Review Article
Volume 1 Issue 3 - 2017
Raft Forming Drug Delivery Systems: A Review
Department of Pharmaceutics, Dr Samuel George Institute of Pharmaceutical sciences
*Corresponding Author: Dr Samuel George Institute of Pharmaceutical Sciences, Tarlupadu Road, Markapur, Prakasam (dt), Andhra Pradesh, India.
Received: May 25, 2017; Published: July 15, 2017
Abstract
To build up an oral drug delivery system, it is essential to optimize both release rate of drug and residence time of system within gastrointestinal tract. Controlled release oral drug delivery system to overcome various physiological difficulties such as variation in gastric retention and emptying time. To overcome this drawback and to maximize the oral absorption of various drugs, novel drug delivery systems have been developed. Gastroretentive drug delivery system is facing many challenges which can be overcome by upcoming newly emerging approach i.e. raft forming system. The purpose of writing this review is to focus on recent development of stomach specific floating drug delivery system to circumvent the difficulties associated with formulation design. Various gastroretentive approaches that have been developed till now are also discussed. The present study provides valuable information & highlights advances in this raft forming system.
This review attempts to discuss various factors like physiological factors, physicochemical factors and formulation factors to be considered in the development of the raft forming system. Different types of smart polymers used for their formulation have also been summarized. The review focuses on the mechanism, formulation and development of the raft forming system. This review also summarizes the studies to evaluate the performance and application of these systems. The study finally highlights advantages, disadvantages, and marketed preparation of the raft forming system.
Keywords: Gastroretentive drug delivery system; Raft forming system; Physiological factors
Introduction
Gastric emptying of dosage forms are extremely variable process and ability to prolong the emptying time is a valuable asset for dosage forms, which reside in the stomach for a period of longer time than conventional dosage forms. Drug absorption from the gastrointestinal tract is a complex process and is subject to many changes. It is widely agreed that the extent of gastrointestinal tract drug absorption is related to contact time with the small intestinal mucosa. Thus, small intestinal transit time is an important parameter for drugs that are incompletely absorbed.
Basic human physiology with the details of gastric emptying, motility patterns, and physiological and formulation variables affecting the vast emptying are summarized. Gastro retentive systems are remain in the gastric region for several hours and hence significantly prolong the drug’s gastric residence time. Prolonged gastric retention enhances bioavailability, reduces drug waste, and improves solubility for drugs that are less soluble in a high pH environment.
It is used for local drug delivery to the stomach and proximal small intestines. The extended gastric retention of solid dosage forms may be achieved by the mechanisms of mucoadhesion, flotation, sedimentation, expansion, modified shape systems or by the simultaneous administration of pharmacological agents that delay gastric emptying.
Based on the above approaches, the floating drug delivery systems are
Classification of floating drug delivery systems (FDDS):
Classification of floating drug delivery systems (FDDS):
- In vivo characterization of FDDS.
- In vitro characterization of FDDS.
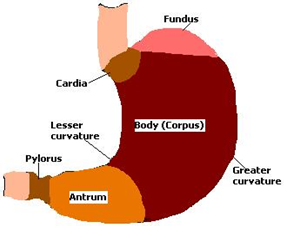
Basic Gastrointestinal Tract Physiology: Anatomically the stomach is divided (Figure1) into 3 regions: fundus, body, and antrum (pylorus). The proximal part made of fundus and body acts as a reservoir for undigested material, whereas the antrum is the main site for mixing motions and act as a pump for gastric emptying by propelling actions. Gastric emptying occurs during fasting as well as fed states.
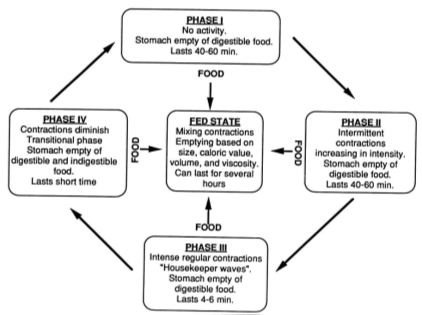
The pattern of motility is however distinct in the 2 states. During the fasting state an inter digestive series of electrical events take place, which cycle both through stomach and intestine every 2 to 3 hours. This is called the inter digestive myloelectric cycle or migrating myloelectric cycle (MMC), which is further divided into following 4 phases as described by Wilson and Washington (Figure2).
- Phase I (basal phase) lasts from 40 to 60 minutes with rare contractions.
- Phase II (preburst phase) lasts for 40 to 60 minutes with achievement action potential and contractions. As the phase progresses the intensity and frequency also increases gradually.
- Phase III (burst phase) lasts for 4 to 6 minutes. It includes intense and regular contractions for short period. It is due to this wave that all the undigested material is swept out of the stomach down to the small intestine. It is also known as the housekeeper wave.
- Phase IV lasts for 0 to 5 minutes and occurs between phases III and I of 2 uninterrupted cycles [2].
After the ingestion of a mixed meal, the pattern of contractions changes from fasted to that of fed state. This is also known as digestive motility pattern and comprises continuous contractions as in phase II of fasted state. These contractions result in reducing the size of food particles (to less than 1 mm), which are propelled toward the pylorus in a suspension form. During the fed state onset of MMC is delayed resulting in slowdown of gastric emptying rate.
