Research Article
Volume 2 Issue 2 - 2018
Syrian Case Study: Behçet’s Disease Clinical Symptomatologies, Ocular Manifestations, and Treatment
1Department of Basic Sciences, Faculty of Dental Medicine, Damascus University, Damascus, Syria
2Department of Chemistry, Faculty of Medicine, Syrian Private University, Damascus, Syria
2Department of Chemistry, Faculty of Medicine, Syrian Private University, Damascus, Syria
*Corresponding Author: Loai Aljerf, Department of Basic Sciences, Faculty of Dental Medicine, Damascus University, Damascus, Syria.
Received: April 03, 2018; Published: April 10, 2018
Abstract
A case history of Behçet's disease (BD) with its complexities of clinical symptomatology has been presented. A 35 year male from Syria was participated in this study. A review of the patient's manifestations was registered. Nephrolithiasis, which merely be a concurrent contribution, had not been formerly depicted as allied with this syndrome but was observed with this BD patient. Furthermore, during this admission criterion, the patient experienced nephrolithiasis and hydronephrosis of the left kidney. After several days, the patient passed a calculus and a follow-up intravenous pyelogram was reported as normal.
Keywords: Behçet's disease; Clinical symptomatology; Nephrolithiasis; Syria; Cortisone; Chlortetracycline
Introduction
Behçet's syndrome disease (BD) is characterized by recurrent attacks of iridocychtis with hypopyon and aphthous lesions of the mucous membranes and genitalia [1,2]. Occasionally, conjunctivitis or keratitis may be the only ocular manifestation [3]. At other times retinitis with extensive retinal hemorrhages occurs. Other retinal changes include periarteritis, endarteritis, and arteriole narrowing [4].
Roizenblatt., et al. [5] believed the ocular lesions first start in the optic nerve and retina and later involve the uveal tract. The acute attacks may spontaneously resolve within a week; however, recurrent insults to ocular tissues may result in blindness due to optic nerve atrophy or phthisis bulbi. The acute inflammatory process of the retinal venules was described by Uysal., et al. [6].
A small venule in the periphery (of the fundus) begins to show signs of involvement. The central reflex becomes dull, the trunk appears engorged and the adjacent retina is edematous [5]. White lines appear on the two sides and finally the vein takes the form of a white cord. The lesion, little by little, spreads centripetally [5]. Blood continues to flow in the interior of the affected vein. Studies of the visual field show a scotoma in the involved area [7].
It is in these trunks with altered walls that there appears from time to time a sudden complication. This is hemorrhage limited in general to the immediate area of the venule [7]. The more important episodes are accompanied by subjective signs, the patient experiencing visual difficulty. Often while areas of exudation accompany the hemorrhage and when the lesion is an important one, involvement of the vitreous is also present [8]. The picture is that of a thrombophlebitis [9]. Reabsorption begins at once and is quickly completed. The vessel remains visibly obliterated and soon the thrombotic area is bridged by new capillaries supplying the involved sector of the retina [10]. The pathological process observed with the ophthalmoscope consists of a small and insidious phlebitis, with recrudescences, complicated from time to time by a more or less obliterating thrombosis and characterized by a strong tendency on the part of tissues towards repair [11,12].
Visual loss may occur after a short period or after many years. Cases do occur in which ocular inflammation subsides spontaneously without permanent effects [13].
The mucous membrane lesions are seen on the tongue, lips, buccal mucosa, hard and soft palates, and tonsils [14]. They are painful, vary in size, and appear as a central area of necrosis surrounded by erythema [14]. These lesions usually heal without scar formation. Small ulcerations of the vulva or scrotum may also appear. These are painful and may present as indurated nodules or erosions. The bases may be clear or filled with yellowish necrotic material. They usually subside in 10 to 14 days without scarring [14].
Of the other manifestations frequently seen, the most common is erythema nodosum accompanied by a high fever. These nodules are small, few in number, and cyanotic in color. They usually disappear within 20 to 30 days [14].
Kisabay., et al. [15] describe occasional nervous system involvement characterized by headaches, nervousness, hypersensitivity, and signs of meningo-encephalitis. When this occurs, the prognosis is grave since 50% of such persons die. Iseki., et al. [16] described the necropsy findings of two cases. Furunculosis, rheumatic pain, swelling of the ankles, papulopustular dermatitis, orchitis, and phlebitis may be associated with BD [17].
