Research Article
Volume 4 Issue 1 - 2019
Chemical Composition, Antimicrobial Activity and Antioxidant Property of Essential Oil Extracted from Artemisia absinthium L. (Ariti)
1Adama Science and Technology Institute, Adama, Ethiopia
2Addis Ababa Institute of Technology, Addis Ababa University, Ethiopia
3Ethiopian Biodiversity Institute, Microbial Biodiversity Directorate, Ethiopia
2Addis Ababa Institute of Technology, Addis Ababa University, Ethiopia
3Ethiopian Biodiversity Institute, Microbial Biodiversity Directorate, Ethiopia
*Corresponding Author: Befekadu Teshome, Addis Ababa Institute of Technology, Addis Ababa University, Ethiopia.
Receieved: March 25, 2019; Published: April 09, 2019
Abstract
Essential oils from plants are used for fragrance, health and beauty applications. This study determined the variations in chemical composition, oil yield, antibacterial, and antioxidant activities of essential oil extracted from aerial parts of Artemisia absinthium collected from Debre Libanos monastery of Ethiopia. The oil extraction was conducted using steam distillation with duplicate experiments. Effects of different parameters on the yield of essential oil extraction were investigated. The chemical composition analysis of the extracted essential oil was done using Gas chromatography-Mass spectrometry (GC-MS) and Fourier Transform Infrared Spectroscopy (FT-IR). The antimicrobial activity of the essential oil was tested using disk- diffusion method. DPPH (1, 1-diphenyl-2-picrylhydrazyl) free radical scavenging method was used to determine the antioxidant activity of extracts. From DESIGN EXPERT software analysis the optimum oil yield obtained was 2.136% at temperature of (85°C), extraction time (4hr) and particle size range (0.5-1.5mm) and the minimum percentage of oil yield obtained 0.411% at temperature of (75°C), extraction time (2hr) and particle size range (2.5-3.5mm). The essential oil composition analyzed using Gas Chromatography/Mass Spectrometric (GC-MS) showed with maximum result of Camphor (43.59%), 5-Hepten-3-one,2-(5-ethenyltetrahydro-5-methyl-2-furanyl)-6-methyl-, [2S- [2.alpha.,5.alpha]] (29.58%), Bornyl acetate (4.22%), 2-propenoic acid, 3-phenyl-, ethylester, (E) (3.32%) from the total essential oil identified 80.71%. The result also showed that essential oil of A. absinthium has high antioxidant property. In general, Gram-positive strains of bacteria tested appeared to be sensitive to the Artemisia absinthium oil and its main compounds, whileGram- negative bacteria were found to be more resistant to essential oils than Gram-positive bacteria.
Keywords: Artemisia absinthium L; Wormwood; Essential oil composition; Antimicrobial; Antioxidant; Steam distillation; GC/MS and FT-IR
Abbreviations: ANOVA:- Analysis of variance; AOAC: Association of Official Analytical Chemists; ATCC:- American Type Culture Collection; B.C:- Before Christ’s Birth; CFU: Colony-Forming Unit; DPPH:- Diphenyl Picrylhydrazyl; FT-IR:- Fourier Transform Infrared Spectroscopy; GC-MS:- Gas Chromatography-Mass Spectrometry; IC50 :-50 percent inhibition concentration; IP:- Inhibition Percentage; NIST: National Institute of Standard and Technology; PCA:- Principal Component analysis; SD:- Standard Deviations.
Introduction
Essential oils are volatile, aromatic oils obtained from plants and used for fragrance, flavoring, and health and beauty applications. Historically, aromatic plants provided important ingredients for perfumes, incense, and cosmetics. They have also been used for ritual purposes and in cooking and medicine (Steffen., 1994).It appears that it was the Egyptians who first made extensive use of herbs with distillation methods dating back 3,500 B.C. Essential oils were used as Egyptian medicine and used in the burial of rulers and pharaohs. As civilizations transferred world power, the essential oil techniques from Greece traveled to Rome who favored aromatherapy and fragrances. After the fall of the Roman Empire, Persia picked up these healing techniques and perfected the essential oil distillation process (Pearlstine, 2011).
Distillation became an important method of obtaining the healing and fragrant components of various plants and was well-studied beginning in the 18th and continuing in the 19th centuries. In the 1900s, during the time of the industrial revolution, component parts of many essential oils were identified. These components could then be synthesized for use in perfume and flavor industries. In recent years, the use of essential oils has increased in many industries and in new applications as awareness of the benefit of naturally derived products grows (Pearlstine, 2011).
Essential oils are composed of different chemical groups of terpenic hydrocarbons and their oxidized derivatives such as aldehydes, esters, ketones and alcohols. Very often the hydrocarbon terpenes represent a large percentage of the components of essential oils of plants and can be found in a remarkable variety of closely related structures. As a common feature, essential oils carry the essence of a plant, the identifiable aroma, flavor or other characteristics that may have some practical use (Ikhlas et al, 2010).
