a highly desirable property for polymers. It permits the utilization of radiography as a non-destructive diagnostic tool for plastics. The triphenylbismuth (Ph3Bi) is an effective X-ray contrast additive for plastics [81-82]. It gives homogeneous blends of high radiopacity with polyacrylates, polystyrene, poly(vinylchloride), polyalkenes, and other polymers. The materials are not affected by moisture and Ph3Bi, unlike the halides, does not interfere with amine accelerators. Moreover the leaching of bismuth can be eliminated by using polymerizable triphenylbismuth derivatives [83].
It was reported that the replacement of phenyl group of triphenylbismuth with styryl or a-methylstyryl moiety prevents leaching of bismuth and the plasticizing effect of free Ph3Bi is largely eliminated. Transparent materials with radiopacities exceeding that of aluminium can easily be obtained [84]. The bismuth-containing polymers are also used as bactericidal paints and coatings in hospitals as reported by some workers [85]. The Farzin Marandi., et al. reported a new 3D coordination polymer of bismuth with nicotinic acid N-oxide {[Bi(NNO)2(NO3)]⋅1.5H2O}n characterized by elemental analysis, IR and 1H-NMR spectroscopy along with single-crystal X-ray diffraction analysis [86]. The bismuth catalysts are also used for synthesis of various biodegradable and biocompatible polymers [87]. The organometallic or metal-oxide compounds of various other metals were also considered and being elucidated at the following metal ranking order: Ti > Ge > Zr ~ Sn > Hf > Sb > Bi [88].
Pharmacological activity of organobismuth compounds
Organometallic compounds played an important role in medicine and other areas for years, ever since humans have walked in the planet, although people have only recently realized their significance [89-93]. The use of organometallic medicinals is widespread. Some examples include:
Organometallic compounds played an important role in medicine and other areas for years, ever since humans have walked in the planet, although people have only recently realized their significance [89-93]. The use of organometallic medicinals is widespread. Some examples include:
| S. No. | Name of Drug | Structure of Drug | Organo-element Present | Ref. | |
| 1. | Cisplatin | 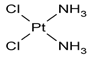 |
Platinum | 89 | |
| 2. | Carboplatin | 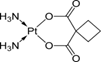 |
Platinum | 90 | |
| 3. | Salvarsan | 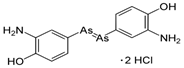 |
Arsenic | 91 | |
| 4. | Silver sulfadizine | 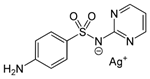 |
Silver | 92 | |
| 5. | Mercurochrome | 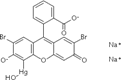 |
Mercury | 93 | |
Table 4: Some organometallic drugs with organo-element present.
Gastroprotective activity of organobismuth compounds
Gastrointestinal Protective
Bismuth drugs such as bismuth subcitrate (BSS) [94], colloidal bismuth subcitrate (CBS) [95] and ranitidine bismuth citrate (RBC) [96] are effective in treating and eradicating Helicobacter pylori together with antibiotics. Colloidal bismuth subcitrate may rearrange from colloidal particles to form a 3-D polymer at neutral pH. This polymeric structure may represent the protective bismuth coating found on ulcer craters [97]. Bismuth compounds hydrolyze in the stomach and form bismuth salts or bismuth polymers, and exert bactericidal effects. For example, bismuth subsalicylate hydrolyzes in gastric juice at pH 3 and releases salicylic acid together with the formation of bismuth oxychloride, these salts interact with the gastric mucus and bind to the proteins within the ulcer crater [98-100]. Bismuth exerts its anti-helicobacter activity by four different ways: (a) forms complexes in the bacterial wall and periplasmic space, (b) H. pylori produces several enzymes including urease, proteases, alcohol dehydrogenase, and phospholipases that promote bacterial colonization. Inhibition of the enzymatic activity of the bacterial urease is an important mechanism of action of bismuth-based drug, (c) inhibits ATP synthesis of bacteria, and (d) inhibits adherence of H. Pylori to the gastric mucosa [101-102].
Gastrointestinal Protective
Bismuth drugs such as bismuth subcitrate (BSS) [94], colloidal bismuth subcitrate (CBS) [95] and ranitidine bismuth citrate (RBC) [96] are effective in treating and eradicating Helicobacter pylori together with antibiotics. Colloidal bismuth subcitrate may rearrange from colloidal particles to form a 3-D polymer at neutral pH. This polymeric structure may represent the protective bismuth coating found on ulcer craters [97]. Bismuth compounds hydrolyze in the stomach and form bismuth salts or bismuth polymers, and exert bactericidal effects. For example, bismuth subsalicylate hydrolyzes in gastric juice at pH 3 and releases salicylic acid together with the formation of bismuth oxychloride, these salts interact with the gastric mucus and bind to the proteins within the ulcer crater [98-100]. Bismuth exerts its anti-helicobacter activity by four different ways: (a) forms complexes in the bacterial wall and periplasmic space, (b) H. pylori produces several enzymes including urease, proteases, alcohol dehydrogenase, and phospholipases that promote bacterial colonization. Inhibition of the enzymatic activity of the bacterial urease is an important mechanism of action of bismuth-based drug, (c) inhibits ATP synthesis of bacteria, and (d) inhibits adherence of H. Pylori to the gastric mucosa [101-102].