This disease is most commonly observed in early adulthood and is probably caused by a virus [18]. Others have implicated an allergy state, sinusitis, syphilis, tuberculosis, and staphylococcus septicemia as the cause [19]. The incidence appears to be higher in the eastern Mediterranean basin [14].
The diagnosis is largely dependent upon the history and presenting signs. Seizer [20] reported the isolation of a virus from the vitreous and sub retinal fluid of several patients. Takeuchi and his group [21] isolated a virus from the fluid of the anterior chamber. During the acute ulcerative stage of the disease, there is an associated viremia. It was during this stage that Sezar isolated the virus from blood and urine specimens [20]. During this stage the complement fixation level reached its maximum and within three weeks gradually decreased. From this he concluded that relapses were caused by auto reinfection and that immunity was of short duration.
Seider [22] states that "this disease is totally resistant to therapy and progression to blindness is almost the rule." To date, no specific therapy has proved effective in arresting the progress of this disease.
Methods
Patient prioritization and categorization
Patient was male, had been chosen on the basis he was permanent residence in Damascus and non-smoker from non-smoker family [14]. This procedure was reported as consensus findings including further recommendations of the National Committee of Environmental Health Sciences (NCEHS) expert panel. These important conditions were highlighted in the patient’s medical record which also included the glycosylated hemoglobin (HbA1c) data.
Patient was male, had been chosen on the basis he was permanent residence in Damascus and non-smoker from non-smoker family [14]. This procedure was reported as consensus findings including further recommendations of the National Committee of Environmental Health Sciences (NCEHS) expert panel. These important conditions were highlighted in the patient’s medical record which also included the glycosylated hemoglobin (HbA1c) data.
An active BD patient (age: 35 yrs) has visited the Rheumatology & Rehabilitation Unit (RRU) in FD-DU since 1990). The patient had confirmed at least one clinical sign according to the International Study Group for BD (ISG) criteria.
The discrimination between active and inactive patients was confirmed by Behçet Syndrome Activity Score (BSAS). The BSAS scores were between 0 and 100, (0 represented inactive disease).
BD Current Activity Form (BDCAF) or named as Leeds Activity Inventory (range 0-12) was used considering the observed clinical signs and symptoms over a predefined time interval (28 days).
Case study
A 35-year-old white man of Syrian extraction was first admitted to the Rheumatology & Rehabilitation Unit (RRU) in FD-DU, 2015, with the chief complaints of recurrent ulcerations of the mouth, migratory arthralgia’s, and intermittent at attacks of bilateral iritis since 2000. In 2000, the patient was hit in the right eye with a rock and was treated for a corneal abrasion. Following this injury, he noted minimal discharge and irritation of the right eye occurring every three months and lasting three to seven days. It was during that year that he also developed recurrent painful ulcerations of the lips, gums, and buccal mucosa. Originally, the mouth lesions occurred singly but subsequently appeared in crops. Each time the ulcerations responded to topical applications of silver nitrate and gentian violet.
A 35-year-old white man of Syrian extraction was first admitted to the Rheumatology & Rehabilitation Unit (RRU) in FD-DU, 2015, with the chief complaints of recurrent ulcerations of the mouth, migratory arthralgia’s, and intermittent at attacks of bilateral iritis since 2000. In 2000, the patient was hit in the right eye with a rock and was treated for a corneal abrasion. Following this injury, he noted minimal discharge and irritation of the right eye occurring every three months and lasting three to seven days. It was during that year that he also developed recurrent painful ulcerations of the lips, gums, and buccal mucosa. Originally, the mouth lesions occurred singly but subsequently appeared in crops. Each time the ulcerations responded to topical applications of silver nitrate and gentian violet.
The following year while stationed in the Aleppo (second largest city in Syria), the lesions of the oral cavity became severe. At that time, the crops of ulcerations appeared mostly on the tongue and with the healing of one area, new ulcerations appeared. Concomitantly, he experienced pain in the lumbo-sacral region, ankles and knees. These joints, although warm to touch and exhibiting some erythema, revealed no evidence of swelling. Shortly after the onset of joint symptoms, a diffuse pustular rash appeared on the body. During his stay in the Aleppo, the patient developed what was subsequently diagnosed as amoebic dysentery.
