Research Article
Volume 2 Issue 2 - 2018
The Impact of the New Metal-Complex (Zn II) Selenium-Containing Compound πQ2721 on the Resistance of Rats to Acute Hypoxic Hypoxia
1Department of Normal Physiology, Smolensk State Medical University, Russia
2Science Investigation Center, Smolensk State Medical University, Russia
3Department of Pathophysiology, Smolensk State Medical University, Russia
4Department of Biochemistry, Smolensk State Medical University, Russia
2Science Investigation Center, Smolensk State Medical University, Russia
3Department of Pathophysiology, Smolensk State Medical University, Russia
4Department of Biochemistry, Smolensk State Medical University, Russia
*Corresponding Author: Evseev Andrey Victorovich, Department of Normal Physiology, Smolensk State Medical University, Smolensk, Russia.
Received: March 26, 2018; Published: April 07, 2018
Abstract
Background: The aim of the study was to confirm in experiments on rats on the model of acute hypoxia with hypercapnia (AH+Hc) the antihypoxic action of the metal-complex (Zn2+) compound πQ2721, which turned out to be the most effective of 11 selenium-containing substances previously studied in experiments on mice. As substances for comparison were used 2 antihypoxants of aminothiol origin – Amtizole and Sunazole and metal-complex compound πQ1983 with confirmed antihypoxic effect.
Materials and methods: Experiments performed on 182 male rats of Wistar line weighing 150-170g. The study of antihypoxic activity of substances was carried out on the model the AH+Hc. The condition of acute hypoxia in rats was formed by placing them in glass airtight containers with a free volume of 1.0 L. Antihypoxic effect was evaluated by the life expectancy of animals in the described conditions.
Substances πQ2721, πQ1983, Amtizole and Sunazole was administered once intraperitoneally at doses of 25, 50 and 100 mg/kg. Previously each substance was dissolved in 0.9% NaCl (1.0 ml). Testing the effectiveness of the substances on AH+Hc model was carried out after 1h after administration of the substances and after 24 h. Animals of control groups were injected with 1.0 ml of 0.9% NaCl.
In animals exposed to test AH+Hc in 1h after administration were performed measurements of the rectal temperature before the experiment and through 1h after administration, i.e. before AH+Hc. In animals selected for 24-hour observation, rectal temperature was measured before the experiment, and then after 1, 3, 6, 12, 18 and 24 h of observation, after which they were exposed to AH+Hc.
Results: The antihypoxic effect of a selenium-containing substance πQ2721 based on Zn2+ was confirmed in experiments on rats. In a number of substances for comparison the πQ2721 proved himself not only as equally effective. It is found that after 1 h after administration at a dose of 50 mg/kg πQ2721 superior to all studied compounds, including antihypoxant with succinate Sunazole. An important advantage of the new promising antihypoxic agent was the preservation of its action for 24 hours after injection.
Conclusion: In the experiment on rats the antihypoxic effect of πQ2721 was fully confirmed. In a number of substances for comparison substance πQ2721 proved itself not only as equally effective. It is found that after 1 h after πQ2721 administration at a dose of 50 mg/kg it superior to all studied compounds, including Sunazole. An important advantage of the new antihypoxic agent was the preservation of its action for 24 hours.
Keywords: Acute hypoxia; Metal-complex compounds antihypoxants; Rats
Abbreviations: AH+Hc: Acute hypoxia with hypercapnia
Introduction
The problem of pharmacological organism protection from complications caused by sudden oxygen deficiency, despite significant achievements in this field, remains relevant today. The most frequently exposed to acute hypoxia people who have related to extreme activities (Vasin., et al. 1992; Whayne, 2014). Acute hypoxic hypoxia may occur in the operation of aircraft, submarines, in the event of failure of systems that provide supply or regeneration of air inhabited enclosed spaces.
In many studies it is noted that adaptation to acute hypoxia can be carried out by changing the level of activity of various functional systems of the body, and is aimed primarily at the delivery of oxygen to brain cells (Bok., et al. 2017).
