Research Article
Volume 3 Issue 1 - 2018
Lewy Body – Unifying Mechanism involving Antioxidant Therapy, Reactive Oxygen Species, and Oxidative Stress.
1Department of Chemistry and Biochemistry, San Diego State University, San Diego, CA, USA
2Library and Information Access, San Diego State University, San Diego, CA, USA
2Library and Information Access, San Diego State University, San Diego, CA, USA
*Corresponding Author: Peter Kovacic, Department of Chemistry and Biochemistry, San Diego State University, San Diego, CA, USA.
Received: November 05, 2018; Published: December 04, 2018
Abstract
Reactive oxygen species (ROS) and oxidative stress (OS) play roles in Lewy Body (LB) disease, as also in Alzheimer’s (AD), Parkinson’s (PD) disease and Stroke. Various sources, including oxidases, serve as generators of ROS-OS, specifically apoptosis, inflammation, and nerve problems. Many novel examples of antioxidants (AOs) exert a positive influence on the harmful effects, namely through a unifying mechanism based on ET-ROS-OS-AO. Drugs for treatment of LB are discussed in relation to the unifying theme including phenolic and phenolic ethers among other types.
Keywords: Lewy Body; α-synuclein; radicals; oxidative stress; reactive oxygen species; antioxidants
abbreviations: (ET), electron transfer; (ROS), reactive oxygen species; (OS), oxidative stress; (AO), antioxidant; (LB), Lewy Body; (α-syn), α-synuclein; (AD), Alzheimer’s disease; and (PD), Parkinson’s disease
Introduction
Symptoms
Symptoms for Lewy Body (LB) are addressed in detail from the literature. Symptoms for AD, PD, and dementia are included in the original reports [1-3]. The probability of the following symptoms for LB is as follows. A decline in thinking abilities that interfere with everyday life is always associated with LB. Planning, problem-solving abilities difficulty with direction and spatial relationships, fluctuating cognitive ability, and severe sensitivity to medicine used to treat hallucinations are likely. It is also possible that there will be significant memory loss, language problems, changes in mood, sleep behavior disorder, hallucinations, changes in gait, slower movement, problems using hands, tremors, and balance problems.
Symptoms for Lewy Body (LB) are addressed in detail from the literature. Symptoms for AD, PD, and dementia are included in the original reports [1-3]. The probability of the following symptoms for LB is as follows. A decline in thinking abilities that interfere with everyday life is always associated with LB. Planning, problem-solving abilities difficulty with direction and spatial relationships, fluctuating cognitive ability, and severe sensitivity to medicine used to treat hallucinations are likely. It is also possible that there will be significant memory loss, language problems, changes in mood, sleep behavior disorder, hallucinations, changes in gait, slower movement, problems using hands, tremors, and balance problems.
LB illness is a neurodegenerative disease resulting in dementia [4]. It has various features in common with PD and AD. The precise diagnosis of LB is often difficult to make and distinguish from PD and AD [5]. Lewy body pathology is a pathognomonic for a spectrum of disorders, the most frequent being PD. PD is the prototypic LB disease, characterized by extrapyramidal symptoms and the presence of Lewy bodies in the brain stem. Widespread Lewy bodies occur in the cerebral cortex of patients with dementia.
Dementia with LB was the recommended term for a syndrome that has been ascribed numerous other names. Pathologically, LB disease is defined by the formation of both brainstem and cortical LB in the presence of sparse or absent neurofibrillary pathology [6].
LB are the main histopathological feature [7]. Classic LB dementia shows tau-negative, spherical, red-staining neuronal inclusion bodies. They may be single or multiple within neurons and may vary in size. Other associative pathological features of LB dementia include neuritis, plaques, neurofibrillary tangles, regional neuronal loss of substantia nigra, locus ceruleus, and nucleus basalis of Meynert, and microvacuolation. There are a few rare familial cases of LB dementia related to an autosomal dominant pattern of inheritance. LB usually ends in death; the mean survival is 3 to 9 years after diagnosis.