Ethics
The study was done in accordance with the Declaration of Helsinki, good clinical practice, and International Organization for Standardization standard 14155. The protocol was approved by the institutional ethics committees of the Faculty of Dental Medicine/Damascus University (IEC-FD-DU) and the Eye Surgical hospital (ESH) in Damascus. A patient provided written informed consent. Completeness and quality of data were assured by 100% source document verification. An independent data monitoring committee adjudicated all adverse clinical events. So, the eligible patient signed his approval to participate in this study and the IEC-FD-DU had provided the participant the ethical clearance and all information regarding risks and benefits of this research. Then, individual’ proforma was prepared to gather adequate information from case sheets of patients including symptomatology and laboratory investigation.
The study was done in accordance with the Declaration of Helsinki, good clinical practice, and International Organization for Standardization standard 14155. The protocol was approved by the institutional ethics committees of the Faculty of Dental Medicine/Damascus University (IEC-FD-DU) and the Eye Surgical hospital (ESH) in Damascus. A patient provided written informed consent. Completeness and quality of data were assured by 100% source document verification. An independent data monitoring committee adjudicated all adverse clinical events. So, the eligible patient signed his approval to participate in this study and the IEC-FD-DU had provided the participant the ethical clearance and all information regarding risks and benefits of this research. Then, individual’ proforma was prepared to gather adequate information from case sheets of patients including symptomatology and laboratory investigation.
Results and Discussion
By early 2002, the oral ulcerations had decreased in severity and frequency but recurred every two to three months. The pustular rash had completely disappeared and the arthralgias were of minimal severity.
In 2005, following service in Aleppo and separation from the service, he suddenly experienced an eruption of flat red isolated blotches affecting the major portion of his body, excepting only his hands, feet, and face. These blotches lasted three months and were associated with swelling and inflammation of the left ankle, left great toe, and right knee. An elevated blood uric acid determination was obtained and the patient was treated for gout. The oral, cutaneous, and joint manifestations continued in varying degrees of severity until 2010. The ocular manifestations until this time were minimal and consisted of a low-grade recurrent iritis.
In 2010, the patient first exhibited aphthous lesions on the glans penis and scrotum. During that year, he noted many black spots floating before his right eye. These lasted one week and would recur every one to two months. This condition progressed until 2012, at which time he was only able to perceive light with his right eye. At that time, he was hospitalized and treated for a sinusitis which was believed to be the cause of his visual difficulties. Other treatment consisted of cortisone ointment and chlortetracycline drops. The ocular manifestations cleared completely within three weeks. One month later, a similar affection of the right eye recurred and was again treated with cortisone. This attack lasted three weeks. In 2013, the diagnosis of gout was reestablished by the presence of an elevated uric acid level and colchicine was started. The patient was unable to tolerate this medication and was then given prednisone. During this year, he again had iritis of the right eye.
In 2014, the diagnosis of rheumatoid arthritis was made elsewhere. Ulcers again appeared on the penis and scrotum. These were associated with marked pain, swelling, and the presence of right testicular nodules. The ulcers were extremely painful and recurred every six months. Late in 2014, the patient noted black spots before his left eye. These became more severe and he was treated with topical and systemic antibiotics (penicillin and tetracycline), topical and subconjunctival cortisone, prednisone and ACTH. The ocular manifestations became less intense and the vision gradually improved. By September, 2014, vision was: O.D. 20/50; O.S. 20/20.
During childhood, the patient had mumps and diphtheria. In 1996, he had dysuria, possibly due to a gonococcal infection. In 2000, a pilonidal cyst was surgically removed. In 2009, a deviated nasal septum was corrected.
His father died at the age of 77years of arteriosclerotic heart disease, tuberculosis, and ankylosing spondylitis. His mother died at the age of 67 years of diabetes mellitus. Three siblings are living and well, one having had rheumatic fever as a child.
Upon admission to the ESH, Damascus, the significant findings of the head and neck were: Corrected visual acuity: O.D., 20/60, O.S., 20/25. The right eye had a two-plus flare with innumerable cells in the anterior chamber. The right pupil was dilated and fixed from medication. Due to a marked vitreous haze, the right fundus was not visible. The left eye revealed a one-plus flare and a moderate number of cells in the anterior chamber. A mild vitreous haze was noted but the fundus appeared normal.
