Thesis
Volume 1 Issue 2 - 2017
Comparitive Studies on Phytochemical Screening, Total Phenolic, Total Flavoid Content and Metal Analysis of Hydroponic (Nft System) and Geoponic Cultivated Spinach
1MLR Institute of pharmacy, Dundigal, Qutbullapur, Ranga Reddy Dist, Hyderabad, TS 500043
2Startech Labs Pvt. Ltd, Madinaguda, Hyderabad, TS-5000050
2Startech Labs Pvt. Ltd, Madinaguda, Hyderabad, TS-5000050
*Corresponding Author: Chandaka Madhu M. Pharm., (Ph.D), Associate Professor, Department of pharmacology, MLR Institute of Pharmacy, Hyderabad.
Received: March 22, 2017; Published: April 29, 2017
| S. No | Contents | Page No |
| 1 | Abstract | 3 |
| 2 | Introduction | 4 |
| 3 | Plant Profile | 9 |
| 4 | Instrumentation | 11 |
| 5 | Aim and Objective | 16 |
| 6 | Literature Review | 17 |
| 7 | Plan of Work | 18 |
| 8 | Method and Methodology | 19 |
| 9 | Results and Discussion | 23 |
| 10 | Conclusion | 34 |
| 11 | Reference | 34 |
Abstract
Today, the agriculture is the key development in the rise of sedentary human civilization, whereby farming of domesticated species create food surpluses that nurture the development of civilization.
For global food security, the agricultural sector of the world economy must achieve a production level that ensures adequate food supply to feed the increasing population as well as provides raw materials for the industries.
For this reasons, now a day the crops are excessively exposed to chemical /synthetic fertilizers and pesticides for getting more yield within the limit of time for money. By consuming such food material, a large proportion of the population has been suffering from a lot of and disorders because they contain arsenic, mercury, nitrates, and lead more than permitted levels.
In view of health hazards posed by indiscriminate use of chemical fertilizers and pesticides, extremely difficult situations of land and water managements and adverse influences of climatic conditions such as heavy rains, air pollution, water pollution, soil pollution, heavy sun effect) on the quality and quantity of agricultural products , we have panned the cultivation of spinach hydroponically. The objective of present study was to compare the phytochemical constituents of geoponic and hydroponic cultivated spinach and screening for antioxidant activity based on their phenolic and flavonoid contents .Further, how much extent the hydroponic cultivation is influencing the presence of metals by carrying out metal analysis by Inductively Coupled Plasma/Optical Emission Spectrometry.
We have observed that the plant, which was cultivated by hydroponics, have a good growth, and also shows a significate content of phenolic and flavonoid than the geoponics. The most important thing was that the plant (hydroponics) don’t have heavy metals .but geoponics have both the Lead and Arsenic which are very dangerous diseases to the human life.
List of abbreviations: %: percentage; ml : millilitre; μg : micro gram; g : gram; kg: Kilogram; min: minute; sec: seconds; W/v:
weight/volume; V/v: volume/volume; fig: figure; conc.: Concentrated; ppt: precipitated
Introduction
Historically, humans secured food through plants and animal source. Food is any substance [1] consumed to provide nutritional support for an organism. Today, the agriculture is the key development in the rise of sedentary human civilization, whereby farming of domesticated species create food surpluses that nurture the development of civilization.
For global food security, the agricultural sector of the world economy must achieve a production level that ensures adequate food supply to feed the increasing population as well as provides raw materials for the industries.
For this reasons, now a day the crops are excessively exposed to chemical/synthetic fertilizers and pesticides for getting more yield within the limit of time for money. By consuming such food material, a large proportion of the population has been suffering from a lot of diseases and disorders because they contain Arsenic, Mercury, Nitrates, and Lead more than permitted levels.
Pesticides may cause acute and delayed health effects in people who are exposed [3]. Their exposure can cause a variety of adverse health effects, ranging from simple irritation of the skin and eyes to more severe effects such as affecting the nervous system, mimicking hormones causing reproductive problems, and causing cancer [4]. A 2007 systematic review found that "most studies on non-Hodgkin lymphoma and leukemia showed positive associations with pesticide exposure" and thus concluded that cosmetic use of pesticides should be decreased [5]. There is substantial evidence of associations between organophosphate insecticide exposures and neurobehavioral alterations [6-9]. Limited evidence also exists for other negative outcomes from pesticide exposure including neurological, birth defects, and fetal death. [10]
Pesticide use raises a number of environmental concerns also. Over 98% of sprayed insecticides and 95% of herbicides reach a destination other than their target species, including non-target species, air, water and soil. [12] Pesticide drift occurs when pesticides suspended in the air as particles are carried by wind to other areas, potentially contaminating them. Pesticides are one of the causes of water pollution, and some pesticides are persistent organic pollutants and contribute to soil contamination.
The World Health Organization and the UN Environment Programme estimate that each year, 3 million workers in agriculture in the developing world experience severe poisoning from pesticides, about 18,000 of whom die. Owing to inadequate regulation and safety precautions, 99% of pesticide related deaths occur in developing countries that account for only 25% of pesticide usage.
In developing countries, there is no thorough control over the usage of pesticides. For example, more than 60 pesticides banned in other countries in use in India. To avoid this problem food safety and food security are monitored by agencies like the International Association for Food Protection, World Resources Institute, World Food. They address issues such as sustainability, biological diversity, climate change, nutritional economics, population growth, water supply, and access to food.)Programme, Food and Agriculture Organization, and International Food Information Council.
Some countries follow the International Maximum Residue Limits-Codex Alimentarius to define the residue limits; this was established by Food and Agriculture Organization of the United Nations (FAO) and World Health Organization (WHO) in 1963 to develop international food standards, guidelines codes of practices, and recommendation for food safety.
Apart from these problems, the other major problems of cultivation are availability of vast land, water supply/scarcity, shortage of labour etc., to avoid indiscriminate usage of pesticides and chemical fertilizers, and water and land problem in conventional cultivation, we have thought of some alternative method of cultivation, which is viable and effective. Therefore, we have contemplated to select a technique called as hydroponics.
Hydroponics is a subset of hydroculture, the method of growing plants without soil, using mineral nutrient solutions in a water solvent. Terrestrial plants may be grown with only their roots exposed to the mineral solution, or the roots may be supported by an inert medium, such as perlite or gravel.
The cultivation of plants by using hydroponics techniques such as Wicks system, Water culture, Ebb and Flow system, Drip system, Nutrient Flim Technique, Aeroponics system. Substrates are also used for support the plant to stay in the hydroponics system. Some of the substrates used in hydroponics were following Grow Stones, Coir Peat,Rice Husks,Perlite, Vermiculite, Pumice, Sand, Gravel, Wood Fibre, Sheep Wool, Rock Wool, Brick Shards, Polystyrene Packing Peanuts Selection of the medium is an important parameter. Different media are appropriate for different growing techniques
Conditions to be maintained in hydroponics technique: All plants need the same basics to survive and grow healthily. In soilless gardening there are definite factors to consider achieving optimal hydroponic growing conditions. These are generally the same basics as with soil-based gardening. However, there are a few specifics of hydroponic growing conditions that all beginners should be aware of before getting started.
Six considerations for the optimum conditions of growth in hydroponics are Water, light, Nutrients, Temperature, oxygen, Structure/Support:
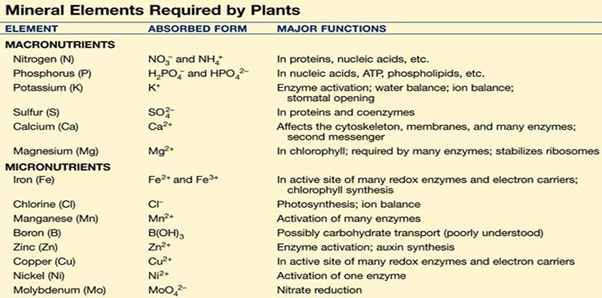
List of nutrition required and its uses:
Advantages of Hydroponic
- Plants can be grown anywhere as long as their growth requirements are met.
- It uses only 1/20th of water compared to traditional (soil based) gardening.
- It provides a sterile environment for plant production. This technique does not require pesticides, fertilizers and other chemicals, as there is no chance of damage due to soil-borne diseases or pests.
- Crops grow two times faster in hydroponic gardening. It provides controlled environment, and yield is doubled leading to more production from same amount of space.
- It needs 20% of less space in comparison to soil-based gardens, as plants with small roots can be grown closer to each other.