Unifying Mechanism
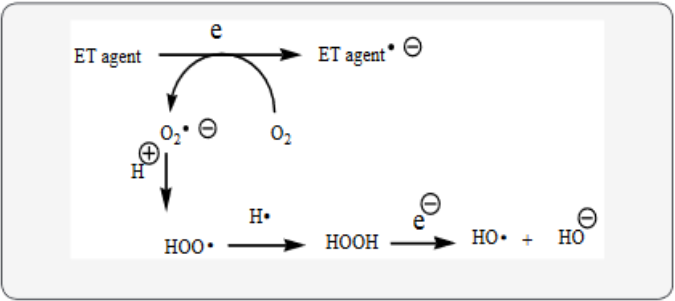
LB fits into the unifying mechanism which has been widely applied in a previous article involving electron transfer (ET), reactive oxygen species (ROS) and oxidative stress (OS) [8]. This unifying mechanism argues that the preponderance of bioactive substances, usually as the metabolites, incorporate ET functionalities. We believe these ET-metabolites play an important role in physiological responses. The main group includes quinones (or phenolic precursors), metal complexes (or complexors), aromatic nitro compounds (or reduced hydroxylamine and nitroso derivatives), and conjugated imines (or iminium species). Resultant redox cycling is illustrated in Scheme 1. In vivo redox cycling with oxygen can occur, giving rise to OS through generation of ROS, such as hydrogen peroxide, hydroperoxides, alkyl peroxides, and diverse radicals (hydroxyl, alkoxyl, hydroperoxyl, and superoxide) (Scheme 1). Cellular and mitochondrial enzymes can also perform catalytically in the reduction of O2.
LB fits into the unifying mechanism which has been widely applied in a previous article involving electron transfer (ET), reactive oxygen species (ROS) and oxidative stress (OS) [8]. This unifying mechanism argues that the preponderance of bioactive substances, usually as the metabolites, incorporate ET functionalities. We believe these ET-metabolites play an important role in physiological responses. The main group includes quinones (or phenolic precursors), metal complexes (or complexors), aromatic nitro compounds (or reduced hydroxylamine and nitroso derivatives), and conjugated imines (or iminium species). Resultant redox cycling is illustrated in Scheme 1. In vivo redox cycling with oxygen can occur, giving rise to OS through generation of ROS, such as hydrogen peroxide, hydroperoxides, alkyl peroxides, and diverse radicals (hydroxyl, alkoxyl, hydroperoxyl, and superoxide) (Scheme 1). Cellular and mitochondrial enzymes can also perform catalytically in the reduction of O2.
In some cases, ET results in involvement with normal electrical effects (e.g. neurochemistry). Generally, active entities possessing ET groups display reduction potentials in the physiologically responsive range. Hence, ET in vivo can occur resulting in production of ROS, which can be beneficial in cell signaling at low concentrations, but produce toxic results at high levels. Electron donors consist of phenols, N-heterocycles or disulfides in proteins, which produce relatively stable radical cations. ET, ROS and OS have been increasingly implicated in the mode of action of drugs and toxins, e.g. anticancer drugs [9], carcinogens [10], cardiovascular toxins [11], toxins [12], ototoxins [13] and various other categories [14].
In addition to the above, there is a plethora of experimental evidence supporting the theoretical framework. This evidence includes generation of the common ROS, lipid peroxidation, degradation products of oxidation, depletion of AOs, effect of exogenous AOs, and DNA oxidation and cleavage products, as well as electrochemical data [8]. This comprehensive, unifying mechanism is consistent with the frequent observation that many ET substances display a variety of activities (e.g. multiple-drug properties), as well as toxic effects.
It is important to recognize that mode of action in the biodomain is often involved with many physiological actions and is multifaceted. In addition to ET-ROS-OS in relation to mechanism, much attention in the literature is paid to AO action entailing physiological effects.
ROS-OS
ROS can be beneficial, but at high levels toxic effects often predominate. There are various sources for these species [15]. NAPDH oxidase is an important producer of the ROS in various organs. The G72 gene increased radical generation in cells. The gene acts as an activator of oxidase. ROS generated by NO synthase have been implicated in an array of harmful behaviors. Mitochondia provide another source of ROS-OS which appears to contribute to aging. Leakage of electrons occurs in the ET chain which react with oxygen to produce superoxide, a precursor of other ROS. Other examples of ROS producers are cytochrome P450, metal complexes, monoamine oxidase and microglia.