Gastro-duodenal Protective
The antibacterial effects of bismuth compounds against H. pylori facilitates healing of gastroduodenal ulcers also [103]. Bismuth salts have both in-vitro and in-vivo against H. pylori as well as exert other effects on various sites on gastric and duodenal mucosa. Organobismuth drugs like CBC etc. exert cytoprotective action with protection against gastric lesions in rats when exposed to stressful agents, this effect is mediated by protaglandins, epithelial growth factor (EGF) & mucosal bi-carbonate secretion [104]. These agents inhibit action of pepsin & gastric juice [105].
The antibacterial effects of bismuth compounds against H. pylori facilitates healing of gastroduodenal ulcers also [103]. Bismuth salts have both in-vitro and in-vivo against H. pylori as well as exert other effects on various sites on gastric and duodenal mucosa. Organobismuth drugs like CBC etc. exert cytoprotective action with protection against gastric lesions in rats when exposed to stressful agents, this effect is mediated by protaglandins, epithelial growth factor (EGF) & mucosal bi-carbonate secretion [104]. These agents inhibit action of pepsin & gastric juice [105].
They show healing effect both as duodenal and gastric ulcer as well as protection against NSAIDS, aspirin and alcohol induced damage is also noted [106]. Colloidal bismuth subcitrate, bismuth subsalicylate, bismuth subnitrate, and ranitidine bismuth citrate are different bismuth salts which are currently under use in the treatment of gastroduodenal ulcers [107].
Antimicrobial activity of organobismuth compounds
In addition to H. pylori, Bi compounds have been effectively used to treat a host of bacterial associated infections such as syphilis (e.g., potassium bismuth tartrate, bismuth quinine iodide and iodobismitol), colitis (bismuth subnitrate, bismuth citrate), diarrhea (BSS and bismuth nitrate) and wound infections (bismuth oxide) [108-109]. In recent a group of Japanese workers synthesized a series of organobismuth compounds which shows potent antimicrobial activity against fungus and bacterial culture responsible for human pathogenic disease [110].
In addition to H. pylori, Bi compounds have been effectively used to treat a host of bacterial associated infections such as syphilis (e.g., potassium bismuth tartrate, bismuth quinine iodide and iodobismitol), colitis (bismuth subnitrate, bismuth citrate), diarrhea (BSS and bismuth nitrate) and wound infections (bismuth oxide) [108-109]. In recent a group of Japanese workers synthesized a series of organobismuth compounds which shows potent antimicrobial activity against fungus and bacterial culture responsible for human pathogenic disease [110].
Antibacterial Activity
It is known that bismuth salts have antibacterial activity against multiple gastrointestinal pathogens such as Escherichia coli, Vibrio cholera, Campylobacter jejuni, Salmonella, Shigella, and Yersinia. Because of this activity, bismuth salts are used in the treatment of some form of gastroenteritis such as traveler’s diarrhea. Synergism between bismuth salts and antibiotics was present like metronidazole and bismuth were administered together [111]. Kotani T. et al. synthesize some Cyclic Organobismuth(III) Compounds and reported them as potent antibacterial [112]. Marzano et.al. synthesize some new Complex of Bismuth(III) with Sulfapyridine [BiCl3(C11H11N3O2S)3]. The structure of the complex reveals a distorted octahedral geometry around the bismuth atom, which is bound to three sulfonamidic nitrogens from sulfapyridine, acting as a monodentate ligand, and to three chloride ions, and report them 3 times more potent than the ligand against Salmonella typhimurium, 4 times against Staphylococcus aureus, Shigella dysenteriae, and Shigella sonnei and 8 times more potent against Pseudomonas aeruginosa and Escherichia coli [113].
It is known that bismuth salts have antibacterial activity against multiple gastrointestinal pathogens such as Escherichia coli, Vibrio cholera, Campylobacter jejuni, Salmonella, Shigella, and Yersinia. Because of this activity, bismuth salts are used in the treatment of some form of gastroenteritis such as traveler’s diarrhea. Synergism between bismuth salts and antibiotics was present like metronidazole and bismuth were administered together [111]. Kotani T. et al. synthesize some Cyclic Organobismuth(III) Compounds and reported them as potent antibacterial [112]. Marzano et.al. synthesize some new Complex of Bismuth(III) with Sulfapyridine [BiCl3(C11H11N3O2S)3]. The structure of the complex reveals a distorted octahedral geometry around the bismuth atom, which is bound to three sulfonamidic nitrogens from sulfapyridine, acting as a monodentate ligand, and to three chloride ions, and report them 3 times more potent than the ligand against Salmonella typhimurium, 4 times against Staphylococcus aureus, Shigella dysenteriae, and Shigella sonnei and 8 times more potent against Pseudomonas aeruginosa and Escherichia coli [113].
Antifungal Activity
A series of hypervalent organobismuth (III) compounds derived from alkyl aryl ketones [XBi(5-R'C6H3-2-COR) (Ar)] was synthesized by Murafuji., et al. & their antifungal activity against the yeast Saccharomyces cerevisiae with their structure activity relationships was repoted, the synthesized compounds were seems to be active towards yeast Saccharomyces cerevisiae [114]. Various new organobismuth compounds like tetraorganobismuth(V) aryloxyacetate [115], new organobismuth amides [116], triorganobismuth (V) amide [117], new diphenylbismuth (III) chloride [118], new tris (Pentafluorophenyl) bismuth (V) dichloride [119] were synthesized and reported for their antibacterial and antifungal activity.