- Run-off in traditional gardening can lead to environment degradation due to high proportion of calcium, phosphorous and potassium content dissolved in it. But in hydroponic systems; water can be reused multiple times leading to water conservation with less expense incurred on it.
- There is no-doubt in the fact that hydroponics involves less labor. Upkeep is also minimal.
- It is simple to get complete control over nutrient balance by using solutions like Olivia’s Growing Solution.
- There are no soil setup and testing hassles.
- Plants grown through this technique are healthy and have better nutritional value. It has been proved that vitamin content is 50% more in hydroponically grown plants as compared to conventional ones.
- It is easy to harvest in this type of gardening.
- There are no worries about the changing seasons, as crops can be grown all year round.
- Hydroponic gardening is amazingly stress-relieving and a relaxing hobby. Moreover, it is a great way to spend quality family time.
Disadvantages of Hydroponic Gardening
- Initial set up cost of hydroponic system is high. It requires constant supervision.
- These gardens can also become susceptible to power outage; in this case plants will dry out. If this ever happens, you have to manually water your garden.
- Water-based microorganism can be easily introduced.
- Technical knowledge is required for growing plants through hydroponics.
Few Plants Which Grown By Hydroponics
Lettuce, Tomatoes, Radishes, Celery, Cucumbers, Watermelon, Cantaloupe (Netted Melons) Strawberries, Blueberries, Grapes, Chives, Oregano, Basil, Sage, Rosemary
Lettuce, Tomatoes, Radishes, Celery, Cucumbers, Watermelon, Cantaloupe (Netted Melons) Strawberries, Blueberries, Grapes, Chives, Oregano, Basil, Sage, Rosemary
Plant Profile
Kingdom: Plante
Unranked: Angiosperms
Unranked: Eudicots
Order: Caryophyllales
Family: Amaranthaceae
Subfamily: Chenopodioideae
Genus: Spinacia
Species: S. Oleracea
Kingdom: Plante
Unranked: Angiosperms
Unranked: Eudicots
Order: Caryophyllales
Family: Amaranthaceae
Subfamily: Chenopodioideae
Genus: Spinacia
Species: S. Oleracea
Botanical source: Spinach (Spinaciaoleracea) is an edible flowering plant in the family Amaranthaceae native to central and western Asia. Its leaves are eaten as a vegetable.
Description
It is an annual plant (rarely biennial) growing to 30 cm (12 in) tall. Spinach may survive over winter in temperate regions. The leaves are alternate, simple, ovate to triangular, and very variable in size from about 2-30 cm (1-12 in) long and 1-15 cm (0.4-5.9 in) broad, with larger leaves at the base of the plant and small leaves higher on the flowering stem. The flowers are inconspicuous, yellow-green, 3-4 mm (0.1–0.2 in) in diameter, maturing into a small, hard, dry, lumpy fruit cluster 5-10 mm (0.2-0.4 in) across containing several seeds.
It is an annual plant (rarely biennial) growing to 30 cm (12 in) tall. Spinach may survive over winter in temperate regions. The leaves are alternate, simple, ovate to triangular, and very variable in size from about 2-30 cm (1-12 in) long and 1-15 cm (0.4-5.9 in) broad, with larger leaves at the base of the plant and small leaves higher on the flowering stem. The flowers are inconspicuous, yellow-green, 3-4 mm (0.1–0.2 in) in diameter, maturing into a small, hard, dry, lumpy fruit cluster 5-10 mm (0.2-0.4 in) across containing several seeds.
Distribution
Native to South- west Asia; cultivated throughout India
Native to South- west Asia; cultivated throughout India
Cultivation: Harvest spinach when the leaves are tender and big enough to eat. Spinach is ready for picking 40 to 65 days after sowing. Cut spinach leaf by leaf-cut the outer leaves first allowing the inner leaves to grow larger–or cut away the whole plant one inch (2.5 cm) above the soil. Either way, the plant will keep producing new leaves as long as temperatures are cool. Spinach grows best between 60° and 65°F (15°-18°C)-commonly during spring or autumn. Plants commonly flower (bolt) and stop producing when temperatures reach the high 70°sF (21°+C). If temperatures rise into the 80°sF (26°+C), start picking outer leaves immediately; this will briefly delay bolting. Very warm temperatures turn spinach bitter. Overwintered spinach will give you an early spring harvest. Harvest spinach as close to meal time as possible for the best flavor. Cut spinach with a garden scissors or serrated bread knife. Store spinach cold and moist, 32°-40°F (0°-5°C) and 95 percent relative humidity. Place spinach in the refrigerator in a perforated plastic bag in the vegetable crisper section. Spinach will keep in the refrigerator for about 10 days. Spinach that is stored too cold or too long will develop brown spots on the midrib and the leaves will wilt and yellow.
Chemical constituents: Flavonoids: Spinaciaoleracea is very rich in the flavonoids. Various Flvonoids reported to be present Arequerecetin; Myricetin; Kampeferol; Apigenin; Luteolin; Patuletin; Spinacetin; jaceidin; 4’-glu-curonide;5,3’,4’-trihydroxy-3-methoxy-6:7-methylenedioxyflavone-4’-glucuronide; 5,4’-dihydroxy-3.3’-dimethoxy-6:7-methylene dioxyflavone-4’-glu-curonide; 5,4’-dihydroxi-3,3’-dimithoxi-6,7-methylene-dioxi-flavone(C18H14O8.); 3,5,7,3’,4’pentahydroxi-6-methoxiflavone.
Phenolic Compounds: The polyphenols isolated from the plant are para-coumaric acid, ferulic acid, ortho- coumaric acid. Carotinods: Spinach shows presence of different carotinoids like lutein, β-carotene, violaxanthin and 9’-(Z)-neoxanhin Vitamins: Spinaciaoleracea contains high concentration of vitamin A, E, C, and K. and also folic acid, oxalic acid. Minerals: Along with these chemicals various minerals present in the spinach. These are magnesium, manganese, calcium, phosphorus, iron, zink, copper and potash.
Traditional Uses
The plant is sweet, cooling, carminative, laxative, alexipharmic; useful in diseases of blood, brain, asthma, leprosy, biliousness; treatment of urinary calculi, hypoglycemic properties. emollient, wholesome, antipyretic, diuretic, maturant, laxative, digestiblle, anthelmentic, useful in urinary concretion, inflammation of the lungs and the bowels, sore throat, pain in joints, thirst, lumbago, cold and sneezing, sore eye, ring worm scabies, leucoderma, soalding urine, arrestvomiting , biliousness, flatulence. It have been used in the treatment of febrile conditions. The seeds are useful in fevers, leucorrhoea, urinary discharges, lumbago, and diseases of the brain and of the heart (Yunani). Seeds are laxative and cooling. They have been used in the treatment of difficulty in breathing, inflammation of the liver and jaundice. The green plant is given for the urinary calculi.
The plant is sweet, cooling, carminative, laxative, alexipharmic; useful in diseases of blood, brain, asthma, leprosy, biliousness; treatment of urinary calculi, hypoglycemic properties. emollient, wholesome, antipyretic, diuretic, maturant, laxative, digestiblle, anthelmentic, useful in urinary concretion, inflammation of the lungs and the bowels, sore throat, pain in joints, thirst, lumbago, cold and sneezing, sore eye, ring worm scabies, leucoderma, soalding urine, arrestvomiting , biliousness, flatulence. It have been used in the treatment of febrile conditions. The seeds are useful in fevers, leucorrhoea, urinary discharges, lumbago, and diseases of the brain and of the heart (Yunani). Seeds are laxative and cooling. They have been used in the treatment of difficulty in breathing, inflammation of the liver and jaundice. The green plant is given for the urinary calculi.
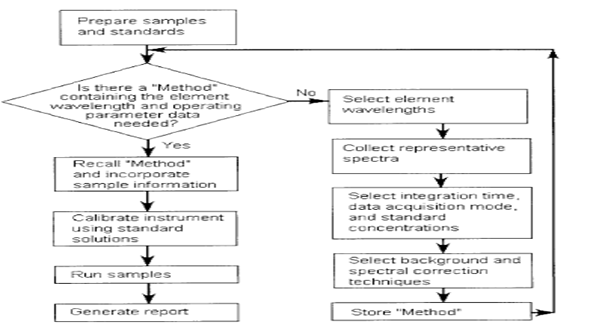
Instrumentation
Inductively Coupled Plasma\Optical Emission Spectroscopy
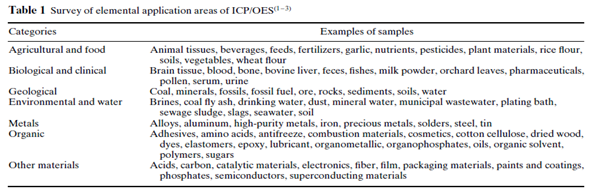
ICP/OES is one of the most powerful and popular analytical tools for the determination of trace elements in a myriad of sample types (Table 1). The technique is based upon the spontaneous emission of photons from atoms and ions that have been excited in a RF discharge. Liquid and gas samples may be injected directly into the instrument, while solid samples require extraction or acid digestion so that the analytes will be present in a solution.