Scintigraphic studies determining gastric emptying rates revealed that orally administered controlled release dosage forms are subjected to basically 2 complications, that of short gastric residence time and unpredictable gastric emptying rate.
Factors Affecting Gastric Retention [3]:
- Gastric residence time of an oral dosage form is affected byseveral factors. To pass through the pyloric valve into thesmall intestine the particle size should be in the range of 1to 2 mm.
- The pH of the stomach in fasting state is ~1.5 to2.0 and in fed state is 2.0 to 6.0. A large volume of wateradministered with an oral dosage form raises the pH ofstomach contents to 6.0 to 9.0. Stomach doesn’t get time toproduce sufficient acid when the liquid empties the stomach,hence generally basic drugs have a better chance ofdissolving in fed state than in a fasting state.
- The rate of gastric emptying depends mainly on viscosity,volume, and caloric content of meals.
- Nutritive density ofmeals helps determine gastric emptying time. It does notmake any difference whether the meal has high protein, fat,or carbohydrate content as long as the caloric content is thesame. However, increase in acidity and caloric value slowsdown gastric emptying time. Biological factors such as age,body mass index (BMI), gender, posture, and diseased states(diabetes, Chron’s disease) influence gastric emptying. Inthe case of elderly persons, gastric emptying is slowed down.
- Generally females have slower gastric emptying rates than males. Stress increases gastric emptying rates while depression slows it down. The resting volume of the stomach is 25 to 50 ml. Volume of liquids administered affects the gastric emptying time. When volume is large, the emptying is faster. Fluids taken at body temperature leave the stomach faster than colder or warmer fluids.
- Studies have revealed that gastric emptying of a dosage form in the fed state can also be influenced by its size. Small-size tablets leave the stomach during the digestive phase while the large-size tablets are emptied during the housekeeping waves.
Advantages of Floating drug delivery system [4]:
- The floating drug delivery systems are advantageous for drugs absorbed through the stomach. Eg: Ferrous salts, antacids.
- Acidic substances like aspirin cause irritation on the stomach wall when come in contact with it. Hence HBS formulation may be useful for the administration of aspirin and other similar drugs.
- Administration of prolongs release floating dosage forms, tablet or capsules, will result in dissolution of the drug in the gastric fluid. They dissolve in the gastric fluid would be available for absorption in the small intestine after emptying of the stomach contents. It is therefore expected that a drug will be fully absorbed from floating dosage forms if it remains in the solution form even at the alkaline pH of the intestine.
- The floating drug delivery systems are advantageous for drugs meant for local action in the stomach. Eg. antacids.
- When there is a vigorous intestinal movement and a short transit time as might occur in certain type of diarrhea, poor absorption is expected. Under such circumstances it may be advantageous to keep the drug in floating condition in stomach to get a relatively better response.
Disadvantages of floating drug delivery system [5]:
- Floating system is not feasible for those drugs that have solubility or stability problem in GI tract.
- These systems require a high level of fluid in the stomach for drug delivery to float and work efficiently.
- The drugs that are significantly absorbed through out gastrointestinal tract, which undergo significant first pass metabolism, are only desirable candidate.
- Some drugs present in the floating system causes irritation to gastric mucosa.
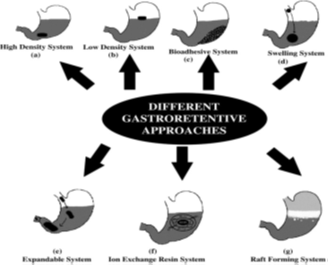
Different gastroretentive forms [6]:
Many technological approaches have been made to develop a dosage form that can be retained in the stomach. The approaches that have been proposed to increase the retention of an oral dosage form in the upper part of the gastrointestinal tract are described in Figure3.
Many technological approaches have been made to develop a dosage form that can be retained in the stomach. The approaches that have been proposed to increase the retention of an oral dosage form in the upper part of the gastrointestinal tract are described in Figure3.
1. High-density systems/non-floating system
Gastric contents have a density close to water (˜ ≈ 1.004 g/cm3). These systems have density higher than the gastric content. A density close to 2.5 g/cm3 is necessary for significant prolongation of gastric residence time (Figure 3a). Barium sulfate, zinc oxide, iron powder, and titanium dioxide are excipients used to formulate such type of dosage form
Gastric contents have a density close to water (˜ ≈ 1.004 g/cm3). These systems have density higher than the gastric content. A density close to 2.5 g/cm3 is necessary for significant prolongation of gastric residence time (Figure 3a). Barium sulfate, zinc oxide, iron powder, and titanium dioxide are excipients used to formulate such type of dosage form
2. Low density system/floating system
Low density system or floating drug delivery system has bulk density lower than that of the gastric fluid, and thus remain buoyant in the stomach for a prolonged period (Figure 3b). Floating drug delivery system is one of the important approaches to achieve gastric retention to obtain sufficient drug bioavailability. This delivery system is desirable for drugs with an absorption window in the stomach or in the upper small intestine.