A series of hypervalent organobismuth (III) compounds derived from alkyl aryl ketones [XBi(5-R'C6H3-2-COR) (Ar)] was synthesized by Murafuji., et al. & their antifungal activity against the yeast Saccharomyces cerevisiae with their structure activity relationships was repoted, the synthesized compounds were seems to be active towards yeast Saccharomyces cerevisiae [114]. Various new organobismuth compounds like tetraorganobismuth(V) aryloxyacetate [115], new organobismuth amides [116], triorganobismuth (V) amide [117], new diphenylbismuth (III) chloride [118], new tris (Pentafluorophenyl) bismuth (V) dichloride [119] were synthesized and reported for their antibacterial and antifungal activity.
Organobismuth compounds in cancer treatment
Ten most active metals towards anticancer activity are arsenic, antimony, bismuth, gold, vanadium, iron, rhodium, titanium, gallium and platinum [120]. Bismuth complexes of 6-mercaptopurine were the first antitumour compounds tested. They yielded promising results, as compared to platinum(II) analogues [121]. Treatment with inorganic bismuth results in regression of gastric lymphoma caused by H. pyroli & reduction of toxic side effects of cisplatin by tissue specific induction of metallothionein [122]. A number of recent publications from Henan University, Kaifeng, China contribute to the story in relation to bismuth thisosemicarbazone or thiocarbonohydrazone complexes as anti-cancer agents [123-124].
Ten most active metals towards anticancer activity are arsenic, antimony, bismuth, gold, vanadium, iron, rhodium, titanium, gallium and platinum [120]. Bismuth complexes of 6-mercaptopurine were the first antitumour compounds tested. They yielded promising results, as compared to platinum(II) analogues [121]. Treatment with inorganic bismuth results in regression of gastric lymphoma caused by H. pyroli & reduction of toxic side effects of cisplatin by tissue specific induction of metallothionein [122]. A number of recent publications from Henan University, Kaifeng, China contribute to the story in relation to bismuth thisosemicarbazone or thiocarbonohydrazone complexes as anti-cancer agents [123-124].
Bismuth(V) complexes of lapachol have been synthesized by the reaction of Ph3BiCl2 with lapachol (Lp) and characterized by several physicochemical techniques such as IR, and NMR spectroscopy and X-ray crystallography by Oliveira., et al. Bismuth (V) complex formed is a dinuclear compound bridged by an oxygen atom, (Lp)2(Ph3Bi)2O. The compound inhibited the growth of a chronic myelogenous leukemia cell line and the complex of Bi(V) was also about five times more active than free lapachol [125].
Luchi., et al. synthesized some novel heterocyclic organobismuth compounds that have potent antibacterial properties, they also examined their anticancer activity and found that these compounds have particularly potent anticancer activities against leukemia cell lines. One of them, bi-chlorodibenzo [c,f] [1,5] thiabismocine, inhibited the growth of the human promyelocytic leukemia cell line HL-60 at a concentration of 0.22 microM [126].
Cui L,. et al. synthesized three novel organobismuth(V) complexes Ph3Bi(OOCC6H3F2)2, Ph3Bi(OOCC6H4CF3)2 and Ph3Bi(OOCC4H3S)2. The interaction of the first complex with calf-thymus DNA (CT-DNA) was investigated by UV absorption spectroscopy, fluorescence emission spectroscopy and viscosity. All results revealed that this complex binds to DNA via intercalative mode. Furthermore the proliferation inhibitory activities of the all three complexes on MDA-MB-231 breast cancer cells were investigated. The results indicated that all of the three complexes have superior inhibition of cellular proliferation [127]. The organobismuth compounds are extremely potent cytotoxic agent when attached to a monoclonal antibody as these can target leukemia, lymphoma and other tumors [128].
Anti-Leishmaniasis activity of organobismuth compounds
Leishmaniasis constitutes a spectrum of diseases that range in severity from self-healing to fatal. It is caused by protozoan parasites of the Trypanosomatidae family and typically contracted by the bite of an infected female sand fly. Potassium antimony tartrate, a Sb(III) compound was developed at the start of the 20th century, was initially used and increased survival rates but was highly toxic to the patient [129].
Leishmaniasis constitutes a spectrum of diseases that range in severity from self-healing to fatal. It is caused by protozoan parasites of the Trypanosomatidae family and typically contracted by the bite of an infected female sand fly. Potassium antimony tartrate, a Sb(III) compound was developed at the start of the 20th century, was initially used and increased survival rates but was highly toxic to the patient [129].
Rocha., et al. by using the classic microscopic in vitro model have analyzed the effects of a series of lapachol and chlorides complexes with antimony (V), bismuth (V), and tin (IV) against L. amazonensis and found that all seven compounds exhibited antileishmanial activity, but most of the antimony (V) and bismuth (V) complexes were toxic against human HepG2 cells and murine macrophages [130].
Andrews., et al. recently reported a series of Bi(III) β-thioxoketonate complexes as anti-leishmanial agents. They hypothesised that Bi complexes with a more thermodynamically stable Bi-S bond would be less labile than for example a carboxylate analogue and in turn possess improved hydrolytic stability which should positively influence purity, reproducibility and activity in biological systems [131].
Although different complexes of bismuth like β-thioxoketones complexes of formula R1C-(=O)CH2(=S)R2 and their Bi complexes of formula [Bi{R1C(=O)CHC(=S)R2}3], the bismuth derivatives of free acid and BiPh3, naproxen tris-carboxylato Bi(III)complex were reported for the activity by different workers but in general the Bi complexes displayed little or no selectivity, which would suggest that they are not suitable candidates for the treatment of Leishmaniasis and the development of compounds with selective anti-leishmanial activity is the challenge.