ROS can be beneficial, but at high levels toxic effects often predominate. There are various sources for these species [15]. NAPDH oxidase is an important producer of the ROS in various organs. The G72 gene increased radical generation in cells. The gene acts as an activator of oxidase. ROS generated by NO synthase have been implicated in an array of harmful behaviors. Mitochondia provide another source of ROS-OS which appears to contribute to aging. Leakage of electrons occurs in the ET chain which react with oxygen to produce superoxide, a precursor of other ROS. Other examples of ROS producers are cytochrome P450, metal complexes, monoamine oxidase and microglia.
Discussion
A variety of pathological processes may underlie various brain diseases, including OS, such as apoptosis, inflammation, and nerve problems [16]. Patients with LB disorders show cognitive decline resulting from a variety of pathologies. The severity of PD dementia correlates significantly with the density of LB in the brain [17]. Associated OS may play a role in the pathogenesis. Various studies demonstrated enhanced markers of OS in the brain of LB patients [18]. Studies of LB disorders revealed OS damage to a serious extent [19]. Various pathogenic processes are associated with LB diseases [16]. Other factors are inflammation and apoptosis.
A variety of pathological processes may underlie various brain diseases, including OS, such as apoptosis, inflammation, and nerve problems [16]. Patients with LB disorders show cognitive decline resulting from a variety of pathologies. The severity of PD dementia correlates significantly with the density of LB in the brain [17]. Associated OS may play a role in the pathogenesis. Various studies demonstrated enhanced markers of OS in the brain of LB patients [18]. Studies of LB disorders revealed OS damage to a serious extent [19]. Various pathogenic processes are associated with LB diseases [16]. Other factors are inflammation and apoptosis.
α-Synuclein
Immunohistochemical visualization of ubiquitin-positive and α-synuclein (α-syn) in LB has played an important part in revealing the prevalence of this condition [6]. α-Syn, a key pathology of the brain, is a major protein component of LB [20]. The review deals with α-syn aggregation and the mechanism involved in the role played by mitochondrial dysfunction. α-Syn is the major protein in LB [21]. α-Syn is upregulated in response to toxicity. Acute OS leads to the accumulation of α-syn. There is further support for a “two hit” hypothesis for LB involving protective upregulation of α-syn from mild stress and degeneration of neurons. Data suggest the possibility that OS upregulates α-syn [22]. This finding supports OS as a factor in LB disorders. OS, inflammation and α-syn overexpression are risk factors in LB disorders [19]. OS enables α-syn aggregation leading to a pathological cycle.
Immunohistochemical visualization of ubiquitin-positive and α-synuclein (α-syn) in LB has played an important part in revealing the prevalence of this condition [6]. α-Syn, a key pathology of the brain, is a major protein component of LB [20]. The review deals with α-syn aggregation and the mechanism involved in the role played by mitochondrial dysfunction. α-Syn is the major protein in LB [21]. α-Syn is upregulated in response to toxicity. Acute OS leads to the accumulation of α-syn. There is further support for a “two hit” hypothesis for LB involving protective upregulation of α-syn from mild stress and degeneration of neurons. Data suggest the possibility that OS upregulates α-syn [22]. This finding supports OS as a factor in LB disorders. OS, inflammation and α-syn overexpression are risk factors in LB disorders [19]. OS enables α-syn aggregation leading to a pathological cycle.
α-Syn assembly has been implicated as an important step in development of brain disease, such as LB [23]. Evidence shows extensive lipoxidative alteration of α-syn [19]. Mutation in α-syn may induce protein aggregation [24]. Mutations or oxidative modification of α-syn in LB causes aggregation [25]. This result, in addition to neurodegeneration, may proceed by mechanisms involving OS and excitotoxicity. These findings may reveal several novel therapeutic targets. The goal is to downregulate α-syn and related genes, with benefits regarding OS, mitochondrial health, apoptosis and inhibition of inflammation [26]. “Such treatment would reduce α-syn accumulation and LB.”.