Low density system or floating drug delivery system has bulk density lower than that of the gastric fluid, and thus remain buoyant in the stomach for a prolonged period (Figure 3b). Floating drug delivery system is one of the important approaches to achieve gastric retention to obtain sufficient drug bioavailability. This delivery system is desirable for drugs with an absorption window in the stomach or in the upper small intestine.
Based on the mechanism of buoyancy two distinctly different technologies, i.e. non-effervescent and effervescent systems have been utilized for the development of the floating drug delivery system.
iii. Approaches to Design Floating Dosage Forms [7]: The following approaches have been used for the design of floating dosage forms of single- and multiple-unit systems.
a. Single-Unit Dosage Forms: In Low-density approach the globular shells apparently having lower density than that of gastric fluid can be used as a carrier for drug for its controlled release. A buoyant dosage form can also be obtained by using a fluid-filled system that floats in the stomach. In coated shells popcorn, poprice, and polystyrol have been exploited as drug carriers. Sugar polymeric materials such as metha crylic polymer and cellulose acetate phthalate have been used to undercoat these shells. These are further coated with a drug-polymer mixture. The polymer of choice can be either ethyl cellulose or hydroxyl propyl cellulose depending on the type of release desired. Finally, the product floats on the gastric fluid while releasing the drug gradually over a prolonged duration.
Single-unit formulations are associated with problems such as sticking together or being obstructed in the gastrointestinal tract, which may have a potential danger of producing irritation.
b. Multiple-Unit Dosage Forms: The purpose of designing multiple-unit dosage form is to develop a reliable formulation that has all the advantages of a single-unit form and also is devoid of any of the above mentioned disadvantages of single-unit formulations. In pursuit of this endeavor many multiple-unit floatable dosage forms have been designed.
These dosage forms are excluded from the passage of the pyloric sphincter if a diameter of ~12 to 18 mm in their expanded state is exceeded.
Classification of Floating Drug Delivery Systems (FDDS) [8]: Floating drug delivery systems are classified depending on the use of 2 formulation variables: effervescent and non effervescent systems.
1. Effervescent Floating Dosage Forms: These are matrix types of systems prepared with the help of swellable polymers such as methylcellulose and chitosan and various effervescent compounds, Eg: sodium bicarbonate, tartaric acid, and citric acid.
They are formulated in such a way that when in contact with the acidic gastric contents, CO2 is liberated and gets entrapped in swollen hydrocolloids, which provides buoyancy to the dosage forms (Figure 4 & 5).
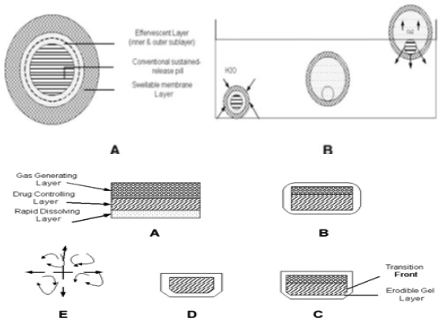
Figure 4: Schematic presentation of working of a triple-layer system. (A) Initial configuration of triple-layer tablet. (B) On contact with the dissolution medium the bismuth layer rapidly dissolves and matrix starts swelling. (C) Tablet swells and erodes. (D) and (E) Tablet erodes completely.
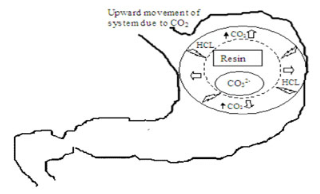
Figure 5: Pictorial presentation of working of effervescent floating drug delivery system based on ion exchange resin.
1.1. Bioadhesive systems [1,9]
Bioadhesive systems are those systems which bind to the gastric epithelial cell surface which serve as a potential means of extending the gastric retention of drug delivery system in the stomach by increasing the intimacy and duration of contact of drug with the biological membrane (Figure 3c). These systems permit a given drug delivery system to be incorporated with a bioadhesive agent. A bioadhesive substance is a natural or synthetic polymer that enables a device to adhere to the stomach and capable of producing an interaction based on hydration mediated, receptor mediated or bonding mediated adhesion with the biological membrane of the gastrointestinal mucosa. Some promising excipients that have been used are carbopol, chitosan, polycarbophil, lectins etc.
Bioadhesive systems are those systems which bind to the gastric epithelial cell surface which serve as a potential means of extending the gastric retention of drug delivery system in the stomach by increasing the intimacy and duration of contact of drug with the biological membrane (Figure 3c). These systems permit a given drug delivery system to be incorporated with a bioadhesive agent. A bioadhesive substance is a natural or synthetic polymer that enables a device to adhere to the stomach and capable of producing an interaction based on hydration mediated, receptor mediated or bonding mediated adhesion with the biological membrane of the gastrointestinal mucosa. Some promising excipients that have been used are carbopol, chitosan, polycarbophil, lectins etc.
1.2. Swelling systems
These are the dosage forms, which after swallowing, swell to an extent that prevents their exit from the pylorus (Figure 3d). As a result, the dosage form retains in the stomach for a longer period of time. These systems may be named as ‘plug type systems’. Sustained and controlled drug release may be achieved by selection of polymer of proper molecular weight and swelling of the polymer retards the drug release.