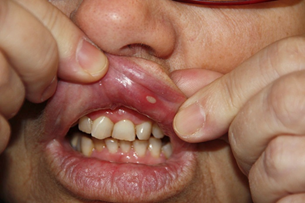
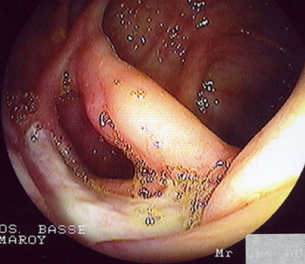
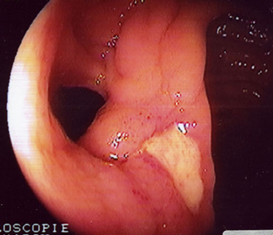
The oral cavity exhibited several aphthous lesions. The lesions had a red base with a grayish white center and were extremely tender to touch (Figures 1-3). They were located on the inner cheek, inner lips, palate and on the under surface of the tongue. The remainder of the examination was negative except for a disseminated popular follicular rash in the nuchal region of the scalp, on the chest, wrists, buttocks, and extensor surfaces of the legs. These lesions had a red base with a vesicular or pustule like center.
The laboratory studies included a complete blood count, urinalysis, stool cultures, lupus cell preparations, liver tests, serology, urine cultures, fasting blood sugar, blood uric acid, C - reactive protein, and an antistreptolysin titer. These studies were essentially negative.
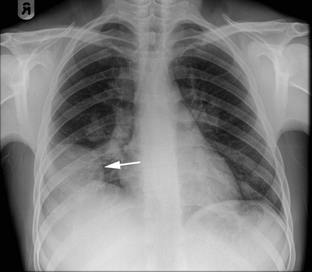
A chest X-ray (Figure 4) examination and intravenous pyelogram were within normal limits. Sinus X-ray films revealed polypoid defects of the right maxillary sinus.
The total circulating eosinophil count was 242 per c.mm. A repeat urine culture revealed coagulase-positive staphylococcus aureus. The skin tests gave negative reactions to histoplasmosis, coccidioidmycosis, brucellosis, and lymph granuloma venereum. The P.P.D. first strength was strongly positive. Staphylococcus skin test 1:100 was negative.
In addition to the topical therapy, he received 20 cc. of gamma globulin weekly intramuscularly. This was started on March 5, 2015. Chlortetracycline (250 mg. four times daily) was given for 10 days. The weekly dosage of gamma globulin was maintained, with rapid improvement of the oral and cutaneous manifestations. These lesions subsided within five days of their appearance. Previously, they had lasted as long as two to three weeks. Since there were continual occurrences of the ocular signs, systemic steroid therapy was started. On April 24, 2015, the electrophoretic pattern was repeated on the patient's serum to evaluate the effects of gamma globulin therapy. The pattern revealed a slight increase in the gamma globulin fraction with the other protein fractions remaining normal.
Oral prednisone was started (5.0 mg. four times daily). There was gradual objective and symptomatic improvement of the ocular signs and the dosage of prednisone were gradually reduced to 5.0 mg. twice daily. By May 25, 2015, both eyes had a visual acuity of 20/20 and there was no longer any evidence of the pre-existing inflammation. The patient was discharged from the ESH on July 10, 2015.
He remained completely asymptomatic on 5.0 mg. of prednisone twice daily and gamma globulin (10 cc. twice weekly). On October 23, 2015, the dosage of gamma globulin was decreased to 5.0 cc. twice weekly and seven days later the patient returned with an acute pan uveitis of the left eye. The dosage of prednisone was then increased to 5.0 mg. four times daily. Topical steroids and mydriatics were utilized and by November 27, 2015, the eye was clear.
On December 11th, several new aphthous ulcers were noted and on December 16th, the patient was readmitted to this hospital and viral studies were performed on blood, urine and fluid aspirated from the anterior chamber. These cultures were reported as negative.
Ophthalmic examination revealed two plus cells in the anterior chamber of both eyes. Several aphthous mouth ulcers were present (Figures 2 and 3). The extremities exhibited minimal swelling and warmth of the left ankle. Small lumps were noted on the pretibial region of the right leg. These had appeared intermittently for the past two years, suggesting the diagnosis of erythema nodosum. Numerous popular-follicular lesions were, seen on the skin and some appeared to be pustular.