The Asteraceae is one of the largest plant families, and more than 28,000 compounds have been identified in chemical studies of this family. The genus Artemisia L., commonly known as wormwood, is one of the largest and most widely distributed genus of the family Asteraceae. It includes perennial, biennial, and annual herbs plus small shrubs with strong aromatic leaves (Watson et al., 2002; Iranshahi ., et al. 2007; Mukul, 2013).This genus is of special interest because many Artemisia species have botanical and pharmaceutical properties, characterized scents and tastes due to the content of monoterpenes and sesquiterpenes. The plants have folk and conventional medicine applications (Mucciarelli et al., 1995; Kordali et al., 2005a, b).They have medicinal importance and are used in traditional medicine for the treatment of a variety of diseases and complaints (Burits ., et.al. 2001).
Artemisia essential oils have been used for various purposes such as flavorings, fragrances, and rodent and mite repellents and as folk medicine for antispasmodic, anti-pyretic, anti- inflammatory and abortifacient activities. The essential oils of some Artemisia species are also used in soaps, detergents, cosmetics and perfumes, and in aromatherapy (Burits. ,et al. 2001). The major classes of phyto-constituents of Artemisia species are terpenoids, flavonoids, coumarins, caffeoylquinic acids, and sterols (Bora and Sharma, 2011); making the genus an important source of biological compounds used in insecticides, antimalarials, cytotoxins, antihepatotoxic, fungicides, antibacterials, and allelochemicals (Bora and Sharma, 2011).A notably important drug found in this genus is artemisinin, the anti-malarial drug isolated from A. annua (Bora and Sharma, 2011). Other species of Artemisia have also been noted for their potential use at in- depth investigations on biological activities, especially those species that affect the central nervous and cardiovascular systems (Bora and Sharma, 2011).A. absinthium is known to contain high amount of thujon and historically this plant was used to produce a popular banned commercial drink called Absinthe during the 19th century. This drink was banned in different countries because of its association with a syndrome called Absinthism which had different neurological symptoms (Abegaz and Paulos, 1980).
There are only four species of Artemisia found in the Ethiopian flora (A. afra, A. annua, and A. absinthium and A. abyssinica). Their phyto-constituent chemical compositions include glycosides, terpenes, alkaloids and essential oils. Artemisia absinthium (Artemisia rehan), locally known as “Ariti”, is a perennial odorous herb which is widespread in and native to Ethiopia. A. absinthium for which the botanists suggest the name “Ethiopian Wormwood” is cultivated in home gardens in the Northern and Central Parts of Ethiopia.Itgrows mostly in areas having an elevation from 1500 - 2300m a.s.l. which covers the “Woina – dega” area according to Ethiopian traditional agro-ecological zone classification (Gorfu and Ahmed, 2011).Ethiopian wormwood is used to for its aroma at coffee ceremonies and in rituals called “Adbar” and for flavoring a locally distilled alcoholic drink called Areki. It is also a tradition for Ethiopian women to carry large bunches of this plant to church because the aromatic scent helps prevent feeling of drowsiness (Mukul, 2013).
The essential oil from A. absinthium has potential to be used in perfumery, cosmetics and aromatherapy and has been reported that it has an antifungal and antimicrobial effects (Mukul, 2013). It is also used to spice meal. It was also described that the dried leaves, flowering tops, and essential oil of the plant have traditionally been used as an anthelmintic, antiseptic, antispasmodic, carminative, sedative, stimulant, and stomachic. The plant has also been used to improve blood circulation as a cardiac stimulant, as a pain reliever for women during labor, and as an agent against tumors and cancers. The plant is also recognized as a moth and insect repellent (Mihret., et al, 2015).
A few main chemical components of the plant that have been isolated include thujone, absinthin, caffeoylquinic acids, phenols, and select flavonoids, all of which have different physiological effects and in some cases, beneficial health properties(Quave, 2012). Parts used, the leaves and flowering tops (fresh and dried), are harvested just before or during flowering; from these a volatile oil is obtained by steam distillation (Ikhlas., et al, 2010).
The essential oil content of Artemisia absinthium varies considerably in composition from 0.2% to 1.5% (E/S/C/O/P Monographs, 2003). Ghasemi et al (2013) stated that several studies have shown that some medicinal and aromatic plants’ genetic characteristics can be affected by ecological factors, including precipitation, temperature, plant competition, harvesting and post- harvest schedules, and nitrogen concentration in the soil. Thus, the essential oil composition of Artemisia may be expected to vary with genetic, chemotype, environmental conditions, harvesting time, and geographic origin; including climate, topography, elevation, and edaphic factors.
The chemical compositions of essential oil, oil yield, antibacterial and antioxidant activities of A. absinthium are not well documented and available in Ethiopia. The present study determined the variations in chemical composition, oil yield, antibacterial, and antioxidant activities of essential oil extracted from populations of A. absinthium collected from Debre Libanos monastery of Ethiopia.