These are the dosage forms, which after swallowing, swell to an extent that prevents their exit from the pylorus (Figure 3d). As a result, the dosage form retains in the stomach for a longer period of time. These systems may be named as ‘plug type systems’. Sustained and controlled drug release may be achieved by selection of polymer of proper molecular weight and swelling of the polymer retards the drug release.
On coming in contact with gastric fluid, the polymer imbibes water and swells. The extensive swelling of these polymers is due to the presence of physical/chemical cross-links in the hydrophilic polymer network. These cross-links prevent the dissolution of the polymer and hence maintain the physical integrity of the dosage form.
1.3. Expandable systems
A dosage form in the stomach will withstand gastric transit if it is bigger than the pyloric sphincter. Thus, three configurations are required: a small configuration for oral intake, an expanded gastroretentive form and a final small form enabling evacuation following drug release (Figure 3e). Thus, gastroretentivity is improved by the combination of substantial dimension with high rigidity of dosage form to withstand peristalsis and mechanical contractility of the stomach. Unfoldable and swellable systems have been investigated and recently tried to develop an effective gastroretentive drug delivery.
A dosage form in the stomach will withstand gastric transit if it is bigger than the pyloric sphincter. Thus, three configurations are required: a small configuration for oral intake, an expanded gastroretentive form and a final small form enabling evacuation following drug release (Figure 3e). Thus, gastroretentivity is improved by the combination of substantial dimension with high rigidity of dosage form to withstand peristalsis and mechanical contractility of the stomach. Unfoldable and swellable systems have been investigated and recently tried to develop an effective gastroretentive drug delivery.
1.4. Ion exchange resin system
Ion exchange resin system is a system which is formulated to have gastroretentive properties. Ion exchange resin beads are loaded with bicarbonate and a negatively charged drug is bound to the resin (Figure 3f). The resultant beads were then encapsulated in a semipermeable membrane to overcome the rapid loss of carbon dioxide. Upon arrival in the acidic environment of the stomach, an exchange of chloride and bicarbonate ions takes place.
Ion exchange resin system is a system which is formulated to have gastroretentive properties. Ion exchange resin beads are loaded with bicarbonate and a negatively charged drug is bound to the resin (Figure 3f). The resultant beads were then encapsulated in a semipermeable membrane to overcome the rapid loss of carbon dioxide. Upon arrival in the acidic environment of the stomach, an exchange of chloride and bicarbonate ions takes place.
As a result of this reaction carbon dioxide was released and trapped in the membrane thereby carrying beads towards the top of gastric content and producing a floating layer of resin beads in contrast to the uncoated beads, which will sink quickly.
1.5. Raft forming system [1,10]
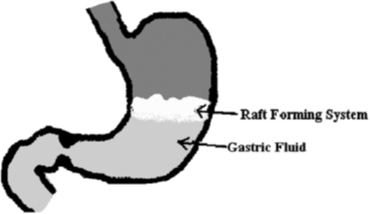
The raft forming system is one of the approaches which involve the formulation of effervescent floating liquid with in situ gelling properties, which has been assessed for sustaining drug delivery and targeting. Moreover, the gels formed in situ remained intact for more than 48h to facilitate sustained release of drugs. The mechanism of the raft forming system involves the formation of continuous layer called a raft. The system involves the formation of a viscous cohesive gel in contact with gastric fluids, wherein each portion of the liquid swells forming a continuous layer called a raft.
The raft forming system is one of the approaches which involve the formulation of effervescent floating liquid with in situ gelling properties, which has been assessed for sustaining drug delivery and targeting. Moreover, the gels formed in situ remained intact for more than 48h to facilitate sustained release of drugs. The mechanism of the raft forming system involves the formation of continuous layer called a raft. The system involves the formation of a viscous cohesive gel in contact with gastric fluids, wherein each portion of the liquid swells forming a continuous layer called a raft.
The layer of the gel floats on the gastric fluid because it has bulk density less than the gastric fluid, as low density is created by the formation of CO2. So the system remains buoyant in the stomach without affecting the gastric emptying rate for a prolonged period of time. The gel formed from in situ gelling, being lighter than gastric fluid, floats over the stomach contents or adheres to the gastric mucosa due to the presence of a bioadhesive nature of the polymer and prevents the reflux of gastric content into the esophagus by acting as a barrier between the stomach and the esophagus (Figure 6). Thus it produces retention of dosage form and increases gastric residence time resulting in prolonged drug delivery in gastrointestinal tract. When the system is floating on the gastric contents, the drug is released slowly at the desired rate from the system. After release of the drug, the residual system is emptied from the stomach. This results in an increased gastro retention time and a better control of the fluctuations in plasma drug concentration.
1.5.1 The design of the raft forming system
The formulation of the raft forming system depends on the physicochemical properties of the drug molecule, the diseased condition for which treatment is required, the patient population and the marketing preference. Physico-chemical factors include molecular weight, lipophilicity and molecular charge; an anatomical and physiological factor includes membrane transport and pH of tissue fluid; formulation factors include pH, gelation temperature, viscosity, osmolarity, and spreadability.
The formulation of the raft forming system depends on the physicochemical properties of the drug molecule, the diseased condition for which treatment is required, the patient population and the marketing preference. Physico-chemical factors include molecular weight, lipophilicity and molecular charge; an anatomical and physiological factor includes membrane transport and pH of tissue fluid; formulation factors include pH, gelation temperature, viscosity, osmolarity, and spreadability.