A 2015 review by Minami, A., et al. deals with LB disease, which is a group of neurodegenerative disorders involving α-syn accumulation characteristic of LB dementia and PD [20]. This aggregation is an important pathogenesis of neurodegenerative disorders, such as LB dementia. α-Syn interacts with many proteins, hormones, neurotransmitters, and metals that can influence aggregation. LB dementia is the most common form of dementia and movement disorders, after AD. LB are composed of intraneural cells inclusion with α-syn as the main component. Mitochondrial dysfunction may lead to increase in protein aggregate, including α-syn. Various reports emphasize that OS may be a likely factor in α-syn pathology.
In LB formation, Fe induced α-syn is partly dependent on OS [27]. Fe may regulate α-syn aggregation. A report supports the notion that Fe chelation and AO therapy may play a significant favorable role in neuroprotection in neurodegenerative disease, such as LB [28]. Fe accumulation has been linked to NO mechanism. Cytochrome C, and ET agent and mediator of apoptotic cell death, may play a role in OS-induced aggregation of α-syn in LB [29]. Fe catalyzed oxidative processes may be involved in α-syn aggregation. Treatment includes Fe chelation, neuroprotection and anti-inflammatory drugs.
Transition metals
Transition metals, such as Cu, are involved in LB [30]. The relationship between α-syn and metal catalyzed oxidation represent a concern in treatment of these diseases. Cu-dependent enzymes are important contributors to defense by AOs. Free Cu is related to LB formation [31]. Decreased bound Cu may enhance Fe levels and associated OS. Regarding therapy, Cu supplementation may represent a therapy, while Cu chelation may aggravate the pathology. However, excess intake of Cu is not a significant factor in these disease studies [32]. AGE are markers of OS induced by transition metals, inducers of protein crosslinking, and free radical formation by various processes [33]. Evidence indicated that AGE promotion of LB reflects early causative changes. Therefore, transition metals can function as both AOs and generators of ROS-OS.
Transition metals, such as Cu, are involved in LB [30]. The relationship between α-syn and metal catalyzed oxidation represent a concern in treatment of these diseases. Cu-dependent enzymes are important contributors to defense by AOs. Free Cu is related to LB formation [31]. Decreased bound Cu may enhance Fe levels and associated OS. Regarding therapy, Cu supplementation may represent a therapy, while Cu chelation may aggravate the pathology. However, excess intake of Cu is not a significant factor in these disease studies [32]. AGE are markers of OS induced by transition metals, inducers of protein crosslinking, and free radical formation by various processes [33]. Evidence indicated that AGE promotion of LB reflects early causative changes. Therefore, transition metals can function as both AOs and generators of ROS-OS.
AO Drugs
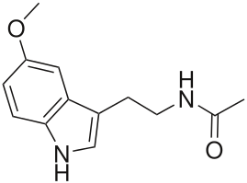
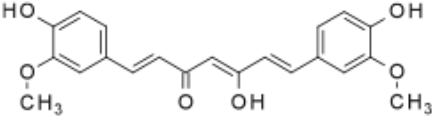
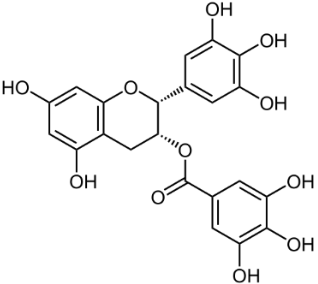
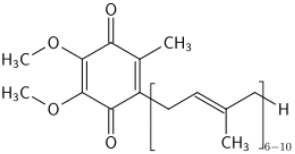
One of the most important classes of AOs for brain illnesses are comprised of phenols and phenolic ethers. Melatonin (figure 1), a phenolic ether, from the pineal gland, exhibits beneficial effects as an AO and in neuroprotection [34]. Polyphenols, such as curcumin (figure 2) and masoprocol (figure 3), phenol and phenolic ether, can function as prophylactics for ROS induced damage, protein misfolding, and neurodegenerative diseases, e.g. LB [35]. Phenolic ethers can undergo cleavage to AO phenols [2].