Materials and Methods
Plant material
The aerial parts of Artemisia absinthium L. (Ariti) were collected from Ficheworeda (administrative area) which is located in the Northern Shoa Zone of Oromia regional state, Ethiopia. The plant material was bought from farmers who live in Debre Libanos Gedam (Monastery) and sell it at market found in the nearby town, Fiche. The samples were collected in January 2017. The botanical identification of plant sample was carried out by Botanists at Addis Ababa University National Herbarium, Addis Ababa, Ethiopia and voucher specimens (Herbarium No. CNCS-CN-No-001) were deposited at the institute.
The aerial parts of Artemisia absinthium L. (Ariti) were collected from Ficheworeda (administrative area) which is located in the Northern Shoa Zone of Oromia regional state, Ethiopia. The plant material was bought from farmers who live in Debre Libanos Gedam (Monastery) and sell it at market found in the nearby town, Fiche. The samples were collected in January 2017. The botanical identification of plant sample was carried out by Botanists at Addis Ababa University National Herbarium, Addis Ababa, Ethiopia and voucher specimens (Herbarium No. CNCS-CN-No-001) were deposited at the institute.
Essential oil extraction
The aerial parts of plant were washed by tap water in order to remove dust and other contaminant debris. Then, the plant parts were air dried at room temperature for five days in a dust free environment under a shade (Aram and Unnithan, 2016). In order to prevent the dried plant material from absorption of moisture and microbial deterioration, it was kept in plastic bag until use. This sample was ground to a fine powder using grinder and passed through a mesh sieve (with sieve size of 1-2mm, 2-3mm, and 3-4mm) to remove large pieces of debris. The oil extraction from Artemisia absinthium L. (Ariti) was conducted using steam distillation with duplicate experiments. 40g of Ariti with different conditions or factor like temperature, time and particle size was fed in steam distillation at three different times: 2hr, 3hr and 4hr with three different temperature 75°C, 85°C and 95°C and with different particle size of 1-2mm, 2-3mm and 3-4mm to compare their efficiency both on the essential oil extraction yield. Anhydrous sodium sulphate was used for drying or removing water from oil. Then, products were measured by micro pippet volumetric meter and the essential oil physicochemical properties were determined.
The amounts of dried plant used in the experiment and the yield of essential oil (as a percentage) were determined (AOAC, 2000).Yield percentage was calculated as mg of essential oil per 100g of plant dry matter. All experiments were done in duplicate.
Chemical composition analysis of the extracted essential oil
The chemical composition analysis of the extracted essential oil was done by GC-MS and FT-IR.
Gas Chromatography-Mass Spectroscopy (GC-MS)
Gas chromatography-mass spectrometry (GC-MS) analysis was performed using an Agilent Technologies 7820A Gas chromatograph system equipped with a HP-5 capillary column (30m x 0.25; coating thickness, 0.25 µm) and an Agilent technologies 5977E Mass spectroscopy ion trap detector. Analytical conditions were as follows: injector and transfer line temperature, 220°C and 240°C, respectively; oven temperature, programmed from 60°C to 240°C at 3°C/min; carrier gas, helium at 1mL/min; injection, 5µL (10% hexane solution); and split ratio, 1:30 (Martinez et al, 2004).Identification of the constituents was based on comparison of the retention time along with their percentage peak areas and quantitative data was obtained from electronic integration of area percentages without the use of correction factors. All chemical compositions were identified searching through mass\hunter\library\NIST14.L and comparing with other previous work.
Fourier Transform Infrared Spectroscopy (FT-IR)
The FT-IR spectrum of the essential oil was obtained using PerkinElmer spectrum 65 (FT-IR) Spectrometer found in Addis Ababa University, College of Natural Science. The functional groups were determined with the help of IR correlation charts. The IR spectra reported in % transmittance with wave number region for the analysis 4000 - 400 cm-1 (in the mid-infrared range).
Antimicrobial activity test
In vitro antibacterial studies were carried out against 4 bacteria strains and 1 fungus strain. Gram positive strains; Staphylococcus aureus (ATCC 25923), Bacillus subtilis (From Ethiopian Biodiversity Institute microbial gene bank) and Gram negative strains: Pseudomonas aeruginosa (ATCC 27853), and Escherichia coli (ATCC 25922), and 1 fungus: Candida albicans (From Ethiopian Biodiversity Institute microbial gene bank), were used. All microorganism cultures were obtained from Ethiopian Biodiversity Institute, Microbial Gene Bank, Addis Ababa, Ethiopia.
The antimicrobial activity of the essential oil was tested using paper disk-diffusion method (Imelouane et al., 2010). An overnight culture of each microbial strain was adjusted to 0.5 McFarland standards (1X108 CFU/ml) for bacterial inoculums and 2 McFarlands for yeast inoculums. The bacteria strains were inoculated on Mueller Hinton agar (Oxoid) and the fungus strain was inoculated on Potato Dextrose agar (Oxoid) by using sterile swab. Under asceptic conditions, blank filter paper disks (Whatman 6 mm in diameter) were impregnated with 10 μl of the essential oil and placed on the inoculated plates. The plates were incubated at 35+2°C for 18h - 24h. The results of the antimicrobial activity were recorded by measuring the diameters of zones of growth inhibition in millimeters. All tests were done in triplicate. The results were expressed as average values. Tetracycline (30µg) was used as a positive control agent whereas, water as a negative control for bacteria and Co- trimoxazole 25µg used as a positive control of Candida albican.