To achieve the gastric retention of the dosage form, the dosage form must be able to satisfy the following criteria.
- The drug should be released slowly from the system.
- The dosage form must be able to withstand the force exerted by peristaltic waves in the stomach and the constant contractions, grinding and churning moments.
- Should maintain specific gravity lower than gastric contents (1.004–1.01 g/cm3).
- The dosage form must remain in the stomach for a prolonged period of time.
- Better patient compliance.
- Easy for administration for the patient.
- After the release of the drug the device should be easily evacuated from the stomach.
1.5.2 Drugs used for the raft forming system
Raft forming systems have received much attention for the delivery of antacids and drug delivery for gastrointestinal infections and disorders. The raft forming system is the potential approach for heart burn and esophagitis. This system is suitable for acid soluble drugs that are poorly soluble or unstable in intestinal fluids. Various drugs that can be used for the raft forming system are summarized in Table 1 with their category.
Raft forming systems have received much attention for the delivery of antacids and drug delivery for gastrointestinal infections and disorders. The raft forming system is the potential approach for heart burn and esophagitis. This system is suitable for acid soluble drugs that are poorly soluble or unstable in intestinal fluids. Various drugs that can be used for the raft forming system are summarized in Table 1 with their category.
Thus the criteria of the drug to be considered for the selection of the drug for gastro retention are as follows:
- Drugs that are locally active in the stomach.
- Drugs that have narrow absorption window in the gastrointestinal tract.
- Drug that are absorbed from the stomach and upper part of the gastrointestinal tract.
- Drugs that are unstable in the intestinal or colonic environment.
- Drugs that disturb normal colonic microbes.
- Drugs that degrade in the colon.
- Drugs that exhibit low solubility at high pH values (or are poorly soluble at alkaline pH).
| Category | Drugs |
| Proton pump inhibitor | Omeprazole, Lansoprazole, Pantoprazole, Rabeprazole, Esomeprazole |
| H2 receptor antagonist | Cimetidine, Ranitidine, Famotidine, Nizatidine |
| Antacids | Aluminum hydroxide, aluminum phosphate, magnesium silicate, magnesium hydroxide, calcium carbonate. |
| Anti-cholinergic | Oxyphenonium, Propantheline, telezepine |
| Anti-helicobacter | Amoxicillin, Clarithromycin, Tetracycline |
| pylori drugs | Metronidazole, Tinidazole, colloidal bismuth |
Table 1: Drugs that can be used for the raft forming system.
1.5.3 The criteria of the drug that are not suitable for gastric retention are;
- Drugs that have very limited acid solubility.
- Drugs that suffer instability in the gastric environment.
- Drugs intended for selective release in the colon.
1.5.4 Polymer used for formulation
Various polymers are employed in floating drug delivery systems so as to target the delivery of the drug to a specific region in the gastrointestinal tract i.e. stomach. Various natural and synthetic polymers are used in the formulation of the raft forming drug delivery system.
Various polymers are employed in floating drug delivery systems so as to target the delivery of the drug to a specific region in the gastrointestinal tract i.e. stomach. Various natural and synthetic polymers are used in the formulation of the raft forming drug delivery system.
Natural polymer such as alginic acid, guar gum, gellan gum, xyloglucan, pectin, chitosan etc. and synthetic polymer such as poly (DL lactic acid), poly (DL-lactide-co-glycolide) and poly-caprolactone, HPMC etc. are used for formulation development of the raft forming drug delivery system.
A polymer used for in situ gels should have the following characteristics
- It should be biocompatible.
- It should have pseudo plastic behavior.
- The polymer should be capable of increasing the viscosity with increasing the shear rate.
Advantages of floating raft forming gastroretentive drug delivery system:
- Raft forming system forms a low density viscous layer on gastric contents and hence provides more effective surface area than a tablet.
- These lead to more drug release and improve bioavailability.
- Floating obtained faster than the other floating dosage form.
- Improve patient compliance by making a once a day therapy.
- Improve therapeutic efficacy.
- Easy to administer to a patient.
Limitation of floating raft forming gastroretentive drug delivery system:
- These systems are formulated in the form of solution which is more susceptible to stability problems. These are due to chemical degradation (oxidation, hydrolysis, etc.) or microbial degradation.
- The formulation must be stored properly because if the formulation is not stored properly it may cause stability problem. This is due to change in the pH of the system on prolonged storage or on storing inappropriate temperature conditions.
- Exposure of certain polymer to radiations (e.g. UV, Visible, electromagnetic, etc.) induces the formation of gel within the package.
2. Non-Effervescent Floating Dosage Forms [11]: Non-effervescent floating dosage forms use a gel forming or swellable cellulose type of hydrocolloids, polysaccharides, and matrix-forming polymers like polycarbonate, polyacrylate, polymethacrylate, and polystyrene. The formulation method includes a simple approach of thoroughly mixing the drug and the gel-forming hydrocolloid. After oral administration this dosage form swells in contact with gastric fluids and attains a bulk density of GIT. The air entrapped within the swollen matrix imparts buoyancy to the dosage form. The so formed swollen gel-like structure acts as a reservoir and allows sustained release of drug through the gelatinous mass.