One of the most important classes of AOs for brain illnesses are comprised of phenols and phenolic ethers. Melatonin (figure 1), a phenolic ether, from the pineal gland, exhibits beneficial effects as an AO and in neuroprotection [34]. Polyphenols, such as curcumin (figure 2) and masoprocol (figure 3), phenol and phenolic ether, can function as prophylactics for ROS induced damage, protein misfolding, and neurodegenerative diseases, e.g. LB [35]. Phenolic ethers can undergo cleavage to AO phenols [2].
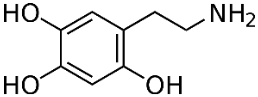
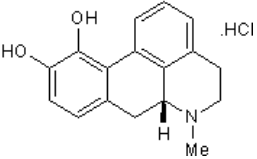
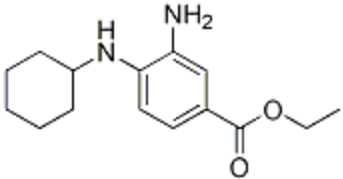
6-Hydroxydopamine (6-OHDA) (figure 4) toxicity is associated with protein degradation [24]. Radical scavengers, such as R-apomorphine (figure 5) and green tea catechin polyphenol (figure 6), are neuroprotective against neurotoxins and prevent the accumulation of Fe and α-syn [28].
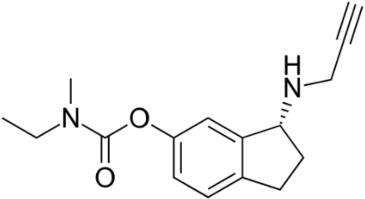
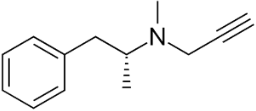
The AO ladostigil (figure 7), a phenolic ether, confers neuroprotection in aged rates [36]. It may be useful in potential treatment of LB and other brain illnesses. Another study deals with neuroprotective effect of ferrostatin-1 (Fer-1) (figure 8), an aromatic ether [37]. Fer-1 inhibits generation of ROS/RNS and quenched radical formation. Additionally, there was mitigation of α-syn aggregation.
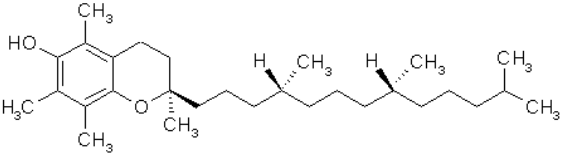
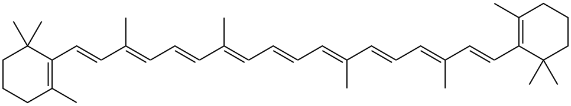
AOs, such as vitamin E (figure 9), a phenolic, and coenzyme Q10 (figure 10), have been used with some success for treatment of LB disorders [23]. Reduction of coenzyme Q10 would yield AO groups comprising hydroquinone bearing two phenolic ether substituents. β-carotene (figure. 11), a polyene, and coenzyme Q10 inhibited formation of α-syn fibrils. Another physiologically active polyene is amphotericin B [38].
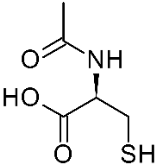
AO agents such as N-acetyl-L-cysteine (figure 12), a thiol AO, blocked harmful pathways [29]. Thiols, such as GSH and disulfide precursors are well known AO agents [39]. Administration of the AO N-acetyl-L-cysteine to the 6-OHDA treated cells increased cell survival and reduced protein degradation.
There is substantial loss of the AO GSH in LB [40]. The AO participates in removal of H2O2 and various toxins. Evidence points to various mechanisms playing a role. Levels of GSH in substantia nigra were reduced in LB disease [41]. Indicators of OS and mitochondrial function were examined. Similar findings concerning of levels of GSH in LB disease were reported, including OS as a cause of nigrial cell death [42].