Inductively Coupled Plasma\Optical Emission Spectroscopy
ICP/OES is one of the most powerful and popular analytical tools for the determination of trace elements in a myriad of sample types (Table 1). The technique is based upon the spontaneous emission of photons from atoms and ions that have been excited in a RF discharge. Liquid and gas samples may be injected directly into the instrument, while solid samples require extraction or acid digestion so that the analytes will be present in a solution.
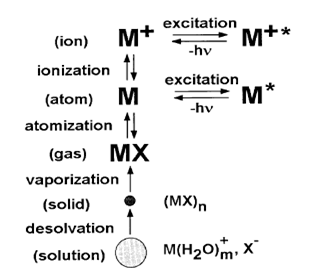
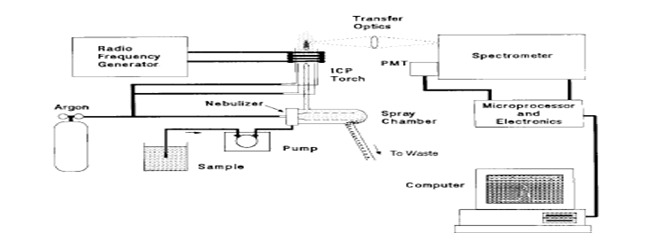
The sample solution is converted to an aerosol and directed into the central channel of the plasma. At its core, the inductively coupled plasma (ICP) sustains a temperature of approximately 10000K, so the aerosol is quickly vaporized. Analyte elements are liberated as free atoms in the gaseous state. Further collisional excitation within the plasma imparts additional energy to the atoms, promoting them to excited states. Sufficient energy is often available to convert the atoms to ions and subsequently promote the ions to excited states. Both the atomic and ionic excited state species may then relax to the ground state via the emission of a photon. These photons have characteristic energies that are determined by the quantized energy level structure for the atoms or ions. Thus, the wavelength of the photons can be used to identify the elements from which they originated. The total number of photons is directly proportional to the concentration of the originating element in the sample. The instrumentation associated with an ICP/OES system is relatively simple. A portion of the photons emitted by the ICP is collected with a lens or a concave mirror. This focusing optic forms an image of the ICP on the entrance aperture of a wavelength selection device such as a monochromator. The particular wavelength exiting the monochromator is converted to an electrical signal by a photo detector. The signal is amplified and processed by the detector electronics, then displayed and stored by a personal computer.
The characteristics of the ICP as an analytical atomic emission source are so impressive that virtually all other emission sources [such as the flame, microwave induced plasma (MIP), direct current plasma (DCP), laser-induced plasma (LIP), and electrical discharge] have been relegated to specific, narrowly defined application niches. Indeed, even much of the application field originally assigned to atomic absorption spectrometry (AAS), using both the flame and graphite furnace atomic absorption spectrometry (GFAAS), has been relinquished to the ICP. Compared to these other techniques, ICP/OES enjoys a higher atomization temperature, a more inert environment, and the natural ability to provide simultaneous determinations for up to 70 elements. This makes the ICP less susceptible to matrix interferences, and better able to correct for them when they occur. In cases where sample volume is not limited, ICP/OES provides detection limits as low as, or lower than its best competitor, GFAAS, for all but a few elements. Even for these elements, the simplicity with which the ICP/OES instrument is operated often outweighs the loss in sensitivity.
Inductively coupled plasma operation
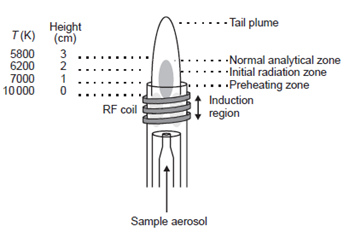
As shown in Figure the so-called ICP ‘‘torch’’ is usually an assembly of three concentric fused-silica tubes. These are frequently referred to as the outer, intermediate, and inner gas tubes. The diameter of the outer tube ranges from 9 to 27 mm. A water-cooled, two- or three-turn copper coil, called the load coil, surrounds the top section of the torch, and is connected to a RF generator. The outer argon flow (10–15 L min1) sustains the high temperature plasma, and positions the plasma relative to the outer walls and the induction coil, preventing the walls from melting and facilitating the observation of emission signals. The plasma under these conditions has an annular shape. The sample aerosol carried by the inner argon flow (0.5-1.5L min1) enters the central channel of the plasma and helps to sustain the shape.
As shown in Figure the so-called ICP ‘‘torch’’ is usually an assembly of three concentric fused-silica tubes. These are frequently referred to as the outer, intermediate, and inner gas tubes. The diameter of the outer tube ranges from 9 to 27 mm. A water-cooled, two- or three-turn copper coil, called the load coil, surrounds the top section of the torch, and is connected to a RF generator. The outer argon flow (10–15 L min1) sustains the high temperature plasma, and positions the plasma relative to the outer walls and the induction coil, preventing the walls from melting and facilitating the observation of emission signals. The plasma under these conditions has an annular shape. The sample aerosol carried by the inner argon flow (0.5-1.5L min1) enters the central channel of the plasma and helps to sustain the shape.
The intermediate argon flow (0-1.5 Lmin1) is optional and has the function of lifting the plasma slightly and diluting the inner gas flow in the presence of organic solvents. The ICP is generated as follows. RF power, typically 700-1500W, is applied to the load coil and an alternating current oscillates inside the coil at a rate corresponding to the frequency of the RF generator. For most ICP/OES instruments, the RF generator has a frequency of either 27 or 40 MHz. The oscillation of the current at this high frequency causes the same high-frequency oscillation of electric and magnetic fields to be set up inside the top of the torch. With argon gas flowing through the torch, a spark from a Tesla coil is used to produce ‘‘seed’’ electrons and ions in the argon gas inside the load coil region. These ions and electrons are then accelerated by the magnetic field, and collide with other argon atoms, causing further ionization in a chain reaction manner. This process continues until a very intense, brilliant white, teardrop shaped, high-temperature plasma is formed. Adding energy to the plasma via RF-induced collision is known as inductive coupling, and thus the plasma is called an ICP. The ICP is sustained within the torch as long as sufficient RF energy is applied. 1/In a cruder sense, the coupling of RF power to the plasma can be visualized as positively charged Ar ions in the plasma gas attempting to follow the negatively charged electrons flowing in the load coil as the flow changes direction 27 million times per second.
The temperature gradient within the ICP with respect to height above the load coil. It also gives the nomenclature for the different zones of the plasma as suggested by Koirtyohann., et al. 7/The induction region (IR) at the base of the plasma is ‘‘doughnut-shaped’’ as described above, and it is the region where the inductive energy transfer occurs. This is also the region of highest temperature and it is characterized by a bright continuum emission. From the IR upward towards to the tail plume, the temperature decreases. An aerosol, or very fine mist of liquid droplets, is generated from a liquid sample by the use of a nebulizer. The aerosol is carried into the center of the plasma by the argon gas flow through the IR. Upon entering the plasma, the droplets undergo three processes. The first step is desolvation, or the removal of the solvent from the droplets, resulting in microscopic solid particulates, or a dry aerosol. The second step is vaporization, or the decomposition of the particles into gaseous state molecules. The third step is atomization, or the breaking of the gaseous molecules into atoms. These steps occur predominantly in the preheating zone (PHZ). Finally, excitation and ionization of the atoms occur, followed by the emission of radiation from these excited species. These excitation and ionization processes occur predominantly in the initial radiation zone (IRZ), and the normal analytical zone (NAZ) from which analytical emission is usually collected. 1/.