Antioxidant property test
DPPH Assay: The electron donation ability of the aerial parts extracts was measured by bleaching of the purple colored solution of 1, 1-diphenyl-2-picrylhydrazyl radical (DPPH) according to the method (Hatano et al., 1988).One-half mL of 0.2mM DPPH methanolic solution was added to aerial parts extracts of A. absinthium (2 mL, 10 -1000 𝜇g/mL). After an incubation period of 30min at room temperature, the absorbance was read against a blank at 517nm. The inhibition percentage of free radical DPPH (IP %) was calculated.
% IP = Ab - As x 100
Ab
% IP = Ab - As x 100
Ab
Where, 𝐴b is the absorbance of the control reaction (absorbance blank) and 𝐴s is the absorbance in the presence of plant extract (absorbance of sample). Extract concentration providing 50% inhibition (IC50) was calculated from the regression equation prepared from the concentration of the extracts and the inhibition percentage. Ascorbic acid was used as a positive control.
Statistical analysis
All analyses were performed in triplicate and the results were expressed as mean values ± standard deviations (SD). The data were subjected to statistical analysis using statistical program package Design Expert Version 6.8. Univariate and multivariate analysis of variance (ANOVA) were employed and the differences between individual mean values were deemed to be significant at 𝑃< 0.05. In addition, a principal component analysis (PCA) was performed in order to discriminate between different region on the basis of their essential oils and phenolic composition.
Results and Discussion
Essential oil yield
The essential oil extracted from A. absinthium produced a clear, dark blue opaque liquid with strong aromatic fragrance. The number of experiments was with two replicates according to general factorial design expert. The yield obtained taken as mean value of two replicates (AOAC, 2000). The maximum value for extracted A. absinthiumoil obtained was 2.136% at extraction temperature 85°C, time 4hr and particle size ranges (0.5 -1.5) mm and the minimum yield obtained was 0.411% at temperature of 75°C, extraction time 2hr and particle size ranges from (2.5 -3.5) mm. This result was higher when compared with previous reports in different Tunisia Regions by hydro-distillation method, where BouSalem (1.46 ± 0.06%), region of Kairouan (1.12 ± 0.08%) and Boukornine and J´erissa regions (1.10 ± 0.04% and 1.00 ± 0.03%), respectively (Kamel et al., 2015). At different European regions, the oil yields were ranged from 0.1 to 1.1% w/w as reported in literature prepared by E/S/C/O/P Monographs (2003). A report in Ethiopia, from different parts of Muhonny, Tigray Regional States of the Northern Ethiopia, showed that oil percentage found using Clevenger-type apparatus extracted was 1.996% (Aram & Unnithan, 2016). The result on this paper was higher than that obtained in other country. This shows that the environmental factors such as seasonal, geographical area and extraction techniques affects the essential oils yield (Kamel et al., 2015).
The essential oil extracted from A. absinthium produced a clear, dark blue opaque liquid with strong aromatic fragrance. The number of experiments was with two replicates according to general factorial design expert. The yield obtained taken as mean value of two replicates (AOAC, 2000). The maximum value for extracted A. absinthiumoil obtained was 2.136% at extraction temperature 85°C, time 4hr and particle size ranges (0.5 -1.5) mm and the minimum yield obtained was 0.411% at temperature of 75°C, extraction time 2hr and particle size ranges from (2.5 -3.5) mm. This result was higher when compared with previous reports in different Tunisia Regions by hydro-distillation method, where BouSalem (1.46 ± 0.06%), region of Kairouan (1.12 ± 0.08%) and Boukornine and J´erissa regions (1.10 ± 0.04% and 1.00 ± 0.03%), respectively (Kamel et al., 2015). At different European regions, the oil yields were ranged from 0.1 to 1.1% w/w as reported in literature prepared by E/S/C/O/P Monographs (2003). A report in Ethiopia, from different parts of Muhonny, Tigray Regional States of the Northern Ethiopia, showed that oil percentage found using Clevenger-type apparatus extracted was 1.996% (Aram & Unnithan, 2016). The result on this paper was higher than that obtained in other country. This shows that the environmental factors such as seasonal, geographical area and extraction techniques affects the essential oils yield (Kamel et al., 2015).