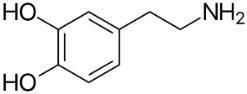
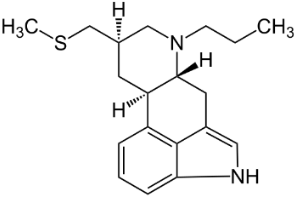
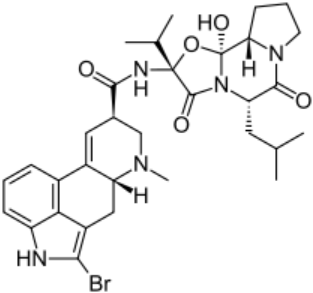
Anti-Parkinsonian agents, such as selegiline (figure 13), a phenethylamine, dopamine (figure 14), an AO phenol, pergolide (figure 15), a sulfide, bromocriptine (figure 16) and trihexyphenidyl (figure 17), were studied in relation to fibril formation and destabilization [43]. All molecules destabilized the fibrils; these agents should be examined as therapies for LB dementia. Evidence points to the possibility of attaining neuroprotection by dopamine agonists, a part of the AO strategy approach. Dopamine is a well known member of the AO phenolic category [2].
Uric acid (UA) (figure 18), a natural AO, was studied for its role in LB disorders [44]. Lower serum levels in LB patients. Findings show lowered serum UA levels in LB patients. α-Syn damage is augmented by dopamine (see figure 14) and paraquat. Paraquat is able to participate in ET and redox cycling with formation of ROS, due to its favorable reduction potential. The herbicide contains a highly conjugated iminium-like structure, making it part of one of the important ET classes [45]. These data suggest the need for multiple oxidative insults in concert with α-syn action in LB.
Metallothionein (MTs), a cysteine rich protein, may protect in therapy by acting as an AO [46]. MTs, as free radical scavengers, inhibit α-syn formation involved in LB disease. Thus, MTs may be useful in nanomedicine
References
- Kovacic P and Weston W. “Phenolic antioxidants as drugs for Alzheimer’s disease: oxidative stress and selectivity”. Novel Approaches in Drug Designing and Development 3.20 (2017): 555606.
- Kovacic P and Weston W. “Treatment of Parkinson’s disease with phenolic antioxidant drugs: Oxidative stress, reactive oxygen species and selectivity”. Chronicles of Pharmaceutical Science 1.4 (2017): 193.
- Kovacic P and Weston, W. “Dementia – Unifying Mechanism Involving Antioxidant Therapy: Reactive Oxygen Species, Oxidative Stress, and Phenolics”. Chronicles of Pharmaceutical Science, 2.6 (2018): 710.
- Foguem, C. and Manckoundia, P. “Lewy Body Disease: Clinical and Pathological "Overlap Syndrome" Between Synucleinopathies (Parkinson Disease) and Tauopathies (Alzheimer Disease).” Current Neurology and Neuroscience Reports 18.5 (2018):24. DOI: 10.1007/s11910-018-0835-5.
- Lewy Body Dementia Association “Early Differentiating Symptoms”, (2018).
- Harrington, C.R. "Dementia: Lewy Body." The Encyclopedia of Aging, Richard Schulz, Springer Publishing Company, 4th edition, (2006).
- Dilli, E. and Miller, B.L. "Non-Alzheimer Dementias." Encyclopedia of Cognitive Science, L. Nadel, Wiley, 1st edition, (2005).
- Kovacic, P. “Unifying mechanism for nutrients as anticancer agents: Electron transfer, reactive oxygen species and oxidative stress”. Global Journal of Health Science 9.8 (2017: 66.
- Kovacic, P., et al. “Mechanisms of anticancer agents: Emphasis on oxidative stress and electron transfer”. Current Pharmaceutical Design: Ingenta Connect Publication 6 (2000): 277.
- Kovacic, P and Jacintho JD. “Reproductive toxins. Pervasive theme of oxidative stress and electron transfer”. Current Medicinal Chemistry 8.7(2001): 863.
- Kovacic, P and Thum LA. “Cardiovascular toxicity from the perspective of oxidative stress, electron transfer, and prevention by antioxidants”. Current Pharmaceutical Design: Ingenta Connect Publication 3.2 (2005): 107.