It should be noted that under these conditions, the general orientation of adaptation processes does not exclude the possibility of parallel negative reactions. In this regard, as an integral criterion of adaptation of the organism to the lack of oxygen, the indicator of the life expectancy of the organism in the hypoxygenated environment is usually used (Khachatur'yan and Panchenko, 2002).
Many authors assume that an effective way to increase the human survival in conditions of acute hypoxic hypoxia is to limit physical activity that limits consumption of oxygen and substrates for biological oxidation (Levchenkova., et al. 2018; Moore, et al. 2014; Żebrowska., et al. 2018; Lühker., et al. 2018). The decrease in metabolism can also be achieved through the use of pharmacological substances from the class of antihypoxants. In this capacity positively proved themselves derivatives of aminothiols – Amtizole and its succinate modification Sunazole (Levchenkova., et al. 2018). Unfortunately, ready-made dosage forms of these compounds are still not available, which requires further research.
In the last 10 years it became known about high antihypoxic activity of metal-complex compounds containing various endogenous biologically active substances (vitamins, antioxidants, amino acids, etc.) as ligands (de Souza., et al. 2016, Evseev, et al. 2006).
For the first time the synthesis of such compounds has been carried out in Russia E. Parfenov at the end of the XX century, and the substances themselves, marked with laboratory code "πQ", was initially stated by the author as physiologically compatible antioxidants (PCAO) (Parfenov and Zaikov, 2000). In the course of studying of PCAO of various groups besides antihypoxic effect other types of their biological activity were found. However, the antihypoxic effect of metal-complexes was especially noticeable and often surpassed in this respect already known antihypoxants. The main disadvantage of PCAO in their use as antihypoxic agents remained high toxicity (Evseev., et al. 2007).
Nevertheless, during the search of low-toxic metal-complex compounds, it was found that the most successful combination of activity-toxicity give compounds containing as a metal-complexing agent Zn2+, and as a part of ligand (ligands) – selenium. For example, in experiments on mice, the compound πQ2721 at a dose of 50 mg/kg increased the life expectancy of animals in acute hypoxia with hypercapnia (AH+Hc) by almost 3 times, which is 20% higher than the effect of the standard – Amtizole used in the same dose (Evseev., et al. 2017).
It is important to note that often obtained in the experiments on mice, the results of the screening are not reproduced or reproduced to a small extent in larger animals, e.g. in rats. In this regard, the aim of the study was to confirm in experiments on rats antihypoxic action of metal-complex (Zn2+) compound πQ2721, which turned out to be the most effective of 11 selenium-containing substances previously studied in experiments on mice. It was also necessary to compare its activity with the activity of reference compounds – Amtizole and Sunazole, and with antihypoxic effect of the substance πQ1983 studied a few years earlier.
Material and Methods
Design of Study: Experiments performed on 182 male rats of Wistar line weighing 150-170 g. As previously in experiments on mice, the study of antihypoxic activity of substances carried out on the AH+Hc model (Luk'janova, 1990). The condition of acute hypoxia was formed by rats placing in glass airtight containers with a free volume of 1.0 L.
In the described conditions, the life expectancy of animals was an indication of antihypoxic effect. After the appearance of the second agonal breath was recorded the death of rats.
Drugs and their Introduction: During the experiments, rats were injected once intraperitoneally 4 substance, namely πQ2721, πQ1983 (Table 1, Figure 1), Amtizole and Sunazole (Figure 2) at doses of 25, 50 and 100 mg/kg. Each substance was dissolved in 0.9% NaCl (1.0 ml) before injection. Each group included 7 rats. Testing the effectiveness of substances on the AH+Hc model was carried out after 1 h after injection of substances (12 groups) and after 24 h (12 groups). Animals of 2 control groups were injected with 1.0 ml of 0.9% NaCl.