Antispermatogenic Activity of organobismuth compounds
Rising human population throughout the world has detrimental effects on the life supporting system on the earth. Fertility regulation comprising contraception and management of infertility forms an important component of reproductive health. Though considerable progress has been made in development of contraception among females, progress and possibilities in males are still slow and limited [132]. The chemical compounds affecting testicular function includes different groups: Serotonin, Melatonin, Levenogestral, Cyproterone Acetate, Depot Medroxy progesterone Acetate etc and various steroidal drugs but they all have various side effects and hazards at reproductive organs.
Rising human population throughout the world has detrimental effects on the life supporting system on the earth. Fertility regulation comprising contraception and management of infertility forms an important component of reproductive health. Though considerable progress has been made in development of contraception among females, progress and possibilities in males are still slow and limited [132]. The chemical compounds affecting testicular function includes different groups: Serotonin, Melatonin, Levenogestral, Cyproterone Acetate, Depot Medroxy progesterone Acetate etc and various steroidal drugs but they all have various side effects and hazards at reproductive organs.
A series of Bi(III) and As(III) complexes with two N & S donor ligands, 1-(4-chloro-2-oxo-2H-chromen-3-yl)-methylene)-thiosemicarbazide(L1H)andN`-[1-(2-oxo-2H-chrome-3yl-ethylidene]-hydrazinecarbodithionic acid benzyl ester (L2H) have been synthesized by the reaction of BiCl3 and Ph3As with ligands in 1:1 and 1:2 molar ratios byDawara., et al. Antimicrobial activity and antifertility activity in male albino rats of the synthesized compounds was tested and the metal complexes have shown to be more antimicrobial against the microbial species as compared to free ligands. Also marked reduction in sperm motility and density resulted in infertility. Significant alterations were found in biochemical parameters of reproductive organs in treated animals as compared to control group. It is concluded that all these effects may finally impair the fertility of male rats [133].
Acknowledgment
The authors are thankful to the faculty of Pharmacy, Integral University for providing all the necessary facilities related to the present work (Manuscript Communication Number: IU/R&D/2018-MCN000351) along with Managing Director Aryakul College of Pharmacy & Research, Lucknow for providing the valuable support for the research work.
The authors are thankful to the faculty of Pharmacy, Integral University for providing all the necessary facilities related to the present work (Manuscript Communication Number: IU/R&D/2018-MCN000351) along with Managing Director Aryakul College of Pharmacy & Research, Lucknow for providing the valuable support for the research work.
References
- Carlin J F., et al. “Bismuth Minerals Yearbook Vol. 1-Metals and Minerals”. United State Geological Survey (2011).
- Geology. “Economic Minerals: A Review of their Characteristics and Occurrence - Pierfranco Lattanzi”.
- Ojebuoboh FK. “Bismuth-Production, Properties, and Applications, Review of extractive metallurgy, Review of extractive metallurgy”. 44.4 (1992): 46-49.
- Naumov AV. “World market of bismuth”. A review, Russian Journal of Non Ferrous Metals”. 48 (2007): 10-16.
- Habashi F. “Bismuth, Physical and Chemical Properties. In: Kretsinger R.H., Uversky V.N., Permyakov E.A. (eds) Encyclopedia of Metalloproteins. Springer, New York, NY, (2013).
- Cotton FA., et al. “Advanced Organic Chemistry”. New York John Wiley and Sons, Inc (1999).
- Busev AI., et al. “Handbook of the Analytical Chemistry of Rare elements”. Ann Arbor - Hunphrey Science Publishers, Ann Arbor (1970).
- Ulvenlund S., et al. “Univalent Bismuth a subvalent main group metal ion stable in aqueous solution”. Acta Chemica Scandinavica 48 (1994): 635-639.
- Bhatki K S., et al. “Radiochemistry of bismuth”. Springfield Press: USA (1997).
- Cheng KL., et al. “Complexometric titration of bismuth”. Analytical Chemistry 26.121 (1996): 977-1978.
- Raroot S., et al. “Selective complexometric determination of bismuth with mercaptans as masking agents, and its estimation in alloys”. Talanta (1985): 1011-1012.
- Sant BR., et al. “Gravimetric estimation of bismuth. Fresenius' Zeitschrift für analytische Chemie”. 159.6 (1958): 427-429.
- Muir MMP., et al. “On the volumetric estimation of bismuth”. Journal of the Chemical Society, Transactions 41 (1882): 1-4.
- Lisicki NM., et al. “Ultraviolet spectrophotometeric determination of bismuth by iodide and thiourea method”. Analytical Chemistry 27.11 (1955): 1722-1724.
- Willis RB., et al. “Determination of Bismuth by forced flow liquid chromatography”. Talanta 1974, 21.5 (1974): 347-354.
- Phule SR., et al. “Chemistry for sustainable development”. Springer (2011): 209-217.
- Mudi SY., et al. “Clinical and Industrial Application of Organometallic Compounds and Complexes: A Review”. American Journal of Chemistry and Applications 2.6 (2015): 151-158.
- Suzuki H., et al. “Organobismuth Chemistry”. Elsevier New York (2001).
- Bailar JC., et al. “Comprehensive Inorganic, Chemistry Pergamon Press”. ScienceDirect New York, 1973.
- Du X., et al. “Metal - Insulator - Like Behavior in Semimetallic Bismuth and Graphite”. Physical Review Letters 94 (2005):166601.
- Postel M., et al. “Bismuth derivatives for the oxidation of organic compounds”. Coordination Chemistry Reviews 155 (1996): 127-144.