- Kovacic, P., et al. “Mechanism of mitochondrial uncouplers, inhibitors, and toxins: Focus on electron transfer, free radicals, and structure-activity relationships”. Current Pharmaceutical Design: Ingenta Connect Publication 12.22 (2005): 2601.
- Kovacic, P., et al. “Ototoxicity and noise trauma: Electron transfer, reactive oxygen species, cell signaling, electrical effects, and protection by antioxidants: Practical medical aspects”. Medical Hypotheses 70.5 (2008): 914.
- Halliwell, B and Gutteridge J.M.C. “Free Radicals in Biology and Medicine (3rd ed., Oxford science publications) Oxford: New York: Clarendon Press”. Oxford University Press (1999): 192.
- Kovacic, P. and Weston, W. “Cause and treatment of schizophrenia: Electron transfer, reactive oxygen species, oxidative stress, antioxidants, and unifying mechanism”. Chronicles of Pharmaceutical Science, 1.6 (2017):332.
- Cummings, J.L. “Lewy body diseases with dementia: pathophysiology and treatment”. Brain Cogn., 28.3 (1995): 266.
- Kim, E.M., et al. “Effects of intrahippocampal NAC 61-95 injections on memory in the rat and attenuation with vitamin E”. Progress in Neuro-psychopharmacology & Biological Psychiatry, 33.6 (2009): 945.
- Jabbour-Wadih, T., et al. “The neurochemistry and neuropharmacology of diffuse Lewy body disease”. Rev Neurol., 42.9 (2006): 549.
- Dalfó, E. and Ferrer, I. “Early alpha-synuclein lipoxidation in neocortex in Lewy body diseases”. Neurobiol Aging., 29.3 (2006): 408.
- Minami, A., et al. “Function of α-synuclein and PINK1 in Lewy body dementia”. Int J Mol Med., 35.1 (2015), 3. DOI: 10.3892/ijmm (2014):1980.
- Musgrove R.E., et al. “Neuroprotective upregulation of endogenous α-synuclein precedes ubiquitination in cultured dopaminergic neurons”. Neurotox Res., 19.4 (2011), 592. DOI: 10.1007/s12640-010-9207-x.
- Qing, H. , et al. “Lrrk2 interaction with α-synuclein in diffuse Lewy body disease”. Biochem Biophys Res Commun 390.4 (2009): 1229. DOI: 10.1016/j.bbrc.2009.10.126.
- Ono, K. and Yamada, M. “Vitamin A potently destabilizes preformed α-synuclein fibrils in vitro: implications for Lewy body diseases.” Neurobiol Dis 25.2 (2007): 446.
- Elkon, H., et al. “6-Hydroxydopamine increases ubiquitin-conjugates and protein degradation: implications for the pathogenesis of Parkinson's disease”. Cell Mol Neurobiol., 21.6 (2001): 771.
- Sherer, T.B., Betarbet, R., and Greenamyre, J.T. “Pathogenesis of Parkinson's disease”. Curr Opin Investig Drugs., 2.5 (2001), 657.
- Titze-de-Almeida, R. and Titze-de-Almeida, S.S. “miR-7 Replacement Therapy in Parkinson's Disease”. Curr Gene Ther 18.3 (2018): 143. DOI: 10.2174/1566523218666180430121323.
- Li W., et al. “Oxidative stress partially contributes to iron-induced α-synuclein aggregation in SK-N-SH cells”. Neurotox Res., 19.3 (2011), 435. DOI: 10.1007/s12640-010-9187-x.
- Mandel, S., et al. “Iron and α-synuclein in the substantia nigra of MPTP-treated mice: effect of neuroprotective drugs R-apomorphine and green tea polyphenol (-)-epigallocatechin-3-gallate”. J Mol Neurosci., 24.3 (2004), 401.
- Hashimoto M., et al. “Role of cytochrome c as a stimulator of α-synuclein aggregation in Lewy body disease”. J Biol Chem., 274.41 (1999), 28849.