Chemical composition of essential oil
A. Gas Chromatography-Mass Spectroscopy
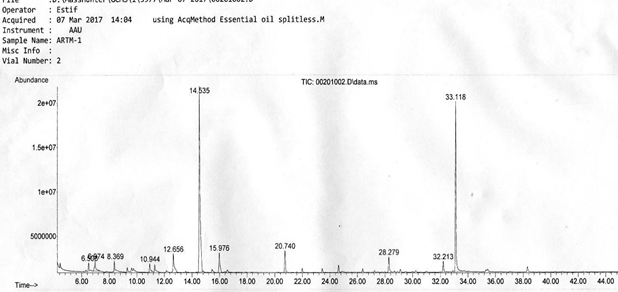
The result of the major composition analysis of Gas Chromatography-Mass Spectroscopy (GC- MS) of essential oil of A. absinthium is presented in table 1. The Gas chromatogram mass spectroscopy of essential oil of Artemisia absinthium is shown in fig. 1. The total components of
The result of the major composition analysis of Gas Chromatography-Mass Spectroscopy (GC- MS) of essential oil of A. absinthium is presented in table 1. The Gas chromatogram mass spectroscopy of essential oil of Artemisia absinthium is shown in fig. 1. The total components of
A. absinthium oil identified by GCMS were 14. Only four of them are shown in the table 1, that match from the library and had quality higher than 90%. The other had minimum library match quality less than 90% and those which are not reported as essential oils. Thus, the major composition of Artemisia absinthiumoil found were Camphor (43.59%), 5-Hepten-3-one,2-(5- ethenyltetrahydro-5-methyl-2-furanyl)-6-methyl-,[2S-[2.alpha.(R*), 5.alpha.]]-29.58%), Bornyl acetate (4.22%), 2-propenoic acid, 3-phenyl-, ethylester, (E)-(3.32%) where, the total sum of 80.71% essential oil.
| S.No. | Compound | Library Match Quality (%) |
Retention time (min) |
Area (%) |
| 1 | Camphor | 98 | 14.535 | 43.59 |
| 2 | Bornyl acetate | 97 | 20.740 | 4.22 |
| 3 | 2-propenoic acid, 3-phenyl-, ethylester, (E)- | 97 | 28.279 | 3.32 |
| 4 | 5-Hepten-3-one, 2-(5-ethenyltetrahydro-5- methyl-2-furanyl)-6-methyl-, [2S-[2.alpha. (R*), 5.alpha.]]- |
95 | 33.11 8 | 29.58 |
Table 1: The chemical compositions of A. absinthium essential oil.
This result had higher essential oil content from composition detected by GC/MS than reported on literature 56% of essential oil and Camphor 41% by Mukul (2013). It is also not in line with the components that obtained in Iran except lower Camphor compound, where camphor (14.83%), p-cymene (10.35%), isoledene (8.52%), caryophyllene (6.92%), isopulegol acetate (6.09%), hysterol (5.64%), isocaryophillene (5.53%), diisoamylene (5.09%), β-farnesene (3.94%) and cyclohexane,2,4-diisopropyl-1,1-dimethyl (3.07%) given by Nezhadali & Parsa (2010) and the reported 2-(5-ethenyltetrahydro-5-methyl-2- furany-6-methyl-[2S- [2α (R⃰), 5α]]- 5-hepten-3-one (71.501 %), bornyl acetate (5.117 %), ethyl-2-propenoate (5.059 %), mercenyl acetate (1.371 %), 7-ethyl-1, 4-dimethylazulene, (4.861 %) and 4,11,11-trimethyl-8- methylene- [1R-(1R⃰, 4Z, 9S⃰)]bicyclo[7, 2, 0]undec-4-ene (3.56 %) given by Aram & Unnithan (2016). Where, the result of Bornyl acetate compound higher than the result on this paper. The variation in the composition of essential oils occurred on their genetic variations, geography, time of collection, stages of plant growth, seasonal and environmental factors (Mariana, et al., 2015).
B. Fourier Transform Infrared Spectroscopy (FT-IR)
Table 2 shows the result of functional group of Artemisia absinthium oil using Fourier Transform Infrared Spectroscopy (FT-IR). When this result compared with previous reported as; at band position (the vibration frequencies in wave numbers) 3462 cm-1 it was observed that O-H stretching vibration of hydroxyl groups (alcohol, phenol, carboxylic acid), but band structures observed between 3150 and 3000 cm-1 were almost exclusively an indication of the unsaturation (C=C-H) and/or a presence of aromatic rings. There was absence of aromatic compounds in the obtained FTIR spectrogram. At 2961.65 (-CH3 asymmetric and symmetric stretching vibration or hydrogen bonds stretching) and the bands situated at 2926.17cm-1 and 2871.99 cm-1 correspond to methylene C-H asymmetric and symmetric stretching vibrations respectively.
Table 2 shows the result of functional group of Artemisia absinthium oil using Fourier Transform Infrared Spectroscopy (FT-IR). When this result compared with previous reported as; at band position (the vibration frequencies in wave numbers) 3462 cm-1 it was observed that O-H stretching vibration of hydroxyl groups (alcohol, phenol, carboxylic acid), but band structures observed between 3150 and 3000 cm-1 were almost exclusively an indication of the unsaturation (C=C-H) and/or a presence of aromatic rings. There was absence of aromatic compounds in the obtained FTIR spectrogram. At 2961.65 (-CH3 asymmetric and symmetric stretching vibration or hydrogen bonds stretching) and the bands situated at 2926.17cm-1 and 2871.99 cm-1 correspond to methylene C-H asymmetric and symmetric stretching vibrations respectively.