- Suresh., et al. “Recent developments on bismuth (III) in carbon-carbon bond formation chemistry”. Rasayan journal of chemistry 4.1 (2011): 73-85.
- Yang N., et al. “Biocoordination chemistry of bismuth: Recent advances”. Coordination Chemistry Reviews 251(2007): 2354-2366.
- Mehring M. “From molecules to bismuth oxide-based materials: Potential homo- and heterometallic precursors and model compounds”. Coordination Chemistry Reviews 251 (2007): 974-1006.
- Sadler PJ., et al. “Coordination chemistry of metals in medicine: target sites for bismuth”. Coordination Chemistry Reviews 185.186 (1995): 689-709.
- Sun H., et al. “Bismuth Antiulcer Complexes”. Journal of Biological Inorganic Chemistry 2 (1999) 159-184.
- Pourshahrestani S., et al. “Bismuth triflate, Bi(OTf)3, as an efficient and reusable catalyst for synthesis of dihydropyrano [3,2-b] chromenediones”. Journal of the Iranian Chemical Society 12.4 (2015): 573-580.
- Bailey AD., et al. “Environmentally friendly organic synthesis using bismuth compounds: bismuth (III) iodide catalyzed deprotection of acetals in water”. Tetrahedron Letters49 (2008): 691-694.
- Alam MM., et al. “Bi(OTf)3-Catalyzed Baeyer–Villiger Oxidation of Carbonyl Compounds with m –CPBA. Synth”. Commun 33 (2008): 3035-3040.
- Garje S S., et al. “Chemistry of arsenic, antimony and bismuth compounds derived from xanthate, dithiocarbamate and phosphorus based ligands”. Coordination Chemistry Reviews 236 (2003): 35-56.
- Spafford MJ., et al. “Environmentally friendly organic synthesis using bismuth compounds. Bismuth trifluoromethanesulfonate catalyzed allylation of dioxolanes”. Australian Journal of Chemistry 61.6 (2008): 419-421.
- Yu L., et al. “Synthesis, characterization and cytotoxicity of some triarylbismuth(V) di(N-p-toluenesulfonyl) aminoacetates and the crystal structure of (4-CH3C6H4SO2NHCH2CO2)2Bi(C6H4Cl-4)3. Appl. Organometal”. Chemistry 18 (2004): 187-190.
- Kirija NV., et al. “Bi(CF3)3/Cu(OCOCH3)2 -A new system for the synthesis of 2-trifluoromethylcycloalkan-1-ones, trifluoromethylanilines and phenyl (trifluoromethyl) sulfane”. Journal of Fluorine Chemistry 106 (200): 217-221.
- Sigma-Aldrich Chemical Co. Catalog, (2009-10).
- Elschenbroich C. “Organometallics (3rd edition)”. Weinheim: Wiley-VCH. ISBN 3-527-29390-2939.
- Bertazzi N., et al. “Complexes of organometallic compounds : XXXI. Studies on organolead (IV) thiocyanate compounds”. Journal of Organometallic Chemistry 37 (1972): 281-284.
- Feham K., et al. “Synthesis and structural study of Triphenylbismuth Bis (Salicylate)”. Crystal Structure Theory and Application 2 (2013): 28-33.
- Li JS., et al. “Synthesis, spectroscopic characterization, and in vitro antitumor activity of tetraphenylantimony derivatives of analogues of demethylcantharidin and demethyldehydrogen-cantharidin”. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry 32.3 (2002): 583-593.
- Doak., et al. “Organometallic compounds of Arsenic, Antimony and Bismuth”. Wiley Interscience New York 1970.
- Maslowsky E. “Vibrational spectra of the organic derivatives of the group VB elements”. Journal of Organometallic Chemistry 70 (1974): 153-227.
- Ooi T., et al. “Fluorotetraphenylbismuth: A new reagent for efficient regioselective α-phenylation of carbonyl compounds”. Journal of the American Chemical Society 125 (2003): 10494-10495.
- Zhang X W., et al. “Synthesis, structure, and in vitro antiproliferative activity of cyclic hypervalent Organobismuth (III) chlorides and their triphenylgermylpropionate derivatives”. Journal of Organometallic Chemistry 694 (2009): 3019-3026.
- Sharma P., et al. “Synthesis and crystal structures of mixed halophenylbismuthates (III)”. Zeitschrift für anorganische und allgemeine Chemie 626 (2000): 921-924.
- Zhang XW., et al. “Air-stable hypervalent Organobismuth (III) tetrafluoroborate as effective and reusable catalyst for the allylation of aldehyde with tetraallyltin”. Tetrahedron Letters 51 (2010): 153-156.
- Matano Y., et al. “Water-soluble non-ionic triarylbismuthanes”. First synthesis and properties”. Journal of the Chemical Society, Perkin Transaction 1 (1998): 2511-2518.
- Gagnon A., et al. “Direct N-cyclopropylation of cyclic amides and azoles employing a cyclopropylbismuth reagent”. Journal of the American Chemical Society 129 (2007): 44-45.
- Finet JP., et al. “Tris (polymethoxyphenyl) bismuth derivatives: Synthesis and reactivity”. Journal of Organometallic Chemistry 691 (2006): 2386-2393.
- Shimada S., et al. “A unique Bi-Bi bond forming reaction using organobismuth oxides and phosphorus compounds bearing a P (=O) H group”. Chemical Communications (2009): 6168-6170.
- Qiu RH., et al. “Synthesis and structure of an air-stable cationic organobismuth complex and its use as a highly efficient catalyst for the direct diastereoselective Mannich reaction in water”. Chemical Communications (2009): 4759-4761.