Inductively Coupled Plasma Characteristics
The main analytical advantages of the ICP over other excitation sources originate from its capability for efficient and reproducible vaporization, atomization, excitation, and ionization for a wide range of elements in various sample matrices. This is mainly due to the high temperature, 6000-7000K, in the observation zones of the ICP. This temperature is much higher than the maximum temperature of flames or furnaces (3300K). The high temperature of the ICP also makes it capable of exciting refractory elements, and renders it less prone to matrix interferences. Other electrical-discharge-based sources, such as alternating current and direct current arcs and sparks, and the MIP, also have high temperatures for excitation and ionization, but the ICP is typically less noisy and better able to handle liquid samples. In addition, the ICP is an electrode less source, so there is no contamination from the impurities present in an electrode material. Furthermore, it is relatively easy to build an ICP assembly and it is inexpensive, compared to some other sources, such as a LIP. The following is a list of some of the most beneficial characteristics of the ICP source.
The main analytical advantages of the ICP over other excitation sources originate from its capability for efficient and reproducible vaporization, atomization, excitation, and ionization for a wide range of elements in various sample matrices. This is mainly due to the high temperature, 6000-7000K, in the observation zones of the ICP. This temperature is much higher than the maximum temperature of flames or furnaces (3300K). The high temperature of the ICP also makes it capable of exciting refractory elements, and renders it less prone to matrix interferences. Other electrical-discharge-based sources, such as alternating current and direct current arcs and sparks, and the MIP, also have high temperatures for excitation and ionization, but the ICP is typically less noisy and better able to handle liquid samples. In addition, the ICP is an electrode less source, so there is no contamination from the impurities present in an electrode material. Furthermore, it is relatively easy to build an ICP assembly and it is inexpensive, compared to some other sources, such as a LIP. The following is a list of some of the most beneficial characteristics of the ICP source.
High temperature (7000–8000k), high electron density (1014-1016 cm3), appreciable degree of ionization for many elements , simultaneous multi element capability (over 70 elements including p and s) ,Low background emission, and relatively low chemical interference ,.high stability leading to excellent accuracy and precision , .excellent detection limits for most elements (0.1-100 ngml),. Wide linear dynamic range (ldr) (four to six orders of magnitude), applicable to the refractory elements. Cost-effective analyses.
Principle
Instrumentation
Process
Applications
It is mainly used to estimate metals in following felids;
It is mainly used to estimate metals in following felids;
- Agricultural& food
- Biological& clinical
- Geological
- Environmental & water
- Pharma drugs
Aim and objective
Agriculture is the key development in the rise of sedentary human civilization, whereby farming of domesticated species created food surpluses that nurtured the development of civilization. In Modern agronomy, plant breeding, agrochemicals such as pesticides and fertilizers, and technological developments have in many cases sharply increased yields from cultivation, but at the same time have caused widespread ecological damage and negative human health effects. Despite what government agencies and corporations tell you, pesticide products currently on the market are not safe, even when they are used legally. There are many flaws in the way that pesticides are registered and in our political process that allows corporations to influence pesticide policy to allow the continued use of their poisonous products. Since the publication of Rachel Carson’s landmark 1962 book Silent Spring, the impacts of pesticides on the environment have been well known. Pesticides are toxic to living organisms. Some can accumulate in water systems, pollute the air, and in some cases have other dramatic environmental effects. Scientists are discovering new threats to the environment that are equally disturbing.
Agriculture is the key development in the rise of sedentary human civilization, whereby farming of domesticated species created food surpluses that nurtured the development of civilization. In Modern agronomy, plant breeding, agrochemicals such as pesticides and fertilizers, and technological developments have in many cases sharply increased yields from cultivation, but at the same time have caused widespread ecological damage and negative human health effects. Despite what government agencies and corporations tell you, pesticide products currently on the market are not safe, even when they are used legally. There are many flaws in the way that pesticides are registered and in our political process that allows corporations to influence pesticide policy to allow the continued use of their poisonous products. Since the publication of Rachel Carson’s landmark 1962 book Silent Spring, the impacts of pesticides on the environment have been well known. Pesticides are toxic to living organisms. Some can accumulate in water systems, pollute the air, and in some cases have other dramatic environmental effects. Scientists are discovering new threats to the environment that are equally disturbing.
Pesticide use can damage agricultural land by harming beneficial insect species, soil microorganisms, and worms which naturally limit pest populations and maintain soil health; Even if we know that a pesticide causes severe health and environmental impacts, including cancer and genetic damage, it may still be allowed for use. The EPA may determine that a cancer-causing chemical may be used despite its public health hazard if its "economic, social or environmental" benefits are deemed greater than its risk. According to the US EPA, more than 70 active ingredients known to cause cancer in animal tests are allowed for use.
Agricultural food production and water management are increasingly becoming global issues that are fostering debate on a number of fronts. Significant degradation of climatic conditions, land and water resources, including the depletion of aquifers, has been observed in recent decades, and the effects of global warming on agriculture. In view of health hazards posed by indiscriminate use of chemical fertilizers and pesticides, extremely difficult situations of land and water managements and adverse influence of climatic conditions such as heavy rains, air pollution, water pollution, soil pollution, heavy sun effect) on the quality and quantity of agricultural products ,we have panned the cultivation of spinach hydroponically.
The objective of present study was to compare the phytochemical constituents of geoponic and hydroponic cultivated spinach and screening for antioxidant activity based on their phenolic and flavonoid contents .Further, how much extent the hydroponic cultivation is influencing the presence of metals by carrying out metal analysis by Inductively Coupled Plasma/Optical Emission Spectrometry.
Literature Review
About spinach
About spinach
- Bhatia AL and Jain M. “Spinaciaoleracea L. protects against gamma radiations: a study on glutathione and lipid peroxidation in mouse liver”. Phytomedicine 11.7 (2003): 607-615.
- Verma RK., et al. “Role of Spinaciaoleracea as Antioxidant: A Biochemical Study oncMice Brain after Exposure of Gamma Radiation”. Asian Journal of Experimental Sciences 17 (2003): 51-57.
- Grossman S., et al. “The antioxidant activity of aqueous spinach extract: chemical identification of active fractions”. Phytochemistry 58.1 (2001): 143-152.
- Mizushina Y., et al. “Effect of glycolipidfrom spinach on mammalian DNA polymerases”. Biochemical Pharmacology 65 (2003): 259-267.
About hydroponics
- George pattenson, A Brief History of Hydroponics, http://ezinearticles.com. (Lastcited on 2010 Dec 28).
- www.Grodan.com (Last cited on 2010 Dec 28).
- Noucittakehdi, Hydroponics and medicinal plant our research, info@eurohydro.com. (Last cited on 2010 Dec 28).
- Keith Roberto, How to Hydrophonics, 4th edition, The Future garden press 59 (2003).
Plan of work
- Designing and fabricating a mini NFT system
- Collection and purchase of spinach seeds.
- Procurement of hydroponics nutrition medium, mineral water, pots, LED, Metal halide light and chemicals.
- Germination
- Plantation/cultivation of spinach in NFT system
- Study pH, TDS, conductivity, light intensity, humidity and relative humidity for 2 times a day.
- Measurement of the growth of spinach.
- Extraction with Ethanol for both hydropincs and geopinics
- Phytochemical screening
- Estimation of total phenolic and total flavonoid content in the extract
- Estimation of metals by ICPOES.
Method and Methodology
Materials Required
Chemicals Required: Ethanol, Milli Q water, Standard Reagent Bottles 1000ppm, Conc Nitric Acid.
Chemicals Required: Ethanol, Milli Q water, Standard Reagent Bottles 1000ppm, Conc Nitric Acid.
Instruments Required ICPOES, UV spectroscopy, pH & conductivity meter, TDS meter, luxmeter, hygrometer, Heating Mantles, Hot plate
Glassware Required: Beaker (50 ml & 100 ml), Volumetric Flasks (20 ml,25 ml, 50 ml & 100 ml), Glass Rods, Micro Pipettes, Pipettes (1 ml, 5 ml,10 ml), (500 ml) Round Bottom Flask and Condenser.
Micillaneous: Test tube stand, test tube holders, filter paper, butter paper, spatula, thermometers, stands, tissue paper, zip pouches, markers, gloves, labels, cotton swabs, disinfectant etc.
Instrumentation of nft
Cultivation of spinach by hydroponics technique
Germination: the seeds of spinach, vermicompost, cocoa pit and colonies were purchased from local store. The colonies were filled with vermicompost and cocoa pit with the ratio of 30:70. The seed were sown at a depth of 1 cm and it was covered. The water was sprinkled twice a day and the colonies were placed in dark place for a week.