At the band functional group observed were 1735.52, 1646.01, 1451.96, 1376.40, 1293.55, 1240.80, 1185-1020, 900-800, 800-750; C=O stretching in non-conjugated ketone, carbonyl and in ester groups, C=C vibration of aromatic structure & C=O stretching of carboxylic acid, C-H asymmetric and symmetric bend and OH bend (phenol or tertiary alcohol), C-H asymmetric and symmetric bend; OH bend (phenol or tertiary alcohol), CH2=CH alkane bending vibration, C-O- C stretching bend (aromatic acid ester), C-OH (from phenol) stretching vibration, C-O stretching vibration (primary, secondary and tertiary alcohol) or C-O-C stretching bend (alkyl-substituted ether), =C-H out of plane bending vibration from aromatics and C-H out of plane bending vibration from aromatics (-CH2-)n rocking (n ≥ 3); skeletal C-C vibration respectively. Therefore, the variation in the composition of essential oils occurred on their genetic variations, geography, time of collection, stages of plant growth, seasonal and environmental factors (Mariana., et al. 2015).
Antioxidant activity
DPPH free radical scavenging method was used to determine the concentrations of extracts at which they scavenge the 50% of the DPPH solution termed as IC50 values. Ascorbic acid was used as standard for this purpose. Ascorbic acid standard was prepared by using 7.5mg of Ascorbic acid dissolving it in 25ml of methanol and then DPPH reagent was added to the Ascorbic acid standard. The same step was repeated for sample of oil where DPPH is added. After an incubation period of 30min at room temperature, the absorbance was read against a blank at 517nm. The percentage inhibition was calculated by the following equation.
% IP = Ab - As x 100
Ab
DPPH free radical scavenging method was used to determine the concentrations of extracts at which they scavenge the 50% of the DPPH solution termed as IC50 values. Ascorbic acid was used as standard for this purpose. Ascorbic acid standard was prepared by using 7.5mg of Ascorbic acid dissolving it in 25ml of methanol and then DPPH reagent was added to the Ascorbic acid standard. The same step was repeated for sample of oil where DPPH is added. After an incubation period of 30min at room temperature, the absorbance was read against a blank at 517nm. The percentage inhibition was calculated by the following equation.
% IP = Ab - As x 100
Ab
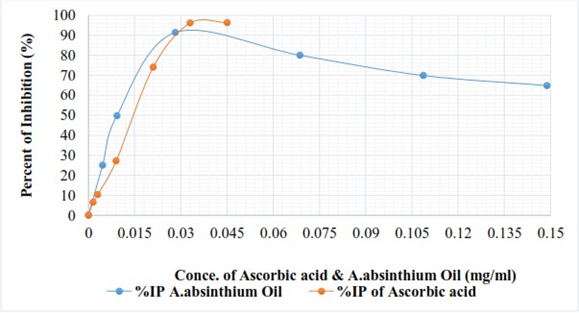
Where, 𝐴b is the absorbance of the control reaction (absorbance blank) and 𝐴s is the absorbance in the presence of plant extract (absorbance of sample). Extract concentration providing 50% inhibition (IC50) was calculated from the regression equation prepared from the concentration of the extracts and the inhibition percentage. Ascorbic acid was used as a positive control. The experiment was done in duplicate and mean values were recorded (Table 3 and fig 2).
The absorbance of blank was (Ab) = 0.657nm, the inhibition percent (%IP) of Ascorbic acid and essential oil are shown in table 3.
| Concentration (mg/µl) | Ascorbic acid | Essential oil |
| 5 | 6.39 | 24.96 |
| 10 | 10.27 | 49.77 |
| 30 | 27.17 | 91.40 |
| 70 | 73.97 | 79.98 |
| 110 | 96.04 | 69.86 |
| 150 | 96.27 | 64.84 |
Table 2: DPPH radical scavenging activity (%IP) of ascorbic acid and essential oil of A. absinthium.
From figure 2 and table 3 it can be seen that, at varies concentration of A. absinthiumoil and Ascorbic acid standards, the DPPH scavenging varied. The inhibition percent graph of antioxidant activity versus concentration of sample and standards increased, in the first phase until it reached maximum inhibition effect and starts slight down with concentration increased. From figure 2, A. absinthiumoil higher than Ascorbic acid standards until the concentration 0.029mg/ml attained. This shows that the antioxidant of A. absinthiumoil higher than Ascorbic acid standards at low concentration. DPPH scavenging activity usually presented by IC50 value, defined as the concentration of the antioxidant needed to scavenge 50% of DPPH present in the test solution. Therefore, as it can be seen from figure 4.10 also, the extracted concentrations provided 50% inhibition (IC50) were obtained at 0.00923mg/ml. This result was lower concentration than that of standards where, IC50 obtained at 0.0148mg/ml. Therefore, the lower value of IC50 the better DPPH radical-scavenging activity and has strong antioxidant potential.
The maximum inhibition effect of Ascorbic acid was 96.27% at high concentration whereas, the maximum inhibition effect of A. absinthiumoil 91.4% at low concentration. This result has high antioxidant of IC50 than that of reported from four Tunisia Region, (0.00938 ± 0.82, 0.01899 ± 0.38, 0.03174 ± 1.23 and 0.04426 ± 1.92) mg/ml respectively by Kamel, et al. (2015) and IC50 = 0.129 mg/ml reported by Harminder, et al. (2009).