- Matano Y., et al. “Synthesis, structure, and reactions of (acylimino) triaryl-λ5-bismuthanes: First comparative study of the (acylimino) pnictorane series”. Journal of the American Chemical Society 123 (2001): 10954-10965.
- Bolshakov AV., et al. “Three-step one-pot organobismuth-mediated synthesis of benzo[b]pyran compounds”. Tetrahedron Letters 43 (2002): 8245-8248.
- Zhan ZP., et al. “Bismuth Trichloride–Catalyzed C‐Alkylation of Pyrroles with Electron‐Deficient Olefins. Synth”. Commun definition and meaning 36 (2006): 1373-1382.
- Samajdar S., et al. “Surface-mediated highly efficient oxidation of alcohols by bismuth nitrate Synth”. Commun definition and meaning31 (2001): 2691-2695.
- Lee YJ., et al. “Organometallic reactions in aqueous media Bismuth-mediated crossed aldol type reactions”. Canadian Journal of Chemistry 81(2003): 1406-1412.
- Postel M., et al. “Studies on the catalytic oxidation of epoxides to α-diketones by Bi(0)/O2 in DMSO”. Journal of Molecular Catalysis A: Chemical 208.1 (2004): 135-145.
- Boyer B., et al. “BiBr3, an efficient catalyst for the benzylation of alcohols: 2-phenyl-2-propyl, a new benzyl-type protecting group”. Tetrahedron Letters 41.6 (2000): 2891-2894.
- Shen F., et al. “Bismuth Acetate as a Catalyst for the Sequential Protodeboronation of Di- and Triborylated Indoles”. Organic Letters18.7 (2016): 1554-1557.
- Wu QP., et al. “A facile one-pot procedure for the transformation of acetonides into diacetates catalyzed with Bi (OTf)3·xH2O”. Tetrahedron Letters 49.17 (2008): 2714-2718.
- Leonard NM., et al. “Applications of bismuth (III) compounds in organic synthesis”. Tetrahedron 58 (2002): 8373-8397.
- Khosropour AR., “Mohammadpoor-Baltork, I.; Ghorbankhani, H. Bi(TFA)3 in [nbpy]FeCl4- An efficient catalyst system for the one-pot synthesis of 4,6-diarylpyrimidin-2(III)-ones”. Catalysis Communications 7 (2006): 713-716.
- Le Rouzo G., et al. “Synthesis of 4-tertoctylphenol and 4-cumylphenol by metal triflate and metal triflimidate catalysts”. Journal of Chemical Research, Synopses (2006): 521-526.
- Orita A., et al. “Highly efficient and versatile acylation of alcohols with Bi (OTf) 3 as catalyst”. Angewandte Chemie International Edition 39 (2000): 2877-2879.
- Reese AL., et al. “Reactions of bismuth triacetate with organic compounds”. The Journal of Organic Chemistry 38 (1973): 764-768.
- Samadjar S., et al. “Surface-mediated highly efficient oxidation of alcohols by bismuth nitrate”. Synthetic Communications 31 (2001): 2691-2695.
- De SK., et al. “Bismuth trichloride catalyzed synthesis of α-aminonitriles”. Tetrahedron Letters 45 (2004): 7407-7408.
- Patil SB., et al. “Ultrasound‐Assisted Pechmann Condensation of Phenols With β‐Ketoesters to Form Coumarins, in the Presence of Bismuth (III) Chloride Catalyst. Synth”. Commun definition and meaning 36 (2006): 525-531.
- Postel M., et al. “Bismuth derivatives for the oxidation of organic compounds”. Coordination Chemistry Reviews 155 (1996): 127-144.
- Boisselier VL ., et al. “Bismuth (III)-catalyzed oxidative cleavage of aryl epoxides: Substituent effects on the kinetics of the oxidation reaction”. Journal of Organometallic Chemistry482.1 (1994): 119-123.
- Shamrai VF. “Crystal structures and superconductivity of bismuth high temperature superconductors (Review)”. Inorganic Materials: Applied Research 4.4 (2013): 273-283.
- Horn W., et al. “Holographic data storage in photorefractive bismuth tellurite”. Journal of Physics D: Applied Physics 41 (2008): 224006.
- Mallahi M., et al. “Synthesis and characterization of Bismuth oxide nanoparticles via sol-gel method”. American Journal of Engineering Research 3.4 (2014): 162-165.
- Fei L., et al. “Visible Light Responsive Perovskite BiFeO3 Pills and Rods with Dominant {111}c Facets”. Crystal Growth & Design 11 (2011): 1049-1053.
- Silva MR., et al. “Deposition and characterization of BiVO4 thin films and evaluation as photoanodes for methylene blue degradation”. Journal of Solid State Electrochemistry 16 (2012): 3267-3274.
- Yingana G., et al. “Additive-free controllable fabrication of bismuth vanadates and their photocatalytic activity toward dye degradation”. Applied Surface Science 256 (2010): 2215-2222.
- Wenzong Y., et al. “CTAB-assisted synthesis of monoclinic BiVO4 photocatalyst and its highly efficient degradation of organic dye under visible-light irradiation”. Journal of Hazardous Materials 173 (2010): 194-199.
- Zarycka A., et al. “The sol-gel synthesis of bismuth titanate electroceramic thin films”. Materials Science-Poland 23.1 (2005): 167-175.
- Huang G., et al. “Single-crystalline Bi2Se3 nanowires grown by catalyst-free ambient pressure chemical vapor deposition”. Materials Letters 179 (2016): 198-201.