During this admission, the laboratory studies were within normal limits. Skin tests for brucellosis and toxoplasmosis were positive. The patient was discharged from the hospital on December 19, 2015, on the previously described medication.
Throughout the ensuing months, he continued to have mild recurrent cutaneous eruptions. His eyes remained asymptomatic. The dosage of gamma globulin was gradually reduced to 5.0 cc. weekly and prednisone was changed to triamcinolone (2.0 mg. daily).
The gamma globulin dosage was maintained; however, hospitalizations were necessitated in May, October, December, 2016, and April, 2017. These recurrences occurred each time the steroid dosage was significantly decreased.
During the April admission, the patient had a recurrence of ocular signs. In addition to this, he first complained of loss of balance and vertigo which had been present for nine months. He also noted a tingling sensation of his fingers. After neurologic consultation, this was attributed to the central nervous system manifestation of BD and vitamin-B12 therapy was suggested. Also, during this admission, the patient experienced nephrolithiasis and hydronephrosis of the left kidney. After several days, the patient passed a calculus and a follow-up intravenous pyelogram was reported as normal.
In May, 2017, the patient was again admitted to the hospital for left flank pain and tenderness. He was treated with colchicine and probe amid. An elevated blood uric acid was not obtained. He was discharged three days later and remained asymptomatic while receiving 5.0 cc. of gamma globulin weekly and 4.0 mg. daily of triamcinolone. On August 12, 2017, the patient was again admitted to the hospital when several papules appeared on his face and neck. The dosage of gamma globulin was increased to 10 cc. weekly. The lesions cleared within two days and he was discharged. The dosage of gamma globulin was gradually decreased to 5.0 cc. weekly and the dosage of triamcinolone to 2.0 mg. daily.
On December 30th, the time of his most recent visit, his visual acuity was: O.D., 20/25; O.S., 20/20. The anterior chamber of both eyes revealed a few cells. The right eye showed early posterior cortical and sub capsular lenticular changes. The vitreous in both eyes exhibited a crinkly cellophane like appearance. The fundi were completely normal. The intraocular pressure and visual fields were also normal.
Conclusion
This case is of interest for the following reasons:
- Although this disease is described as totally resistant to therapy and blindness is almost always the end-result, this patient after 17 years exhibits no permanent ocular changes.
- Negative viral culture studies were obtained during the stage of viremia.
- Nervous system symptomatology, although present for several months, cleared spontaneously without permanently disabling the patient.
- Finally, the use of gamma globulin and systemic corticosteroids may have helped to arrest the progress of this disease.
Disclosure
No external funds and no funders have any role in the study design, in the collection, analysis and interpretation of data, in writing of the report, and in the decision to submit the article for publication. In addition, this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
No external funds and no funders have any role in the study design, in the collection, analysis and interpretation of data, in writing of the report, and in the decision to submit the article for publication. In addition, this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing Interests
The authors declare that they have no competing interests.
The authors declare that they have no competing interests.
Acknowledgement
Many thanks for St. Dr. Rawan AlHalak for her valuable collaboration in medical treatment.
Many thanks for St. Dr. Rawan AlHalak for her valuable collaboration in medical treatment.
References
- Katzenellebogen I. “Recurrent Aphthous Ulceration of Oral Mucous Membrane and Genitals Associated with Recurrent Hypopyon Iritis (Behcet’s Syndrome), Report of Three Cases”. British Journal of Dermatology 58.7-8 (1946): 161-172.
- Pekiner FN., et al. “Interleukin-2, Interleukin-6 and T Regulatory Cells in Peripheral Blood of Patients with Behçet’s Disease and Recurrent Aphthous Ulcerations”. Journal of Oral Pathology & Medicine 41.1 (2011): 73-79.
- Ji YS and Yoon KC. “A Rare Case of Peripheral Ulcerative Keratitis Associated with Behçet’s Disease”. International Ophthalmology 34.4 (2014): 979-981.
- Chan CC., et al. “Anti-retinal Auto-antibodies in Vogt-Koyanagi-Harada Syndrome, Behcet's Disease, and Sympathetic Ophthalmia”. Ophthalmology 92.8 (1985): 1025-1028.