Antimicrobial activity
The antibacterial and antifungal activity of Artemisia absinthiumoil against bacteria and fungus is shown in table 4. The results indicated that the highest diameter of inhibition zone was observed against Bacillus subtilisstrain with a diameter of 25mm. This result was higher than the positive control (Tetracycline activity IZ = 16.5mm). The next highest inhibition zone was observed against Staphylococcus aureusstrain with a diameter of 15.5mm which is higher than the positive control (Tetracycline activity IZ = 15mm). This result had less inhibition zone than reported from four Tunisia region observed against Staphylococcus aureus strain with a diameter of (25 ± 1.13mm, 18 ±1.13mm, 20.66mm & 20.66mm) by Kamel, et al., 2015 and wormwood from Canada (25 ± 1.4mm) by Lopes-Lutz, et al., 2008.
The antibacterial and antifungal activity of Artemisia absinthiumoil against bacteria and fungus is shown in table 4. The results indicated that the highest diameter of inhibition zone was observed against Bacillus subtilisstrain with a diameter of 25mm. This result was higher than the positive control (Tetracycline activity IZ = 16.5mm). The next highest inhibition zone was observed against Staphylococcus aureusstrain with a diameter of 15.5mm which is higher than the positive control (Tetracycline activity IZ = 15mm). This result had less inhibition zone than reported from four Tunisia region observed against Staphylococcus aureus strain with a diameter of (25 ± 1.13mm, 18 ±1.13mm, 20.66mm & 20.66mm) by Kamel, et al., 2015 and wormwood from Canada (25 ± 1.4mm) by Lopes-Lutz, et al., 2008.
| Type of micro organism | Description | Average zone of inhibition (IZ in mm) |
| Staphylococcus aureus (ATCC 25923) | GM+ | 15.5 |
| Bacillus subtilis (from Ethiopia Bio- Diversity Inst.) | GM+ | 25 |
| Escherichia Coli (ATCC 25922) | GM- | No |
| Pseudomonas aeruginosa (ATCC 27853) | GM- | No |
| Candida albicans | yeast | 14.33 |
Table 3: The antimicrobial activity of Artemisia absinthium oil.
Moreover, E. coli and Pseudomonas aeruginosa were not susceptibility to the essential oils of A. absinthium. This result is not in line with previously reported inhibition zone of essential oil extracted in Turkey which had a diameter of 10mm (Arzu, et al., 2014). In general, the gram- positive strains of bacteria tested appeared to be more sensitive to the oil and its main compounds than Gram-negative bacteria (Javad., et al. 2012). This might be due to the complex nature of Gram-negative bacteria cell wall and the presence of an outer membrane. It is composed of a double layer of phospholipids that is linked to the inner membrane by lipopolysaccharides (LPS). The peptidoglycan layer is covered by an outer membrane that contains various proteins, as well as LPS. LPS consists of lipid A, the core polysaccharide, and the O-side chain, which provides the “quid” that allows Gram-negative bacteria to be more resistant to essential oils and other natural extracts with antimicrobial activity. Though small hydrophilic solutes are able to pass through the outer membrane via abundant porin proteins that serve as hydrophilic trans-membrane channels, and this is one reason that Gram-negative bacteria are relatively resistant to hydrophobic antibiotics and toxic drugs (Suman, et al., 2014). Therefore, gram-negative bacteria were not affected by essential oil of A. absinthium.
Essential oil of A. absinthium showed significant activity against C. albicansstrain with an inhibition zone diameter of 14.33mm. This result is higher than the inhibition zone exhibited by essential oil extract in Turkey which was reported as 11mm diameter (Arzu, et al., 2014).Due to their antiradical, antioxidant, anti-inflammatory and antimicrobial properties, essential oil of A. absinthium are effective for inhibiting different human diseases (Hatice &Ayse , 2014).
Conclusion
The present study identified fourteen components from the essential oil of Artemisia absinthium using Gas Chromatography-Mass Spectroscopy (GC-MS). Four of them were fitted to the essential oil component with percent of 80.71% which is more oil composition than literature. The major components of Artemisia absinthium were Camphor (43.59%), 5-Hepten-3-one, 2-(5- ethenyltetrahydro-5-methyl-2-furanyl)-6-methyl-, [2S-[2.alpha. (R*), 5.alpha.]]- (29.58%), Bornyl acetate (4.22%), 2-propenoic acid, 3-phenyl-, ethylester, (E)-(3.32%). The presence of high percent of Camphor in essential oil makes it important in application of cosmetics as preservative and antioxidant and in other application. The result also showed that essential oil A. absinthium has high antioxidant property. In general, Gram-positive strains of bacteria tested appeared to be sensitive to the Artemisia absinthium oil and its main compounds, whileGram- negative bacteria were found to be more resistant to essential oils than Gram-positive bacteria.