Germination: the seeds of spinach, vermicompost, cocoa pit and colonies were purchased from local store. The colonies were filled with vermicompost and cocoa pit with the ratio of 30:70. The seed were sown at a depth of 1 cm and it was covered. The water was sprinkled twice a day and the colonies were placed in dark place for a week.
Plantation and conditions for nft system
Firstly an young plant of spinach is taken from colonies. The cocoa pit and vermicompost are thoroughly washed in a beaker. After washing the roots clearly, the plant is placed in to a basket and mud rocks are introduced into the basket which helps the plant to stand still in basket. This basket is introduced into the NFT system.
Firstly an young plant of spinach is taken from colonies. The cocoa pit and vermicompost are thoroughly washed in a beaker. After washing the roots clearly, the plant is placed in to a basket and mud rocks are introduced into the basket which helps the plant to stand still in basket. This basket is introduced into the NFT system.
Preparation of powder
After 30 days weeks. The cultivated plant material of spinach (hydroponics) and marketed spinach (geoponics) which was procured from local market were shade dried and then powdered with a mechanical grinder to obtain a coarse powder. The powder was passed through sieve no 40 and was stored in an air tight container until further use. The powder was used for the extraction process.
After 30 days weeks. The cultivated plant material of spinach (hydroponics) and marketed spinach (geoponics) which was procured from local market were shade dried and then powdered with a mechanical grinder to obtain a coarse powder. The powder was passed through sieve no 40 and was stored in an air tight container until further use. The powder was used for the extraction process.
Preparation of alcholic extract
The ethanoic extract of the plant was prepared using hot condensation process. The coarse powders of both hydroponic plant and geoponic plant 200g each were taken in separate round bottom flasks and ethanol was poured until the powder was soaked. Then refluxed it for 8 hrs at 25c. After 8 hrs, the suspension was filtered through a fine muslin cloth.
The ethanoic extract of the plant was prepared using hot condensation process. The coarse powders of both hydroponic plant and geoponic plant 200g each were taken in separate round bottom flasks and ethanol was poured until the powder was soaked. Then refluxed it for 8 hrs at 25c. After 8 hrs, the suspension was filtered through a fine muslin cloth.
| Plant | Light Conditions |
Hid lamp type | Favorable temp |
Ph | Ppm | Tds |
| Spinach | medium | 400/1000W | Cool to warm | 6.0-7.0 | 1260 | 1610 |
The solvent was removed by heating until plant extracts were obtained and then the percentage yields were calculated.
Phytochemical Screening
| S. No | Phytochemical | Name of the Test |
| 1. | Glycosides | Legals test |
| 2 | Saponins | Froth test |
| 3 | Alkaloids | Dragendorffs test |
| 4 | Tannins | Chromic test |
| 5 | Flavoinids | Shinoda test |
| 6 | Mucilage | Ruthenium red test |
| 7 | Carbohydrates | Molisch test |
| 8 | Proteins | Xanthoproteic test |
| 9 | Phytosterols | Salkowski test |
Determination of Total Phenolic Content: [46]
Total Phenolic content of the extract was determined by Folinciocalteau reagent according to Singleton and Rossi using Gallic acid as a standard. The concentration of total phenols was expressed in terms of mg of Gallic Acid equivalents per gram of extract.
Total Phenolic content of the extract was determined by Folinciocalteau reagent according to Singleton and Rossi using Gallic acid as a standard. The concentration of total phenols was expressed in terms of mg of Gallic Acid equivalents per gram of extract.
Plotting of calibration curve
The stock solution of gallic acid (1000 µg/ml) was diluted to get the various concentrations 10,20,30,40,50,60,70 and 80 µg/ml by diluting with distilled water. About 0.5 ml of Folinciocalteau reagent was added to each test tube, mixed thoroughly and incubated for 3 min at room temperature. Later 3 ml of 20% Na2CO3 was added and mixed thoroughly and incubated in boiling water bath for 1 min. The absorbance was measured at 650 nm and the observations are shown in table: 3. The standard plot was drawn (absorbance Vs concentration.) The amount of total phenolic content s was estimated in both Geoponics and Hydroponics extracts of spinach.
The stock solution of gallic acid (1000 µg/ml) was diluted to get the various concentrations 10,20,30,40,50,60,70 and 80 µg/ml by diluting with distilled water. About 0.5 ml of Folinciocalteau reagent was added to each test tube, mixed thoroughly and incubated for 3 min at room temperature. Later 3 ml of 20% Na2CO3 was added and mixed thoroughly and incubated in boiling water bath for 1 min. The absorbance was measured at 650 nm and the observations are shown in table: 3. The standard plot was drawn (absorbance Vs concentration.) The amount of total phenolic content s was estimated in both Geoponics and Hydroponics extracts of spinach.
Determination of Total Flavanoid Content: [47]
Total Flavanoid content was measured by the aluminium chloride colorimetric assay. Standard solution of catechin (20, 40, 60, 80 and 100 µg/ml) was taken in separate 10 ml volumetric flasks containing 4 ml of distilled water. 0.3 ml of 5% NaNO2 was added. After 5 min, 0.3 ml 10% AlCl3 was added. At 6th min, 2 ml of 1M NaOH was added and the total volume was made up to 10 ml with distilled H2O. The solution was mixed well and the absorbance was measured against prepared reagent as blank at 510 nm and recorded in a suitable table. Table No: 4. A standard plot was drawn (absorbance Vs concentration. Figure No:
Total Flavanoid content was measured by the aluminium chloride colorimetric assay. Standard solution of catechin (20, 40, 60, 80 and 100 µg/ml) was taken in separate 10 ml volumetric flasks containing 4 ml of distilled water. 0.3 ml of 5% NaNO2 was added. After 5 min, 0.3 ml 10% AlCl3 was added. At 6th min, 2 ml of 1M NaOH was added and the total volume was made up to 10 ml with distilled H2O. The solution was mixed well and the absorbance was measured against prepared reagent as blank at 510 nm and recorded in a suitable table. Table No: 4. A standard plot was drawn (absorbance Vs concentration. Figure No:
An aliquot (1 ml) of extracts were taken and colour developed in the same procedure and absorbance were measured and the standard plot was drawn (Absorbance Vs concentration.) The amounts of flavonoids are calculated and expressed as mg catechin equivalents (CE)/g of extract from the interpolation of the curve. Samples were analyzed in duplicates.
Procedure for estimation of metals
Preparation of extract sample (acid digestion)
1g of the extract was weighed and transferred into 50 ml of beaker. Then add 5 ml of conc. HNO3 and placed it on hot plate until the organic fumes were completely stopped. Then 25 ml of water was added for acid digestion on hot plate. Digestion to be taken until 50% of the sample was too evaporated and remaining sample was filtered and made up to 25 ml, and subjected to furthered dilutions.
Preparation of extract sample (acid digestion)
1g of the extract was weighed and transferred into 50 ml of beaker. Then add 5 ml of conc. HNO3 and placed it on hot plate until the organic fumes were completely stopped. Then 25 ml of water was added for acid digestion on hot plate. Digestion to be taken until 50% of the sample was too evaporated and remaining sample was filtered and made up to 25 ml, and subjected to furthered dilutions.
Preparation of standard
The standard reagent 1000 ppm was purchased from The National Institute of Standards and Technology (NIST) from Pune. From the standard reagent (1000 ppm) furthered dilutions were made containing 1,2,3 ppm. Blank, Standards (0.5,1,1.5 ppm), Blank and Sample were aspirated into ICPOES.
The standard reagent 1000 ppm was purchased from The National Institute of Standards and Technology (NIST) from Pune. From the standard reagent (1000 ppm) furthered dilutions were made containing 1,2,3 ppm. Blank, Standards (0.5,1,1.5 ppm), Blank and Sample were aspirated into ICPOES.