In animals exposed to test by AH+Hc in 1h after injection of substances was carried out measurements of the rectal temperature using electrothermometry, immediately before the start of the experiment and through 1h after injection, i.e., before AH+Hc.
In animals selected for 24-hour observation, rectal temperature was measured just before the experiment, and then after 1, 3, 6, 12, 18 and 24 hours of observation. Then they were exposed to AH+Hc.
| Laboratory code | Ligand L1 |
Ligand L2 |
Base B |
Cation |
| πQ2721 | Diselendipropionic acid | Acetic acid | - | Na |
| πQ1983 | 3-Нydroxy-2- ethyl-6-methylpyridine | - | Dibenzylselenide | - |
Table 1: General characteristics of selenium-containing complex zinc compounds πQ2721 and πQ1983.
Statistical Analysis: Statistical processing of the received data have been carried out with the help of Microsoft Excel 2010 and Statistica 7 application packages. Comparison of the significance of the differences in the results was performed using the nonparametric Wilcoxon criterion. The differences between the compared parameters were considered reliable at p < 0.05.
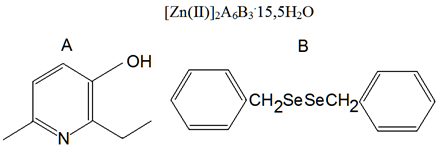
Figure 1: Structural formula of the substance πQ1983 – hexaxis (3-hydroxy-2-ethyl-6-methylpyridine) [tris(dibenzyldiselenid)]dizinc (II)pentadecasemihydrate. A and B – ligands in consist of a complex molecule (Sosin., et al. 2013).
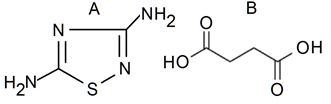
Figure 2: Structural formulae: (А) Amtizole (3,5-diamino-1,2,4-thiadiazolum), (B) succinic acid (ethane-1,2-dicarboxylic acid).
Results
In one way or another in relation to rats, the protective effect demonstrated all included in the study substances was established. The life expectancy of the animals of both control groups was 1 h and 24 h 38.33 ± 3.47 and 35.96 ± 4.08 min respectively, which does not contradict the literature data (Levchenkova., et al. 2018; Shabanov., et al. 2010). Thus practically in all series of experiments with placement of animals in conditions of AH+Hc in 1 h. (Table 2) observed dose-dependent action of substances. At the highest dose (100 mg/kg) most clearly manifested itself Sunazole – Amtizole modified with succinate.
It increases the life expectancy of rats in the conditions of AH+Hc in 2.37 times compared with the control (p < 0.001). The closest in efficiency to Sunazole was πQ2721 metal-complex compound with a result of 1.97 times (p < 0.005). At the same time, at a dose of 50 mg/kg, their protective effect was leveled, and at a dose of 25 mg/kg πQ2721 still had an effect (+17.6%; p < 0.05), while Sunazole lost its activity. It should be noted, both compounds after 1 h after injection reduced rectal temperature to 31.5°C, i.e. 5.5°C lower than in norm be noted.
The relatively modest results demonstrated the substance πQ1983 and antihypoxant Amtizole. At a dose of 100 mg/kg, both tested compounds increased rat life expectancy by an average of 1.7 times, and decreased the rectal temperature by 3.2 and 4.5°C., respectively. Being injected in doses of 50 and 25 mg/kg, they lost their protective effect simultaneously with the ability to cause hypothermia.