Declaration
Authors have no conflict of interest
Authors have no conflict of interest
References
- Abegaz, B., & Paulos, G. “Analysis of the Essential Oils of A. Rehan and C”. Citratus (1980).
- M.Sc. Thesis, AAU, phytochemistry, PP. 23-25.
- AOAC. USA: Association of Official Methods of Analytical Chemist (2000).
- Aram, S. T and Unnithan, C. “Characterization of Essential Oils Extracted from Artemisia absinthium and Their Physicochemical Properties”. International Journal of Modern Chemistry and Applied Science 3. 3 (2016): PP. 427-432.
- Arzu, A., et al. “Determining Essential Oil Composition, Antibacterial and Antioxidant Activity of Water Wormwood Extracts”. GIDA 39. 1 (2014): 17-24.
- Burits, M., et al. “The Antioxidant Activity of the Essential Oils of Artemisia afra, Artemisia abyssinica and Juniperus procera”. Phytotherapy Research 15. 2 (2001): 103-108.
- E/S/C/O/P Monographs. The Scientific Foundation for Herbal Medicinal Products 2nd ed. Stuttgart: Thieme (2003).
- Ghasemi A., et al. “Essential oil compositions, antibacterial and antioxidant activities of various populations of Artemisia chamaemelifolia at two phenological stages”. Revista Brasileira de Farmacognosia 23. 6 (2013): 861-869
- Gorfu, D., & Ahmed, E. Crops and Agro-ecological Zones of Ethiopia. Thesis (2011).
- Hatano, T., et al. “Two New Flavonoids and Other Constituents in Licorice Root: Their Relative Astringency and Radical Scavenging Effects”. Chemical and Pharmaceutical Bulletin 36. 6 (1988): 2090–2097.
- Hatice, Z and Ayse , H. B. “Antibacterial and Antioxidant Activity of Essential Oil Terpenes against Pathogenic and Spoilage-Forming Bacteria and Cell Structure- Activity Relationships Evaluated by SEM Microscopy”. Molecules 19. 11 (2014): 1-26.
- Ikhlas, A. K and Ehab, A. A. “Leung's Encyclopedia of Common Natural Ingredient, Used In Food,Drugs, and Cosmetics”. New Jersey: John Willey & Sons (2010).
- Imelouane, B., et al. “Essential Oil Composition and Antimicrobial Activity of Artemisia Herba-Alba Asso Grown in Morroco”. Banats Journal of Biotechnology 1. 3 (2010): 48-55.
- Hatano, T., et al. “Two New Flavonoids and Other Constituents in Licorice Root: Their Relative Astringency and Radical Scavenging Effects”. Chemical and Pharmaceutical Bulletin 36. 6 (1988): 2090–2097.
- Kamel, M., et al. “Chemical Composition and Antioxidant and Antimicrobial Activities of Wormwood (Artemisia absinthium L.)Essential Oils and Phenolics”. Hindawi Publishing Corporation Journal of Chemistry 10. 2 (2015): 1-12.
- Hatice, Z and Ayse , H. B. “Antibacterial and Antioxidant Activity of Essential Oil Terpenes against Pathogenic and Spoilage-Forming Bacteria and Cell Structure- Activity Relationships Evaluated by SEM Microscopy”. Molecules, 19. 3 (2014): 1-26.
- Kamel, M., et al. “Chemical Composition and Antioxidant and Antimicrobial Activities of Wormwood (Artemisia absinthium L.)Essential Oils and Phenolics”. Hindawi Publishing Corporation Journal of Chemistry 10. 2 (2015): 1-12.
- Lopes-Lutz, D., et al. “Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils”. Phytochemistry 69. 8 (2008): 1732–1738.
- Mariana, D. B., et al. “Chemical composition of the essential oil of Artemisia absinthium from Romania”. Journal Rev. Chim 1814 (Bucharest) 66. 11 (2015): 1-5.
- Mihret, M., et al. “Screening of Botanical Extract for the Control of Artemisia Rehan (Artemisia absinthium)”. Africa Journal of Crop Science 3. 7 (2015): 196-200.
- Mukul, C. “Chemical Investigations of the Essential Oils of Some Artemisia Species of Ethiopia”. IOSR Journal of Applied Chemistry (IOSR-JAC) 6. 4 (2013): 1-7.
- Pearlstine, E. V. The Essential Oils History-Origin. Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences 1. 4 (2011): 1-8.
- Steffen A. Perfume and Flavor Materials of Natural Origin. Allured Publishing Corporation, Carol Stream, IL. (1994): 1-2
- Suman, Sharma., et al. “Evaluation of Antibacterial Properties of Essential Oils from Clove and Eucalyptus”. Asian Journal of Pharmaceutical and Clinical Research 7. 5 (2014): 1-4.
Citation:
Befekadu Teshome., et al. “Chemical Composition, Antimicrobial Activity and Antioxidant Property of Essential Oil Extracted from Artemisia absinthium L. (Ariti)”. Clinical Biotechnology and Microbiology 4.1 (2019): 11-21.
Copyright: © 2019 Befekadu Teshome., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.