Caluculation
Results and Discussion
1. Design of nft system
2. Germination For 1 Week in Dark
B. After 2 weeks
3. Introducing plant into nft:
Study ph, tds, Conductivity, Light intensity, Humidity and Relative Humidity for 2 times a day:
| Morng | Morng | Morng | Morng | Morng | Evng | Evng | Evng | Evng | Evng | |
| Date | Temp | humidity | Lux | Ph | Cond | Temp | Humidity | Lux | Ph | Cond |
| 27/01/17 | 25.9 | 48% | 10000 | 5.7 | 1610 | 23.2 | 28% | 6000 | 5.8 | 1610 |
| 28/01/17 | 25 | 47% | 10004 | 5.9 | 1611 | 23.4 | 27% | 6045 | 5.7 | 1608 |
| 29/01/17 | 26.7 | 49% | 10023 | 5.7 | 1608 | 24.6 | 27% | 6000 | 5.8 | 1608 |
| 30/01/17 | 28.7 | 47% | 10014 | 5.9 | 1610 | 26.4 | 28% | 6004 | 5.6 | 1602 |
| 31/01/17 | 25 | 46% | 10004 | 6.0 | 1611 | 23 | 28% | 6004 | 6.2 | 1610 |
| 01/02/17 | 26.7 | 10004 | 6.2 | 1611 | 24 | 28% | 6045 | 6.3 | 1605 | |
| 02/02/17 | 28 | 48% | 10016 | 6.0 | 1612 | 25 | 27% | 6003 | 5.8 | 1604 |
| 03/02/17 | 25 | 46% | 10023 | 5.7 | 1611 | 23 | 28% | 6000 | 6.2 | 1608 |
| 04/02/17 | 25.9 | 48% | 10004 | 5.9 | 1610 | 26 | 28% | 6045 | 6 | 1610 |
| 05/02/17 | 25 | 46% | 10004 | 6.2 | 1608 | 24.5 | 27% | 6004 | 5.7 | 1608 |
| 06/02/17 | 26.7 | 47% | 10014 | 5.7 | 1611 | 23.2 | 28% | 6000 | 5.8 | 1604 |
| 07/02/17 | 25 | 46% | 10014 | 5.9 | 1608 | 26.5 | 28% | 6045 | 6.2 | 1608 |
| 08/02/17 | 26 | 46% | 10023 | 6.2 | 1610 | 24.2 | 27% | 6009 | 6.2 | 1610 |
| 09/02/17 | 25.9 | 48% | 10023 | 6.2 | 1611 | 23.5 | 28% | 6004 | 6 | 1605 |
| 10/02/17 | 25 | 49% | 10023 | 5.7 | 1608 | 22.4 | 27% | 6000 | 5.7 | 1604 |
| 11/02/17 | 26.7 | 49% | 10014 | 5.9 | 1610 | 22.3 | 28% | 6045 | 5.8 | 1610 |
| 12/02/17 | 25.9 | 48% | 10023 | 6.2 | 1608 | 26.5 | 28% | 6009 | 6.2 | 1608 |
| 13/02/17 | 25 | 49% | 10014 | 6.2 | 1611 | 24.3 | 27% | 6004 | 5.7 | 1604 |
| 14/02/17 | 27 | 47% | 10023 | 5.7 | 1610 | 23.6 | 28% | 6000 | 5.8 | 1605 |
| 15/02/17 | 25 | 47% | 10023 | 5.9 | 1608 | 22.6 | 28% | 6045 | 6.2 | 1605 |
| 16/02/17 | 26.7 | 48% | 10014 | 6.2 | 1610 | 22.3 | 27% | 6004 | 5.8 | 1604 |
| 17/02/17 | 25.9 | 48% | 10000 | 5.7 | 1611 | 22.7 | 28% | 6000 | 6 | 1610 |
| 18/02/17 | 27 | 49% | 10014 | 5.9 | 1608 | 26.5 | 28% | 6045 | 5.7 | 1608 |
| 20/02/17 | 25.9 | 48% | 10000 | 6.2 | 1608 | 24.6 | 27% | 6009 | 5.8 | 1604 |
| 21/02/17 | 25 | 47% | 10014 | 6.2 | 1610 | 26.7 | 28% | 6045 | 6 | |
| 22/02/17 | 25.9 | 48% | 10014 | 5.7 | 1611 | 24.7 | 28% | 6004 | 5.8 | 1608 |
| 24/02/17 | 25 | 47% | 10004 | 5.9 | 1608 | 23.5 | 27% | 6000 | 6 | 1604 |
| 25/02/17 | 26.7 | 49% | 10000 | 5.8 | 1610 | 22.6 | 27% | 6045 | 5.7 | 1610 |
| 26/02/17 | 25 | 47% | 10004 | 5.7 | 1608 | 26.4 | 27% | 6009 | 5.8 | 1605 |
Measurement of the growth of spinach:
Date: 27/01/17
Date: 27/01/17
| S. No | Plant | Activity | Condition | Height (cm) | Pigmentation |
| 01 | 01 | No activity | Dryness | 5 | - |
| 02 | 02 | Root elongation | Normal | 5.5 | - |
| 03 | 03 | Rich root elongation | Normal | 7.5 | Light yellow |
| 04 | 04 | Root elongation | Normal | 6 | - |
| 05 | 05 | Root elongation | Normal | 5.5 | Light yellow |
| 06 | 06 | Root elongation | Normal | 7 | - |
| 07 | 07 | Root elongation | Normal | 6 | - |
| 08 | 08 | - | Normal | 4 | - |
| 09 | 09 | Weak | Dryness | 4.5 | - |
| 10 | 10 | Weak | Living | 5 | Colour change |
| 11 | 11 | Rich root elongation | Normal | 13 | - |
| 12 | 12 | Slight root elongation | Normal | 6.5 | - |
| 13 | 13 | - | Normal | 6 | - |
| 14 | 14 | Weak | Dryness | - | - |
| 15 | 15 | Root elongation | Normal | 6.3 | - |
| 16 | 16 | - | Normal | 7 | - |
| 17 | 17 | - | Normal | 6 | - |
| 18 | 18 | - | Normal | 5.8 | Slight yellow |
| 19 | 19 | Weak | Dryness | - | - |
| 20 | 20 | Weak | Normal | 5.4 | - |
Date: 02/02/17
| S. No | Plant | Activity | Condition | Height (cm) | Pigmentation |
| 01 | 01 | Dead | - | - | - |
| 02 | 02 | Elongation of roots | normal | 7 | - |
| 03 | 03 | Elongation of roots | normal | 6.2 | - |
| 04 | 04 | Elongation of roots | normal | 7 | - |
| 05 | 05 | Elongation of roots | normal | 6.5 | - |
| 06 | 06 | Elongation of roots | normal | 7.5 | - |
| 07 | 07 | Elongation of roots | normal | - | - |
| 08 | 08 | - | Weak | - | Yellow |
| 09 | 09 | Dead | - | - | - |
| 10 | 10 | Weak | weak | - | Yellow |
| 11 | 11 | Elongation of roots | normal | 13.6 | Dark green |
| 12 | 12 | - | normal | 6 | - |
| 13 | 13 | Dead | - | - | - |
| 14 | 14 | Weak | - | - | Light yellow |
| 15 | 15 | Elongation of leaves | active | 6.4 | - |
| 16 | 16 | Active | Active | 7 | - |
| 17 | 17 | - | Normal | - | Slight yellow |
| 18 | 18 | Dead | - | - | - |
| 19 | 19 | Dead | - | - | - |
| 20 | 20 | Elongation of roots | Normal | 6 | - |
Date: 08/02/17
| S. No | Plant | Activity | Condition | Height (cm) | Pigmentation |
| 01 | 01 | Roots initiation | Normal | - | - |
| 02 | 02 | Elongation of roots | Normal | 8 | Dark Brown |
| 03 | 03 | Elongation of roots | Normal | 7 | Light Brown |
| 04 | 04 | Elongation of roots | Normal | 8.6 | Dark Brown |
| 05 | 05 | Elongation of roots | Normal | 7.8 | Light Brown |
| 06 | 06 | Elongation of roots | Normal | 5 | Light Brown |
| 07 | 07 | Roots Intiation | Normal | - | - |
| 08 | 08 | Elongation of roots | Normal | 5 | Light Brown |
| 09 | 09 | Elongation of roots | Normal | 6 | Light Brown |
| 10 | 10 | Weak | Weak | - | Light Yellow |
| 11 | 11 | Elongation of roots | Normal | 14 | Dark Brown |
| 12 | 12 | Elongation of roots | Normal | 5 | Light Brown |
| 13 | 13 | Elongation of roots | Normal | 7 | Light Brown |
| 14 | 14 | Roots Initiation | Normal | - | - |
| 15 | 15 | Elongation of roots | Normal | 5 | Light Green |
| 16 | 16 | Dead | - | - | - |
| 17 | 17 | Elongation of roots | Normal | 7 | Dark Brown |
| 18 | 18 | Roots initiation | Normal | - | - |
| 19 | 19 | Roots initiation | Normal | - | - |
| 20 | 20 | Elongation of Roots | Normal | 6 | Light Brown |
Date: 14/02/17
| S. No | Plant | Activity | Condition | Height (cm) | Pigmentation |
| 01 | 01 | Dead | - | - | |
| 02 | 02 | Normal | Normal | 7.2 | Normal |