Interesting were the results of experiments, which evaluated the possibility of preserving the effect of compounds during the day (table 3). According with the dynamics of rectal temperature (measured 5-fold for 24 h) only the substance πQ2721 after injection at a dose of 100 mg/kg provided the phenomenon of hypothermia in the final part of experiment (-2.2°C), which affected the ability of rats to resist AH+Hc. Life expectancy of animals in this group was 75.38 ± 4.77 min, which is 23% more than the control parameter 35.96 ± 4.08 min (p < 0.05). The effect became statistically insignificant at the lower doses. Other substances in 24 h after injection have been ineffective as protectors of AH+Hc.
| Groups | Dose, mg/kg |
Rectal temperature just before injection (М ± m) |
Rectal temperature in 1h after injection (М ± m) | Temperature difference | Life expectancy, min (М ± m) |
| Control (one group) |
- | 37.0 ± 1.9 | 36.8 ± 1.6 | -0.2 | 38.33 ± 3.47 |
| πQ2721 (three groups) |
25 | 36.7 ± 1.6 | 35.0 ± 1.5 | -1.7 | 50.86 ± 3.42* |
| 50 | 36.6 ± 1.7 | 32.7 ± 1.6** | -3.9 | 62.01 ± 4.12* | |
| 100 | 36.9 ± 1.3 | 31.3 ± 2.0*** | -5.6 | 75.38 ± 4.77** | |
| πQ1983 (three groups) |
25 | 37.1 ± 1.6 | 36.5 ± 1.5 | -0.6 | 45.09 ± 3.03 |
| 50 | 37.0 ± 1.4 | 34.9 ± 1.8* | -2.1 | 53.00 ± 3.52* | |
| 100 | 36.4 ± 1.2 | 33.2 ± 1.6** | -3.2 | 64.18 ± 4.29** | |
| Amtizole (three groups) |
25 | 36.8 ± 1.9 | 36.7 ± 1.5 | -0.1 | 36.65 ± 2.98 |
| 50 | 36.4 ± 1.5 | 35.3 ± 1.4 | -1.1 | 41.27 ± 3.43 | |
| 100 | 36.6 ± 1.5 | 32.1 ± 1.5*** | -4.5 | 65.81 ± 4.26** | |
| Sunazole (three groups) |
25 | 37.0 ± 1.8 | 35.6 ± 1.5 | -1.4 | 43.11 ± 3.75 |
| 50 | 37.0 ± 1.5 | 33.5 ± 1.7** | -3.5 | 54.24 ±3 .85* | |
| 100 | 36.8 ± 1.7 | 31.4 ± 1.6*** | -5.4 | 91.04 ± 5.66*** |
Table 2: Effect of substance πQ2721 and substances for comparison (πQ1983, Amtizole, Sunazole) on the dynamics of rectal temperature and life expectancy of rats undergoes acute hypoxia with hypercapnia in 1h after intraperitoneal injection. There are 7 animals in each group.
Note: *** – p < 0.001; ** – p < 0.005; * – p < 0.05
Note: *** – p < 0.001; ** – p < 0.005; * – p < 0.05
| Groups | Dose, mg/kg |
Rectal temperature just before injection (М ± m) |
Rectal temperature during 24 h after injection | Life expectancy, min (М ± m) |
|||||
| 1h (М ± m) |
3h (М) |
6h (М) |
12h (М) |
18h (М) |
24h (М ± m) |
||||
| Control (one group) |
- | 36.8 ± 1.9 | 36.5 ± 1.3 | 36.6 | 36.6 | 36.5 | 36.4 | 36.5±1.6 | 35.96 ± 4.08 |
| πQ2721 (three groups) |
25 | 37.1 ± 1.5 | 34.5 ± 1.7 | 35.6 | 36.2 | 36.6 | 36.5 | 36.7±1.4 | 38.61 ± 3.69 |
| 50 | 36.6 ± 1.5 | 33.0 ± 1.5 | 33.4 | 34.2 | 35.0 | 35.9 | 36.5±1.5 | 48.43 ± 4.42 | |
| 100 | 36.8 ± 1.7 | 31.6 ± 1.9 | 31.2 | 31.8 | 32.6 | 33.5 | 34.6±1.9 | 55.38 ± 4.72* | |
| πQ1983 (three groups) |
25 | 37.0 ± 1.8 | 36.8 ± 1.9 | 36.6 | 36.5 | 36.6 | 36.7 | 36.6±1.9 | 35.04 ± 3.72 |
| 50 | 37.2 ± 1.5 | 34.2 ± 1.4 | 34.6 | 35.8 | 36.2 | 36.9 | 36.8±1.4 | 38.56 ± 3.24 | |
| 100 | 37.2 ± 1.8 | 32.8 ± 1.4 | 32.5 | 33.3 | 34.9 | 35.6 | 36.3±1.5 | 44.22 ± 3.75 | |
| Amtizole (three groups) |
25 | 36.5 ± 1.4 | 36.2 ± 1.5 | 36.4 | 36.5 | 36.4 | 36.6 | 37.0±1.3 | 40.02 ± 3.50 |
| 50 | 36.8 ± 1.6 | 34.7 ± 1.8 | 34.9 | 35.7 | 36.2 | 36.4 | 36.7±1.6 | 39.18 ± 3.27 | |