2.1. Single layer floating tablets: They are formulated by uniform mixing of a drug with a gel-forming hydrocolloid, which swells in contact with gastric fluid and maintains specific gravity less than one. They are formulated by blending of a drug with low-density enteric materials such as cellulose acetate phthalate and hydroxyl propyl methyl cellulose.
2.2. Bi-layer floating tablets: A bi-layer tablet contains two layers. One is an immediate release layer which releases loading dose from the system while the other is a sustained release layer which releases dose by absorbing gastric fluid, forming an impermeable colloidal gel barrier on its surface, and maintains a specific gravity less than unity and thereby remains buoyant in the stomach.
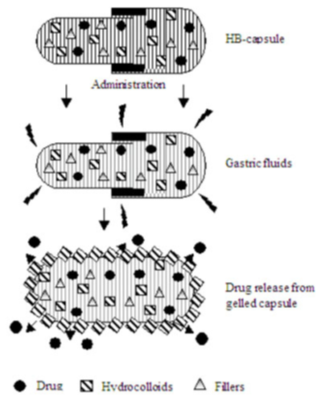
2.3. Hydrodynamically balanced systems: Seth and Tossounian were the first to designate these hydrodynamically balanced systems (Figure 7). These are single-unit dosage forms, containing one or more gelforming hydrophilic polymers. Hydroxypropyl methylcellulose (HPMC) is the most common used excipient, although hydroxyethyl cellulose (HEC), hydroxypropyl cellulose (HPC), sodium carboxymethylcellulose (NaCMC), agar, carrageenan or alginic acid is also used. The polymer ismixedwith a drug and usually administered in a gelatin capsule.
The capsule rapidly dissolves in the gastric fluid, and hydration and swelling of the surface polymers produce a floating mass.
2.4. Microballoons/hollow microspheres: Microballoons/hollow microspheres loaded with drugs in their other polymer were prepared by simple solvent evaporation or solvent diffusion methods to prolong the gastric retention time (GRT) of the dosage form. Commonly used polymers to develop these systems are polycarbonate, cellulose acetate, calcium alginate, Eudragit S, agar, low methoxylated pectin etc. Buoyancy and drug release from dosage form are dependent on quantity of polymers, the plasticizer polymer ratio and the solvent used for formulation
There are several commercial products available based on the research activity of floating drug delivery [2,12] (Table 2).
| Brand Name | API | Dosage form |
| MODAPAR | Levodopa, benserazide | Floating controlled release capsule |
| PROLOPA | Levodopa, benserazide | Floating controlled release capsule |
| VALRELEASE | Diazepam | Floating capsule |
| LIQUID GAVISON | Aluminium hydroxide, Magnesium carbonate |
Effervescent float liquid alginate preparation |
| TOPALKAN | Aluminium-Magnesium antacid |
Floating liquid alginate preparation |
| ALMAGATE FLATCOAT | Aluminium-Magnesium antacid | Floating dosage form |
| CONVIRON | Ferrous sulfate | Colloidal gel forming FDDS |
| CIFRAN | Ciprofloxacin | Gas-generating floating tablets |
| OFLIN OD | Ofloxacin | Gas-generating floating tablets |
| CYTOTEC | Misoprosta | Bilayer floating capsule |
Table 2: Marketed preparations of Floating Drug Delivery System.
II. Evaluation of floating drug delivery systems: Various parameters that need to be evaluated in gastro-retentive formulations include floating duration, dissolution profiles, specific gravity, content uniformity, hardness, and friability in case of solid dosage forms. In the case of multi particulate drug delivery systems, differential scanning calorimetry (DSC), particle size analysis, flow properties, surface morphology, mechanical properties and X-ray diffraction studies are also performed. Hardness, and friability in case of solid dosage forms. In the case of multi particulate drug delivery systems, differential scanning calorimetry (DSC), particle size analysis, flow properties, surface morphology, mechanical properties and X-ray diffraction studies are also performed [13,14].
For Single Unit Dosage Forms (ex: tablets)
Floating lag time [15]: In this the time taken by the tablet to emerge onto the surface of dissolution medium and is expressed in seconds or minutes.
Floating lag time [15]: In this the time taken by the tablet to emerge onto the surface of dissolution medium and is expressed in seconds or minutes.
In-vitro drug release and duration of floating: This is determined by using USP II apparatus (paddle) stirring at a speed of 50 or 100 rpm at 37°C in simulated gastric fluid (pH 1.2 without pepsin). Aliquots of the samples are collected and analyzed for the drug content. The time (h) for which the tablets remain buoyant on the surface of the dissolution medium is the duration of floating and is visually observed.
In vivo evaluation for gastro-retention [1,16]: This is carried out by means of X-ray or Gamma scintigraphic monitoring of the dosage form transition in the GIT. Tablets hardness, weight variation, etc are also evaluated.
Gastroscopy: Gastroscopy is peroral endoscopy used with fiber optics or video systems. Gastroscopy is used to inspect visually the effect of dosage form for prolongation in stomach.