| 03 | 03 | Root elongation | Normal | 9.2 | Normal |
| 04 | 04 | Root elongation | Normal | 7 | - |
| 05 | 05 | Weak | Week | 3 | Yellow |
| 06 | 06 | Root elongation | Rich | 12 | Normal |
| 07 | 07 | Root elongation | Normal | 6 | Normal |
| 08 | 08 | Survived | Normal | 4 | Normal |
| 09 | 09 | Root elongation | Normal | 7 | Normal |
| 10 | 10 | Moderate death | Weak | 3 | Yellowish pink |
| 11 | 11 | Root elongation | Normal | 12 | Normal |
| 12 | 12 | Root elongation | Normal | 5.5 | Normal |
| 13 | 13 | Normal | Week | 6 | Normal |
| 14 | 14 | Dead | - | - | - |
| 15 | 15 | Maximum death | - | - | - |
| 16 | 16 | Normal | Normal | 7.2 | Normal |
| 17 | 17 | Survived | Normal | 4.9 | Normal |
| 18 | 18 | Normal | Normal | 5 | Normal |
| 19 | 19 | Dead | - | - | - |
| 20 | 20 | Normal | Normal | 5 | Normal |
Date: 20/02/17
| S. No | Plant | Activity | Condition | Height (cm) | Pigmentation |
| 01 | 01 | Weak | Weak | 4 | Yellow colour |
| 02 | 02 | Normal | Normal | 5.5 | Yellow spots |
| 03 | 03 | Root elongation | Active | 5.7 | - |
| 04 | 04 | Root elongation | Normal | 6 | - |
| 05 | 05 | Normal | Normal | 6.5 | Yellow colour |
| 06 | 06 | Normal | Normal | 5.5 | - |
| 07 | 07 | Elongation of roots and leaves | Active | 8.7 | Dark green |
| 08 | 08 | Weak | About to death | 4 | Yellow colour |
| 09 | 09 | Normal | Normal | 6.5 | - |
| 10 | 10 | Normal | Normal | 6 | - |
| 11 | 11 | Weak | About to death | 4.5 | Yellow colour |
| 12 | 12 | Active | Active | 9 | - |
| 13 | 13 | Root elongation | Active | 8.8 | - |
| 14 | 14 | Rich root elongation | Active | 14 | Dark green |
| 15 | 15 | Elongation of roots | Active | 14 | - |
| 16 | 16 | Elongation of roots and leaves | Active | 16 | - |
| 17 | 17 | Weak | About to death | 4 | Dryness |
| 18 | 18 | Normal | Normal | 7 | Yellow |
| 19 | 19 | Normal | Normal | 7.2 | - |
| 20 | 20 | Elongation of roots | Active | 9.7 | Dark Brown |
Date: 26/02/17
| S. No | Plant | Activity | Condition | Height (cm) | pigmentation |
| 01 | 01 | Weak | Normal | 4.5 | - |
| 02 | 02 | Normal | Normal | 5.5 | Yellow spots |
| 03 | 03 | Root elongation | Active | 5.7 | - |
| 04 | 04 | Root elongation | Normal | 6.1 | - |
| 05 | 05 | Normal | Normal | 6.5 | - |
| 06 | 06 | Normal | Normal | 5.5 | - |
| 07 | 07 | Elongation of roots and leaves | Active | 9 | Dark green |
| 08 | 08 | Weak | Weak | 4.3 | Yellow colour |
| 09 | 09 | Normal | Normal | 6.6 | - |
| 10 | 10 | Normal | Normal | 6.2 | - |
| 11 | 11 | Weak | Weak | 4.5 | Yellow colour |
| 12 | 12 | Active | Active | 9.4 | - |
| 13 | 13 | Root elongation | Active | 8.5 | - |
| 14 | 14 | Rich root elongation | Active | 15 | Dark green |
| 15 | 15 | Elongation of roots | Active | 14 | - |
| 16 | 16 | Elongation of roots and leaves | Active | 16 | - |
| 17 | 17 | Weak | About to death | 4.1 | Dryness |
| 18 | 18 | Normal | Normal | 7.1 | Yellow |
| 19 | 19 | Normal | Normal | 7.4 | - |
| 20 | 20 | Normal | Active | 9.7 | Dark Brown |
Percentage yield of the extract
| S. No | Name of The Plant | Percentage Yield (%) |
| 1 | Hydropoinc extract | 12.6% |
| 2 | Geopoincs extract | 17.4% |
Table 1:
Preliminary Phytochemical Screening
| Tests for | Hydropoincs Extract | Geoponic Extract |
| Carbohydrates | + | + |
| Glycoside | + | + |
| Saponins | + | + |
| Alkaloids | - | - |
| Phytosterols | - | - |
| Fixed Oils | + | + |
| Gums and Mucilage | - | - |
| Proteins | - | - |
| Phenolic compounds and Tannins | + | + |
| Flavonoids | + | + |
Table 2: Data showing the preliminary phytochemical screening of extract of spinach (+ = present - = absent).
Estimation of Total Phenolic Content
| Standard (Gallic acid) Calibration Curve | |
| Concentration (µg/ml) | Absorbance |
| 10 | 0.184 |
| 20 | 0.214 |
| 30 | 0.244 |
| 40 | 0.273 |
| 50 | 0.304 |
| 60 | 0.334 |
| 70 | 0.364 |
| 80 | 0.414 |
Table 3: Data showing absorbance of various concentration of Gallic acid.
| Sample | |
| Concentration (100 µg/ml) | Absorbance |
| Geoponics extract | 0.166 |
| Hydroponics extract | 0.184 |
Total Phenolic Content
Graph -1
Graph -1
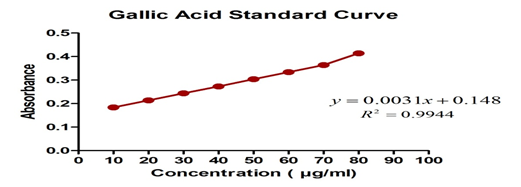
From the Standard Graph of Gallic Acid, The total phenol concentration present in the Geopoincs and Hydropoincs extract was found to be:
Geopoincs Extract: 58 mg GAE/g of extract
Hydroponics Extract: 116.1 mg GAE/g of extract
Geopoincs Extract: 58 mg GAE/g of extract
Hydroponics Extract: 116.1 mg GAE/g of extract
Total Flavanoid Content
| Catechin Standard Curve | |
| Concentration (µg/ml) | Absorbance |
| 10 | 0.060 |
| 20 | 0.113 |
| 30 | 0.166 |
| 40 | 0.219 |
| 50 | 0.272 |
Table 4: Data showing absorbance of various concentration of Catechin.
| Sample Solution | |
| Hydroponics Extract (100 µg/ml) | 0.138 |
| GeopoincsExtract (100 µg/ml) | 0.115 |
Graph -2
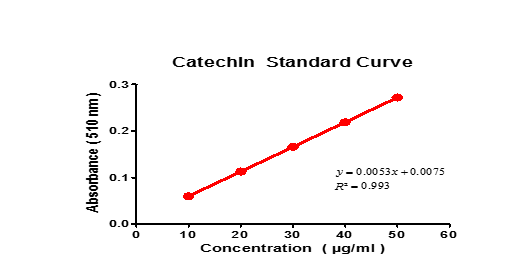
From the Standard Graph of Catechin, The total flavanoid concentration present in the geopoincs and
Hydroponics extract was found to be: Geopoincs Extract: 202.8 mg of CE/g of extract
Hydropoincs Extract: 242.6 mg of CE/g of extract
Metal Analaysis by Using Icpoes for Hydropincs and Geopoincs for 1 Gm of Extract
| Name of the Metal | Hydroponics extract (Conc of Metals in ppm) | Geopoincsextact (Conc of Metals in ppm) |
| Arsenic | Not detected | 1.12 |
| Calcium | 370.70 | 649.73 |
| Cadmium | 4.07 | 0.42 |
| Chromium | 3.06 | 3.48 |
| Cobalt | 0.39 | 0.40 |
| Copper | 6.57 | 2.27 |
| Iron | 16.61 | 31.73 |
| Manganese | 8.92 | 4.96 |
| Nickel | 2.41 | 1.54 |
| Lead | Not detected | 0.54 |
| Vanadium | Not detected | Not detected |
| Zinc | 13.83 | 6.20 |
| Sodium | 800 | 400 |
| Potassium | 5300 | 3800 |
Discussion
Our experiment to cultivate spinach by hydroponics has provided some significant observations. The percentage yield of ethanol extract in hydroponics is relatively low (Hydroponic extract is 12.6% whereas Geopoincs extract was 17.4%). However, there was no variation in the nature of phytoconstituents present in them.