| 100 | 37.0 ± 2.0 | 32.6 ± 1.4 | 33.4 | 34.1 | 35.6 | 36.4 | 36.6±1.6 | 38.46 ± 4.09 | |
| Sunazole (three groups) |
25 | 36.7 ± 1.7 | 35.1 ± 1.6 | 35.7 | 36.2 | 36.3 | 36.3 | 36.4±1.8 | 37.33 ± 3.28 |
| 50 | 36.7 ± 1.8 | 34.2 ± 1.8 | 34.6 | 35.8 | 36.5 | 36.8 | 36.7±1.7 | 36.99 ± 3.60 | |
| 100 | 36.9 ± 1.4 | 32.0 ± 1.9 | 32.9 | 33.7 | 34.68 | 35.40 | 36.1±1.4 | 40.60 ± 4.00 | |
Table 3: Effect of substance πQ2721 and substances for comparison (πQ1983, Amtizole, Sunazole) on the dynamics of rectal temperature and life expectancy of rats undergoes acute hypoxia with hypercapnia in 24 h after intraperitoneal injection. There are 7 animals in each group.
Note: * – p < 0.05
Note: * – p < 0.05
Discussion
It is known that primary researches of new pharmacologically active means are usually carried out by a screening method on small rodents – mice, Mongolian gerbils, etc. (Iasnetsov., et al. 2010; O’Neill and Clemens, 2001). However, literature data and our own results previously obtained, say that the desired effect is often detected at a relatively large laboratory animals (rats, rabbits) much weaker. All this causes the researcher disappointment, especially in the case of premature announcement of the discovery in the press.
In this regard, the main objective of this study was to confirm in an experiment on rats the antihypoxic effect of the substance πQ2721 (metal-complex selenium compound with Zn2+ as a metal complexing agent) earlier established in experiments on mice exposed to acute hypoxia with hypercapnia (Evseev., et al. 2017).
Interest in the substance πQ2721 was explained by the fact that the results of many years of work on the study of antihypoxic properties of metal-complexes led the authors to believe that the effectiveness of this kind of compounds is largely due to the presence in the structure of the complex II-valence zinc, and as a ligand (ligands) – biologically active substances containing selenium. In considered case, selenium was integrated in the molecule in the form of Diselendipropionic acid. It should be noted that selenium-containing metallocomplex compounds not only have a brighter pharmacodynamics in comparison with their metal-free analogues, but also often acquire the ability to penetrate the mucous membranes of the gastrointestinal tract, i.e., to be absorbed. The latter is not typical for most known metal-complex compounds and well-known antihypoxants – Mexidol, Amtizole (Sosin., et al. 2012).
Experiments was carried out not only for investigation of the antihypoxic properties of the substance πQ2721, but also to compare its activity with the effect of the already stated as an antihypoxant substance πQ1983, which is a compound of Zn2+ and substituted 3-hydroxypyridine with diorganodihalcogenide – hexaxis(3-hydroxy-2-ethyl-6-methylpyridine) [tris(dibenzyldiselenid)] dizinc (II)pentadecasemihydrate. The substance previously had been tested on mice, rats and cats (Sosin., et al. 2013). Also, we carried out experiments with the injection of substances known as the standards for this kind of experiments Amtizole and Sunazole. All substances were injected intraperitoneally in typical doses for antihypoxants – 25, 50 and 100 mg/kg.