Magnetic marker monitoring: In this technique, dosage form is magnetically marked with incorporating iron powder inside the dosage form. Image of the dosage form can be taken by very sensitive bio-magnetic measurement equipment. Advantage of this method is that it is radiation less and thus not too much hazardous
For Multiple Unit Dosage Forms [17] (ex: microspheres): From In vitro release, floating duration and in vivo gastro-retention tests, multiple unit dosage forms are also evaluated for
Morphological and dimensional analysis: It is done with the help scanning electron microscopy (SEM). Optical microscope can also be used for measuring size.
% yield of microspheres: the yield of microspheres was calculated from the formula: Weight of microspheres obtained ×100/total weight of drug and polymer.
Entrapment efficiency: By a suitable method the drug is extracted, analyzed and calculated from: Practical amount of drug present ×100/Theoretical drug content.
In vitro floating ability (Buoyancy %): A known quantity of microspheres are spread over the surface of a USP (Type II) dissolution apparatus filled with 900 ml of 0.1 N HCl containing 0.002% v/v Tween 80 and agitated for 12 hours at 100 rpm. when 12 hours are completed, the floating and settled layers are separated, dried in a desiccator and weighed.
The buoyancy is calculated from the following formula
Buoyancy (%) = Wf/(Wf + Ws) × 100
Wf- Weights of floating and Ws- Settled microsphere.
Buoyancy (%) = Wf/(Wf + Ws) × 100
Wf- Weights of floating and Ws- Settled microsphere.
Drug-excipient (DE) interactions: FTIR is used for this type evaluation. The DE interaction is indicated by new peak appearance or disappearance of original drug or excipient peak. From the mentioned evaluation parameters, the effect of ageing on granules is also evaluated with the help of Differential Scanning Calorimeter or Hot stage polarizing microscopy.
III. Characterization Parameters
Size and shape evaluation [18]: Determining solubility rate of the drugs and its bioavailability the particle size and shape plays a major role. The size of particle of the formulation was determined by using Sieve analysis, Photo analysis, Air elutriation analysis, Optical microscope, Sedimentation techniques, Electro resistance counting methods (Coulter counter), Laser diffraction methods, ultrasound attenuation spectroscopy, etc.
Size and shape evaluation [18]: Determining solubility rate of the drugs and its bioavailability the particle size and shape plays a major role. The size of particle of the formulation was determined by using Sieve analysis, Photo analysis, Air elutriation analysis, Optical microscope, Sedimentation techniques, Electro resistance counting methods (Coulter counter), Laser diffraction methods, ultrasound attenuation spectroscopy, etc.
Floating Properties: Effect of formulation variables on the floating properties of gastric floating drug delivery system was determined by using continuous floating monitoring system and statistical experimental design.
Surface Topography 1: The surface topography and structures were determined using scanning electron microscope (SEM) operated with an 10 KV acceleration voltage, Atomic force microscopy (AFM), Contact angle meter, Contact profiliometer.
Determination of Moisture Content: Karl fisher titration was used for determination of moisture content of the prepared formulations. The other methods used are Thermo gravimetric methods, Vacuum drying, Air oven method, Freeze drying, and Moisture Meters.
Sol–gel transition and gelling time [1,19]: Raft forming system is an effervescent liquid which involves the formation of viscous cohesive gel in contact with gastric fluids. The sol–gel transition temperature may be defined as the temperature at which the phase transition of sol meniscus is first noted when kept in a sample tube at a specific temperature and then heated at a specified rate. Gel formation is indicated by a lack of movement of meniscus on tilting the tube.
Gel strength 1: This is used to determine gelling property of prepared formulation. This parameter can be evaluated using a rheometer. In this test a specified amount of gel is prepared in a beaker, from the sol form. Gel containing beaker is raised at a certain rate, then pushing a probe of rheometer slowly through the gel. The changes in the load on the probe can be measured as a function of depth of immersion of the probe below the gel surface
Viscosity and rheology 1: This is an important parameter to be evaluated for the raft forming system. The viscosity and rheological properties of the polymeric formulations, either in solution or in gel made with artificial tissue fluid (depending upon the route of administrations) were determined with a different viscometer. The viscosity can be determined with Brookfield viscometer or some other type of viscometers such as Ostwald's viscometer.
| S. No | Brand Name | Active ingredient | Company name | Delivery system |
| 1 | Liquid Gaviscon® |
Aluminum hydroxide and magnesium carbonate |
GlaxosMithKline, India |
Effervescent floating liquid alginate preparations [20] |
| 2 | Topalkan® | Aluminum and magnesium mixture |
Pierre fabre drug, France |
Effervescent floating liquid alginate preparation [21] |
| 3 | Almagate Flot-Coat® |
Aluminum and magnesium mixture |
-- | Floating dosage form [22] |
Table 3: Marketed formulation of the raft forming system.
Various commercial formulations of the raft forming system 1 are tabulated in Table 3.
Conclusion
Drug absorption in the gastrointestinal tract is a highly variable procedure and prolonging gastric retention of the dosage form extends the time for drug absorption. FDDS promises to be a potential approach for gastric retention. The gastroretentive drug delivery system is a burgeoning field in which most of the researchers are developing various technological approaches in order to produce the desired gastric retention. Various gastric retention approaches are studied such as floating drug delivery systems, high density system, mucoadhesive system, expandable system, swelling system and ion exchange system each having their own advantages and disadvantages.