- Roizenblatt M., et al. “Asymptomatic Progression of Ocular Behçet's Disease”. Ophthalmic Surgery, Lasers and Imaging Retina 43.1 (2017): 18-35.
- Uysal H., et al. “Acute Febrile Neutrophilic Dermatosis (Sweet's Syndrome) in Neuro-Behçet's Disease”. Clinical Neurology and Neurosurgery 95.4 (1993): 319-322.
- Wang ZK. “Confusing Untypical Intestinal Behcet’s Disease: Skip Ulcers with Severe Lower Gastrointestinal Hemorrhage”. World Journal of Gastrointestinal Endoscopy 6.1 (2014): 27.
- Hiraiwa T and Yamamoto T. “Superficial Thrombophlebitis Mimicking Cutaneous Polyarteritis Nodosa as an Early and Sole Cutaneous Manifestation of Behçet’s Disease”. Our Dermatology Online 6.2 (2015).
- Alibaz-Oner F., et al. “Post-Thrombotic Syndrome and Venous Disease-Specific Quality of Life in Patients with Vascular Behçet's Disease”. Journal of Vascular Surgery: Venous and Lymphatic Disorders 4.3 (2016): 301-306.
- Vandergheynst F., et al. “Superior Vena Cava Syndrome without Thrombosis Revealing Behçet's Disease: Two Cases”. Joint Bone Spine 75.3 (2008): 359-361.
- Aksu T. “Intracardiac Thrombosis and Coronary-to-Pulmonary Artery Fistula with Pulmonary Embolism and Budd-Chiari Syndrome in Behçet's Disease: A Case Report”. Turkish Journal of Rheumatology 28.1 (2013): 54-57.
- Ajili F., et al. “C0158: The Venous Thrombosis in Behçet’s Disease (BD)”. Thrombosis Research 133 (2014): 60-61.
- Onal S., et al. “Clinical Course of Ocular Behçet's Disease in Siblings”. Ocular Immunology and Inflammation 9.2 (2001): 111-124.
- Aljerf L and Alhaffar I. “Salivary Distinctiveness and Modifications in Males with Diabetes and Behçet’s Disease”. Biochemistry Research International 2017 (2017): 1-12.
- Kisabay A., et al. “Characteristics of Headaches in Cases Diagnosed with Behcet's Disease”. Ocular Immunology and Inflammation 333 (2013): 396.
- Iseki E., et al. “Two Necropsy Cases of Chronic Encephalomyelitis: Variants of Neuro-Behcet's Syndrome?” Journal of Neurology, Neurosurgery & Psychiatry 51.8 (1988): 1084-1087.
- Diri E. “Papulopustular Skin Lesions are seen more frequently in Patients with Behcet's Syndrome Who Have Arthritis: A Controlled and Masked Study”. Annals of the Rheumatic Diseases 60.11 (2001): 1074-1076.
- Zheng Z., et al. “Serum Reactivity Against Herpes Simplex Virus Type 1 UL48 Protein in Behçet’s DiseasePatients and a Behçet’s Disease-like Mouse Model”. Acta Dermato Venereologica 95.8 (2015): 952-958.
- Mansur A and Frieri M. “Behçet's Disease versus Behçet's Syndrome with Some Criteria for Systemic Lupus Erythematosus”. Pediatric Asthma, Allergy & Immunology 12.1 (1998): 53-59.
- Sezer FN. “The Isolation of a Virus as the Cause of Behçet's Disease”. American Journal of Ophthalmology 36.3 (1953): 301-315.
- Takeuchi M., et al. “Possibility of Inducing Anterior Chamber-Associated Immune Deviation by TGF-β2 Treatment of Monocytes Isolated from Behçet’s Patients”. Experimental Eye Research 83.4 (2006): 981-988.
- Seider N. “Intravenous Immunoglobulin Therapy for Resistant Ocular Behcet's Disease”. British Journal of Ophthalmology 85.11 (2001): 1287-1288.
Citation:
Loai Aljerf and Nuha AlMasri. “Syrian Case Study: Behçet’s Disease Clinical Symptomatologies, Ocular Manifestations, and
Treatment”. Chronicles of Pharmaceutical Science 2.2 (2018): 502-509.
Copyright: © 2018 Loai Aljerf and Nuha AlMasri. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.