But quantitative estimation of total phenolics by Folinciocalteau method proved that the Hydropoinc extract have more amount of total phenolics in comparison to conventional method of cultivated sample. (Geopoincs Extract: 58 mg GAE/g of extract and Hydropoincs Extract: 116.1 mg GAE/g of extract)
Similarly, the total flavanoid concentration present in the geoponics and Hydroponics extract s were compared. They were found to be 202.8 mg of CE/g of extract geoponics Extract and 242.6 mg of CE/g of extract of Hydropoincs. Therefore it is assumed that polyphenolics are excellent antioxidants and free radical scavengers, the spinach cultivated by hydroponics has better medicinal value. Metal analysis by by using ICPOES has proved that the spinach cultivated by hydropincs method is totally free from toxic arsenic and lead. Copper, Cadmium, sodium, potassium and manganese levels are relatively high.
In view of the promising results obtained, further research may be envisaged to understand the role of nutrient medium in enhancing the beneficial contents like iron
Conclusion
The final conclusion is we successfully achieved to produce a healthy organic plant of spinach having 0% of heavy metal content, 0% usage of pesticides or insecticides and other fertilizers.
References
- Lieberson AD. “How long can a person survive without food?” Scientific American Retrieved 17 December 2014.
- CODEX International Food Standards (Oct 23, 2012) [2] Retrieved on October 28, 2012.
- U.S. Environmental Protection Agency (August 30, 2007), Pesticides: Health and Safety. National Assessment of the Worker Protection Workshop #3.
- "Human Health Issues | Pesticides | US EPA". Epa.gov. 2006-06-28. Retrieved 2014-01-28.
- Bassil KL., et al. “Cancer health effects of pesticides: Systematic review”. Canadian Family Physician 53.10 (2007): 704-711.
- Jurewicz J and Hanke W. “Prenatal and childhood exposure to pesticides and neurobehavioral development: review of epidemiological studies”. International Journal of Occupational Medicine and Environmental 21.2 (2008): 121-132.
- Weselak M., et al. “Pesticide exposures and developmental outcomes: the epidemiological evidence”. Journal of Toxicology and Environmental Health 10.1-2 (2007): 41-80.
- Wigle DT., et al. “Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants”. Journal of Toxicology Environment Health 11.5-6 (2008): 373-517.
- Mink PJ., et al. “Epidemiologic studies of glyphosate and non-cancer health outcomes: a review”. Regulatory Toxicology and Pharmacology 61.2 (2011): 172-184.
- Sanborn M., et al. “Non-cancer health effects of pesticides: Systematic review and implications for family doctors”. Canadian Family Physician 53.10 (2007): 1712-1720.
- Council on Environmental Health. “Pesticide exposure in children”. Paediatrics 130.6 (2012): e1757-1763.
- Miller GT (2004), Sustaining the Earth, 6th edition. Thompson Learning, Inc. Pacific Grove, California. Chapter 9, Pages 211-216.
- Santos JD. et al. “Development of a vinasse nutritive solutions for hydroponics”. Journal of Environmental Management 114 (2013): 8-12.
- Douglas James S., Hydroponics, 5th ed. Bombay: Oxford UP (1975): 1-3
- Dunn, HH. (October 1929). "Plant "Pills" Grow Bumper Crops". Popular Science Monthly: 29.
- G. Thiyagarajan., et al., "Hydroponics," Science Tech Entrepreneur, (January 2007), Water Technology Centre, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu 641 003, India.
- Archived December 29, 2009, at the Wayback Machine.
- Bambi Turner, "How Hydroponics Works," HowStuffWorks.com. Retrieved: 29-05-2012.
- Berkeley, biography Archived March 5, 2015, at the Wayback Machine.
- Liddell & Scott, geoponikos Gericke, William F. (1940). The Complete Guide to Soilless Gardening (1st ed). London: Putnam. pp. 9–10, 38 & 84. ISBN 9781163140499.
- “Dennis Robert Hoagland from Encyclopædia Britannica American Botanist”.
- Archived April 30, 2011, at the Wayback Machine.
- The Water Culture Method for Growing Plants without Soil [dead link]
- Nice Clean Gardening, Frank J. Taylor, "The Rotarian", July, 1939, page 14 Nice Clean Gardening
- Anna Heiney, “Farming for the Future”, nasa.gov, 8-27-04.
- Schaefer, Karen. "Canadian greenhouse industry seeks methods to reduce pollution into Lake Erie". Marketplace.org. Marketplace.org. Retrieved 17 January 2017.
- http://www.growthtechnology.com/growtorial/what-is-hydroponic-growing.
- GrowStoneMSDS. http://sunlightsupply.s3.amazonaws.com/documents/product/714230_MSDS.pdf
- Wallheimer, Brian (October 25, 2010). "Rice hulls a sustainable drainage option for greenhouse growers". Purdue University. Retrieved August 30, 2012.
- M Böhme J., et al. “Cucumber grown in sheepwool slabs treated with biostimulator compared to other organic and mineral substrates”. ISHS Acta Horticultural 779: International Symposium on Growing Media. Retrieved December 15, (2012).
- Power house hydroponics. published on june 16,2014 by Ph hydro list.
- http://www.spliffseeds.nl/cannabis-nutrition-and-growing-organic-cannabis.html.
- http://www.flairform.com/hints/nutrient%20ingredients.htm.
- http://www.oliviassolutions.com/blog/advantages-disadvantages-of-hydroponics.
- Prakash P and Gupta N. “Therapeutic uses of ocimum sanctum linn (tulsi) with a note on eugenol and its pharmacological actions: a short review”. Indian Journal of Physiology and Pharmacology 49.2 (2005): 125-131.
- Guha D and Das S. “CNS depressive role of aqueous extract of Spinaciaoleracea L. leaves in adult malemice albino rats”. Indian Journal of Experimental Biology 46.3 (2008): 185-190.
- Kirtikar KR and Basu BD. “Indian Medicinal plants. Vol. 8. Deharadun: International Book Distributors; (2005): 2078-2079.
- Khare CP. Indian Medicinal Plants. 1stEdn. Berlin/Heidelburg: Springer Verlag (2007): 622-623.
- 9 plants.usda.gov [homepage on the internet] Washington, DC: Natural Resources Conservation Service; [updated 2010 July 09].
- Sultana B and Anwar F. “Flavonols (kampeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants”. Food Chemistry 108 (2008): 879-884.
- Ferreres F., et al. “Acylated flavonol glycoside from spinach leaves (spinaciaoleracea)”. Photochemistry 45.8 (1997): 1701-1705.
- Annonymus. The wealth of India. (R-Z). New Delhi: National Institute of Science, Communication & Information Resources (CSIR) 5 (2004): 146-147.
- Andjelkovic M., et al. “Total and individual carotenoids and phenolic acids content in fresh, refrigerated and processed spinach (Spinaciaoleracea L.)”. Food Chemistry 108 (2008): 649-656.
- Chopra RN., et al. “Glossary of Indian Medicinal Plants Text book of concepts, instrumentation and techniques in ICPOES by Charles b.boss and Kenneth j. freden on perkinelmer 8000 series.
- Singleton VL, Rosi JA, Am. J. Enol. Vitic 1965; 10: 144-158 .
- J. Zhishen, T. Mengcheng, W. Jianming, Food Chemistry, 64, 1999, 555-559.
Citation:
Chandaka Madhu M., et al. “Comparitive Studies on Phytochemical Screening, Total Phenolic, Total Flavoid Content and
Metal Analysis of Hydroponic (Nft System) and Geoponic Cultivated Spinach”. Chronicles of Pharmaceutical Science 1.2 (2017): 89-113.
Copyright: © 2017 Chandaka Madhu M., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


































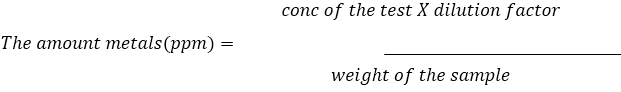




 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.