Among the various approaches the raft forming system has emerged as an efficient means of enhancing the bioavailability and controlling the delivery of many drugs. It provides stomach specific drug release for longer duration which remains buoyant on the gastric surface. As the system remains in the stomach for longer duration, local action of the drug is increased due to prolonged contact time with the gastric mucosa. Thus leads to less frequent dosing and improved efficiency of treatment. Raft forming system is not only helpful for sustained drug delivery but also convenient for pediatric and geriatric patients. This system is helpful as an alternative of oral solid dosage form with the advantages of liquid dosage form. Sustained and prolonged release of the drug, good stability and bioavailability characteristics make the raft forming system very suitable candidate for gastric retention of the drug. Thus the raft forming system promises to be the potential approach for gastric retention drug delivery system. Due to unpredictability of human GIT development of efficient GRDFs is a real challenge to pharmaceutical technology as the drug delivery system must remain for a sufficient time in the stomach which is not compatible with normal physiology.
References
- Vipul D., et al. “Raft forming system-An upcoming approach of gastroretentive drug delivery system”. Journal of Controlled Release 168.2 (2013): 151-165.
- S Arrora., et al. “Floating drug delivery systems: a review”. AAPS PharmSciTech 6.3 (2005): 372-390.
- J Chen., et al. “Gastric retention properties of super porous hydrogel composites”. Journal of Controlled Release 64 1-3 (2000): 39-51.
- J Chen and K Park. “Synthesis and characterization of superporous hydrogel composites”. Journal of Controlled Release 65.1-2 (2000): 73-82.
- R Groning and M Berntgen. “Estimation of the gastric residence time of magnetic dosage forms using the Heidelberg capsule”. Pharmazie 51.5 (1996): 328-331.
- R Groning., et al. “Acyclovir serumconcentrations following peroral administration ofmagnetic depot tablets and the influence of extracorporal magnets to control gastrointestinal transit”. European Journal of Pharmaceutics and Biopharmaceutics 46.3 (1998): 285-291.
- Howida Kamal Ibrahim. “A novel liquid effervescent floating delivery system for sustained drug delivery”. Drug Discoveries & Therapeutics 3.4 (2009): 168-175.
- A Padmasri and S Sandhya. “Approaches for gastroretentive drug delivery systems”. International Journal of Applied Biology and Pharmaceutical 1.2 (2010): 589–601.
- Kumar., et al. “Gastroretentive drug delivery systems: a review”. Asian Journal of Pharmaceutical and Clinical Research 3.1 (2010): 2-10.
- S Sarasija and B Shyamala. “Nasal drug delivery: an overview”. Indian Journal of Pharmaceutical Sciences 6.7 (2005): 19-25.
- RS Paterson., et al. “An assessment of floating raft formation in man using magnetic resonance imaging (MRI)”. Journal of Pharmacy and Pharmacology 8.S2 (2000).
- AK Nayak and B Das. “Gastroretentive drug delivery systems: a review”. Asian Journal of Pharmaceutical and Clinical Research 3.1 (2010): 2-10.
- P S Rajinikanth., et al. “Development and evaluation of a novel floating in situ gelling system of amoxicillin for eradication of Helecobacter pylori”. International Journal of Pharmaceutics 335.1-2 (2007): 114-122.
- R Hejazi and MAmiji. “Stomach-specific anti-H. pylori therapy. I: preparation and characterization of tetracycline of a floating multiple-unit capsule, high-density loaded chitosan microspheres”. International Journal of Pharmaceutics 235.1-2 (2002): 87-94.
- W Sawicki. “Pharmacokinetics of verapamil and nor verapamil from controlled release floating pellets in humans”. European Journal of Pharmaceutics and Biopharmaceutics 53.1 (2002): 29-35.
- S Shah and P Upadhyay. “In situ gel: a novel approach of gastroretentive drug delivery”. Asian Journal of Biomedical and Pharmaceutical Sciences 2.8 (2012): 1-8.
- KG Albhal., et al. “Effect of HPMC K4M, HPMC K15M, Sodium alginate and Carbopol 934 in the formulation of carbonyl ion iron capsule”. Scholars Research Library 4.1 (2012): 364-394.
- K Wataru., et al. “In situ gelling pectin formulations for oral sustained delivery of paracetamol”. Drug Development and Industrial Pharmacy 30.6 (2004): 593-599.
- Wichterle and D Lim. “Hydrophilic gel for biological use”. Nature 185 (1960): 117-118.
- N Thakur., et al. “A comprehensive review on floating oral drug delivery system”. Drug Invention Today 2.7 (2010): 328-330.
- A Sayeed., et al. “Gastroretentive drug delivery system: a review”. Pharmacist's Letter 3.1 (2011): 121-137.
- L Bhardwaj., et al. “A short review on gastroretentive formulation for stomach specific drug delivery: special emphasis on floating in situ gel system”. African Journal of Basic & Applied Sciences 3.6 (2011): 300-312.
Citation:
G. Anucharishma., et al. “Raft Forming Drug Delivery Systems: A Review”. Chronicles of Pharmaceutical Science 1.3 (2017):
135-148.
Copyright: © 2017 G. Anucharishma., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.