- Giani A., et al. “Growth of Bi2Te3 and Sb2Te3 thin films by MOCVD. Mater”. Materials Science and Engineering B 64 (1996): 19-24.
- Giani A., et al. “MOCVD growth of Bi2Te3 layers using diethyltellurium as a precursor”. Thin Solid Films 315 (1998): 99-103.
- Monteiro OC., et al. “ Use of Dialkyldithiocarbamato Complexes of Bismuth(III) for the Preparation of Nano- and Microsized Bi2S3 Particles and the X-ray Crystal Structures of [Bi{S2CN(CH3)(C6H13)}3] and [Bi{S2CN(CH3)(C6H13)}3(C12H8N2)]”. Chemistry of Materials.13.6 (2001): 2103-2111.
- Ignatious F., et al. “Organobismuth polymers as X-ray contrast materials: synthesis, characterization and properties”. Polymer 33.8 (1992): 1724-1730.
- Delaviz Y., et al. “Homogeneous radiopaque polymers with organobismuth compounds”. Journal of Applied Polymer Science 40(1990): 835-843.
- Ignatious F., et al. “X-Ray Contrast Polymers Containing Miscible Organobismuth Compounds”. Integration of Fundamental Polymer Science and Technology-5 (1991): 400-404.
- Ignatious F., et al. “Organobismuth polymers as X-ray contrast materials: synthesis, characterization and properties”. Polymer 33.8 (1992): 1724-1730.
- “M&T Chemicals Inc., Neth. Pat. Appl. 6405309, 1964”. Chemical abstracts 62 (1965): 16300.
- Marandi F., et al. “A new 3D coordination polymer of bismuth with nicotinic acid N-oxide”. Journal of Chemistry (2013)
- Kricheldorf HR. “Syntheses of Biodegradable and Biocompatible Polymers by Means of Bismuth Catalysts”. Chemical Reviews 109.11 (2009): 5579-5594.
- Jacquel N., et al. “Synthesis and properties of poly (butylene succinate): Efficiency of different transesterification catalysts”. Journal of Polymer Science Part A: Polymer Chemistry 49(2011): 5301-5312.
- Florea AM., et al. “Cisplatin as an Anti-Tumor Drug: Cellular Mechanisms of Activity, Drug Resistance and Induced Side Effects.Cancers”. 3(2011): 1351-1371.
- Piccart M J., et al. “Cisplatin Combined With Carboplatin: A New Way of Intensification of Platinum Dose in the Treatment of Advanced Ovarian Cancer”. Journal of the National Cancer Institute 82.8 (1990): 703-707.
- Levinson AS. “The structure of salvarsan and the arsenic-arsenic double bond”. Journal of Chemical Education 54.2 (1977): 98.
- Marek Konop M., et al. “Certain Aspects of Silver and Silver Nanoparticles in Wound Care: A Minireview”. Journal of Nanomaterials 1 (2016).
- Yeh TF., et al. “Mercury poisoning from mercurochrome therapy of an infected omphalocele”. Clinical Toxicology 13.4 (1978): 463-467.
- Holroyde MJ., et al. “Gastric cytoprotection by bismuth subsalicylate. Gastroenterology”. 86 (1984): 1116A40.
- Konturek S J., et al. “Gastrocytoprotection by colloidal bismuth subcitrate (De-nol) and sucralfate”. Role of endogenous prostaglandins Gut 28 (1987): 201-205.
- Tanaka S., et al. “Gastroprotective effect of ranitidine bismuth citrate is associated with increased mucus bismuth concentration in rats Gut”. 39.2 (1996): 164-167.
- Li W., et al. “Structure of colloidal bismuth subcitrate (CBS) in dilute HCl: unique assembly of bismuth citrate dinuclear units ([Bi (cit)(2)Bi](2-))”. Journal of the American Chemical Society 125(2003): 12408-12409.
- Koo J., et al. “Selective coating of gastric ulcer by tripotassium dicitrato bismuthate in the rat”. Gastroenterology 82 (1982): 864-870.
- Philips RH., et al. “Solubility, absorbtion, and anti-Helicobacter pylori activity of bismuth subnitrate and colloidal bismuth subcitrate: in vitro data do not predict in vivo efficacy”. Helicobacter 5 (2000): 176-182.
- Keogan DM., et al. “Current and potential applications of bismuth-based drugs”. Molecules”19 (2014): 15258-15297.
- Ge R., et al. “The actions of bismuth in the treatment of Helicobacter pylori infections: an update”. Metallomics 4 (2002): 239-243.
- Alkim H., et al. “Role of bismuth in the eradication of Helicobacter pyroli”. American Journal of Therapeutics 24.6 (2017): 751-757.
- Lambert JR., et al. “The actions of bismuth in the treatment of Helicobacter pylori infection”. Alimentary Pharmacology & Therapeutics 11.1 (1997): 27-33.
- Beil W., et al. “Studies on the mechanism of action of colloidal bismuth subcitrate. II. Interaction with pepsin”. Pharmacology 47 (1947):135-140.
- Stables R., et al. “Gastric antisecretory, mucosal protective, anti-pepsin and anti- Helicobacter properties of ranitidine bismuth citrate”. Alimentary Pharmacology & Therapeutics 7(1993): 237-246.
- Wagstaff AJ., et al. “Colloidal bismuth subcitrate. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic use in peptic ulcer disease”. Drugs 36 (1988): 132-157.
- Bader JP. “The safety profile of De-Nol.Digestion”. 37 (1987): 53-59.