As important part of the study should be considered the second part, in which an attempt was made to assess the effectiveness of the studied substances after 24h from the moment of injection. Typically, researchers monitor the development of antihypoxic effect during 1h after introduction. The data of periodic rectal thermometry were supposed to serve as an indirect confirmation of the activity presence.
As can be seen from the obtained results, the substance πQ2721 in experiments on rats was effective enough to classify it as an antihypoxant. The substance significantly increased the resistance of animals to the effects of AH+Hc, which in varying degrees of severity shown the other substances. The advantages of the new metal-complexes should include 2 undeniable facts: (1) higher activity at a dose of 50 mg/kg in comparison with other agents; (2) preservation of the effect after 24 hours after administration at a dose of 100 mg/kg, as opposed to substances of comparison.
Results of the study make a fresh look at the theory of mechanisms of protective action of pharmacological substances in the formation of acute hypoxic hypoxia. The concept of "optimization" of the dynamics of redox processes in the electron transport chain of mitochondria in conjunction with the limitation of microsomal oxidation in the cells of the body does not stand criticism when it comes to increasing the life expectancy of animals by more than 2 times (Luk'janova, 1997; Zarubina and Shabanov, 2004). Earlier, applications were made about the ability of metal-complex compounds based on Zn2+ to reverse the processes of oxidative phosphorylation on the mitochondrial matrix with a decrease in ATP production in brain tissue (Evseev and Sosin, 2007; Evseev., et al. 2007).
The decrease in animal body temperature by 5°C, and sometimes more, should be considered in favor of the antimetabolic hypothesis of the formation of the antihypoxic effect, which is most likely to provide the studied metal-complex compounds. It is not excluded that antimetabolic effect is the basis of the protective action of antihypoxic derivatives of aminothiol (Amtizole, Sunazole). In the literature there are timid indications about hypoenergy action of Amtizole. However, to break stereotypes on which the concept of "positive" influence of antihypoxants of metabolic action on the energy metabolism of the organism was based (Shabanov., et al. 2010), it seems, will not be easy.
Conclusion
Thus, in experiments on rats, the antihypoxic effect of a selenium-containing substance πQ2721 based on Zn2+ was confirmed. In a number of substances for comparison the πQ2721 proved himself not only as equally effective. It is found that after 1 h after administration at a dose of 50 mg/kg πQ2721 superior to all studied compounds, including antihypoxant with succinate Sunazole. An important advantage of the new promising antihypoxic agent was the preservation of its action for 24 hours after injection.
The obtained results and literature data suggest that the mechanism of the substance πQ2721 action is mainly due to its ability to slow down the speed of metabolic processes that provide energy-synthetic function at the cellular level, which allows the body in conditions of rapidly increasing oxygen deficiency to significantly reduce its consumption and, thereby, successfully resist the rising hypoxic hypoxia.
References
- Ed Luk'janova LD. “Methodical recommendations for a pilot study of drugs proposed for clinical studies as antihypoxic remedies”. Moscow (1990).
- Bok S., et al. “Hypoxia-inducible factor-1α regulates microglial functions affecting neuronal survival in the acutephase of ischemic stroke in mice”. Oncotarget 8.67 (2017): 111508-111521.
- de Souza IC., et al. “ Investigation of cobalt(III)-triazole systems as prototypes for hypoxia-activated drug delivery”. Dalton Transactions 45.35 (2016): 13671-13674.
- Evseev AV and Sosin DV. “On the possible mechanism of protective action of new derivatives of aminothiols in acute exogenous hypoxia” Vestnik novyh medicinskih tehnologij (in Russian). Journal of New Medical Technologies 14.1 (2007): 185-187.