- Cole, N.B. “Metal catalyzed oxidation of α-synuclein --a role for oligomerization in pathology?”. Curr Alzheimer Res., 5.6 (2008): 599.
- Montes, S., et al. “Copper and copper proteins in Parkinson's disease”. Oxid Med Cell Longev., (2014),147251. DOI: 10.1155/2014/147251.
- Linder, M.C. “The relationship of copper to DNA damage and damage prevention in humans”. Mutat Res., 733.1-2 (2012): 83.
- Münch, G., et al. “Crosslinking of α-synuclein by advanced glycation endproducts--an early pathophysiological step in Lewy body formation?”. J Chem Neuroanat., 20.3-4 (2000): 253.
- Ono K., et al. “Effect of melatonin on α-synuclein self-assembly and cytotoxicity”. Neurobiol Aging., 33.9 (2012): 2172. DOI: 10.1016/j.neurobiolaging.2011.10.015.
- Pal R., et al. “Rescue of ER oxidoreductase function through polyphenolic phytochemical intervention: implications for subcellular traffic and neurodegenerative disorders”. Biochem Biophys Res Commun., 392.4 (2010), 567.
- Bar-Am, O., et al. “The novel cholinesterase-monoamine oxidase inhibitor and antioxidant, ladostigil, confers neuroprotection in neuroblastoma cells and aged rats”. J Mol Neurosci., 37.2 (2009): 135. DOI: 10.1007/s12031-008-9139-6.
- Kabiraj, P., et al. “The neuroprotective role of ferrostatin-1 under rotenone-induced oxidative stress in dopaminergic neuroblastoma cells”. Protein J., 34.5 (2015), 349. DOI: 10.1007/s10930-015-9629-7.
- Kovacic. P., and Cooksy, A. “Novel, unifying mechanism for amphotericin B and other polyene drugs: electron affinity, radicals, electron transfer, autoxidation, toxicity, and antifungal action”. Med. Chem. Commun., 3.3 (2012), 274.
- Kovacic, P. and Weston, W. “Antioxidant therapy by lipoic acid in various illnesses: Reactive oxygen species and oxidative stress”. Novel Approaches in Drug Designing & Development (NAPDD), 3.3 (2018), 555612.
- Zeevalk, G.D., et al.“Glutathione and Parkinson's disease: is this the elephant in the room?”. Biomed Pharmacother., 62.4 (2008): 236. DOI: 10.1016/j.biopha.2008.01.017.
- Dexter D.T., et al. “Indices of oxidative stress and mitochondrial function in individuals with incidental Lewy body disease”. Ann Neurol., 35.1 (1994): 38.
- Jenner, P., et al. “Oxidative stress as a cause of nigral cell death in Parkinson's disease and incidental Lewy body disease”. Ann Neurol 32 (1992): Suppl., S82.
- Ono K.,et al. “Anti-Parkinsonian agents have anti-amyloidogenic activity for Alzheimer's beta-amyloid fibrils in vitro”. Neurochem Int 48.4 (2006): 275.
- Maetzler, W., et al. “Serum and cerebrospinal fluid uric acid levels in lewy body disorders: associations with disease occurrence and amyloid-β pathway”. J Alzheimers Dis 27.1 (2011): 119. DOI: 10.3233/JAD-2011-110587.
- Kovacic, P. and Somanathan, R. “Zolpidem, a clinical hypnotic that affects electronic transfer, alters synaptic activity through potential GABA receptors in the nervous system without significant free radical generation”. Oxid Med Cell Longev 2.1 (2009: 52.
- Sharma, S., et al. “Clinical significance of metallothioneins in cell therapy and nanomedicine”. Int J Nanomedicine., 8 (2013): 1477. DOI: 10.2147/IJN.S42019.
Citation:
Peter Kovacic and Wil Weston. “Lewy Body – Unifying Mechanism involving Antioxidant Therapy, Reactive Oxygen Species,
and Oxidative Stress.” Clinical Biotechnology and Microbiology 3.1 (2018): 577-586.
Copyright: © 2018 Peter Kovacic and Wil Weston. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.