- Keogan DM., et al. “Current and potential application of Bismuth based drugs”. Molecules 19 (2014): 15258-15297.
- Sun H. “Biological Chemistry of Arsenic, Antimony and Bismuth”. John Wiley & Sons: Hoboken, NJ, USA (2011).
- Gryboski JD., et al. “Effect of Bismuth Subsalicylate on Chronic Diarrhea in Childhood: A Preliminary Report”. Reviews of Infectious Diseases12.1 (1990): S36-S40.
- Dore MP., et al. “Colloidal bismuth subcitrate-based twice-a-day quadruple therapy as primary or salvage therapy for Helicobacter pylori infection”. The American Journal of Gastroenterology 97(2002): 857-860.
- Kotani T., et al. “Antibacterial properties of some cyclic Organobismuth (III) compounds.Antimicrob”. Agents Chemother 49.7 (2005): 2729-2734.
- Marzano IM., et al. “Crystal structure, antibacterial and cytotoxic activity of a new complex of bismuth (III) with Sulfapyridine”. Molecules 18 (2013): 1464-1676.
- Murafuji T., et al. “Activity of Antifungal Organobismuth(III) Compounds Derived from Alkyl Aryl Ketones against S. cerevisiae: Comparison with a Heterocyclic Bismuth Scaffold Consisting of a Diphenyl Sulfone”. Molecules 19 (2014): 11077-11095.
- Sushma R., et al. “Synthesis and characterization of some tetraorganobismuth(V) aryloxyacetate for their biological screening”. World Research Journal of Applied Medicinal Chemistry 1.2 (2001): 24-27.
- Tiwari VK., et al. “Synthesis, structural and biological screening of some new organobismuth compounds”. International Journal of Drug Discovery 3.2 (2001): 118-122.
- Tripathi DM., et al. “Biomedicinal studies of some novel triorganibismuth (V) compounds”. International Journal of Chemical Research 4.1 (2012): 118-121.
- Soni KK., et al. “Synthesis and characterization of novel organobismuth compounds: Antimicrobial and antitumour studies”. International Journal of Chemical Research 7 (2015): 159-163.
- Tripathi DM., et al. “Antimicrobial and antitumour studies of some organic derivative of bismuth”. World Research Journal of Applied Medicinal Chemistry 1.1 (2001): 01-06.
- Desoize B. “Metals and metal compounds in cancer treatment”. Anticancer research 24 (2004): 1529-1544.
- Tiekink ER. “Antimony and bismuth compounds in oncology”. Critical Reviews in Oncology/Hematology 42 (2002):217-224.
- Fujiwara Y., et al. “An Organobismuth Compound that Exhibits Selective Cytotoxicity to Vascular Endothelial Cells in Vitro”. Journal of health science 51.3 (2015): 333-340.
- Zhang N., et al. “Main group bismuth (III), gallium(III) and diorganotin (IV) complexes derived from bis (2-acetylpyrazine) thiocarbonohydrazone: Synthesis, crystal structures and biological evaluation”. Dalton Trans 43 (2014): 5182-5189.
- Li MX., et al. “Synthesis, crystal structures, in vitro biological evaluation of zinc(II) and bismuth(III) complexes of 2-acetylpyrazine N(4)-phenylthiosemicarbazone Bioorg”. Medicinal Chemistry Letters22 (2012): 2418-2423.
- Oliveira LG., et al. “Antimony( V) and Bismuth(V) Complexes of Lapachol: Synthesis, Crystal Structure and Cytotoxic Activity”. Molecules 16 (2011): 10314-10323.
- Luchi K., et al. “Heterocyclic Organobismuth (III) induces apoptosis of human promyelocytic leukemic cells through activation of caspases and mitochondrial perturbation”. Biochemical Pharmacology76.8 (2008): 974-986.
- Cui L ., et al. “Synthesis, crystal structures, DNA interaction and anticancer activity of Organobismuth (V) complexes”. Inorganica Chimica Acta 437 (2015): 41-46
- Preeti R., et al. “Biomedicinal aspects of group-15 elements (As, Sb, Bi)”. International Journal of Chemical Research 9.1 (2017): 201-208
- Vianna G. “Tratamento da leishmaniose tegumentar por injeções intravenosas de tártaro emético. Presented at 7 Congresso Brasileiro de Medicina Tropical de São Paulo, São Paulo, Brazil (1912).
- Rocha MN., et al. “Cytotoxicity and invitro Antileishmanial Activity of Antimony (V), Bismuth (V), and Tin (IV) Complexes of Lapachol. Bioinorg”. Bioinorganic Chemistry and Applications (2013): 961783-961789.
- Andrews PC., et al. “Bismuth(III) β-thioxoketonates as antibiotics against Helicobacter pylori and as anti-leishmanial agents”. Dalton Trans 43 (2014): 1279-1291.
- Gupta RS., et al. “A review on medicinal plants exhibiting antifertility activity in males”. Natural Product Radiance 5.5 (2006): 389-410.
- Dawara L., et al. “Synthesis, Characterization, and Antimicrobial and Antispermatogenic Activity of Bismuth (III) and Arsenic (III) Derivatives of Biologically Potent Nitrogen and Sulfur Donor Ligands”. International Journal of Inorganic Chemistry (2012).
Citation:
Shivbhadra Singh., et al. “An Overview of Chemical Properties and Pharmacological Importance of Organobismuth
Compounds”. Chronicles of Pharmaceutical Science 2.5 (2018): 668-682.
Copyright: © 2018 Shivbhadra Singh., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.