- Evseev AV., et al. “Antihypoxant effect of zinc(II) bis(N-acetyl-L-cysteinato)sulfate octahydrate in acute normobaric hypoxia”. Experimental and Clinical Pharmacology 70.5 (2007): 47-51.
- Evseev AV., et al. “Complex compounds of N-acetyl-L-cysteine with biometals as factors of self-protection of biological systems”. Bulletin of Experimental Biology and Medicine. 142.7 (2006): 26-30.
- Evseev AV., et al. “Testing of new selenium containing metal complex compounds by acute hypoxia-hypercapnia method”. Reviews on Clinical Pharmacology and Drug Therapy 15.4. (2017): 46-52.
- Iasnetsov VV., et al. “Investigation of anti-hypoxic action of 3-hydroxypyridine derivatives in animals with some types of experimental pathology”. Aerospace and Environmental Medicine 44.3 (2010): 57-60.
- Khachatur'yan ML and Panchenko LA. “Seasonal variations in rat resistance to hypoxia”. Bulletin of Experimental Biology and Medicine 133.3 (2002): 300-303.
- Levchenkova OS., et al. “Signal Mechanism of the Protective Effect of Combined Preconditioning by Amtizole and Moderate Hypoxia”. Bulletin of Experimental Biology and Medicine 164.3 (2018): 320-323.
- Lühker O., et al. “Acid-base balance during muscular exercise: response to Dr. Böning and Dr. Maassen”. European Journal of Applied Physiology 118.4 (2018): 865-866.
- Luk'janova LD. “Bioenergy hypoxia: concept, mechanisms and methods of correction”. Bulletin of Experimental Biology and Medicine 124.9 (1997): 244-254.
- Moore CM., et al. “The effects of acute hypoxia and exercise on marksmanship”. Medicine & Science in Sports & Exercise 4.4 (2014): 795-801.
- O’Neill MJ and Clemens JA. “Rodent models of global cerebral ischemia”. Current protocols in neuroscience 9.5 (2001): 1-25.
- Parfenov EA and Zaikov GE. “Biotic Type Antioxidants: The Perspective Search Area of Novel Chemical Drugs”. Utrecht-Boston-Tokyo “PSV” (2000): 559.
- Shabanov PD., et al. “Metabolic regulators of hypoxia”. Ed. Belevitin AB./Sankt-Peterburg: Inform-Novigator (2010).
- Sosin DV., et al. “Antihypoxic remedy”. Patent of Russia Federation N2472503 (2013).
- Sosin DV., et al. “The study of antihypoxic activity of selenium-containing metal-complex substances after parenteral and enteral introduction”. Reviews on Clinical Pharmacology and Drug Therapy 10.3 (2012): 28-34.
- Vasin MV., et al. “Human biochemical status and its relation to body resistance to exposure to acute hypoxic hypoxia”. Aerospace and Environmental Medicine 26.5.6 (1992): 43-49.
- Vladimirov IuA., et al. “Antiradical activity of complex copper compounds (II) on coumarin ligand base”. Bulletin of Experimental Biology and Medicine 113.5 (1992): 479-481.
- Whayne TF Jr. “Cardiovascular medicine at high altitude”. Angiology 65.6 (2014): 459-472.
- Zarubina IV and Shabanov PD. “Molecular pharmacology of antihypoxants”/Sankt-Peterburg: OOO «Izd. N-L» (2004).
- Żebrowska A., et al. “The effect of high intensity physical exercise and hypoxia on glycemia, angiogenic biomarkers and cardiorespiratory function in patients with type 1 diabetes”. Advances in Clinical and Experimental Medicine 27.2 (2018): 207-216.
Citation:
Evseev Andrey Victorovich., et al. “The Impact of the New Metal-Complex (Zn II) Selenium-Containing Compound πQ2721 on
the Resistance of Rats to Acute Hypoxic Hypoxia”. Chronicles of Pharmaceutical Science 2.2 (2018): 493-501.
Copyright: © 2018 Evseev Andrey Victorovich., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.