Review Article
Volume 2 Issue 5 - 2018
Genetics of Eunuchs: A Review
1Genetics Group of Gujarat Diagnostic Centre, Mehsana, Gujarat
2Sandor Animal Biogenics Pvt. Ltd., Hyderabad, Telangana
2Sandor Animal Biogenics Pvt. Ltd., Hyderabad, Telangana
*Corresponding Author: RK Patel, Sandor Animal Biogenics Pvt. Ltd., Hyderabad, Telangana.
Received: June 28, 2018; Published: August 24, 2018
Abstract
Eunuchs are the people who are uncategorized sex, or more specifically a male to female psychosexual. Estimated 5 to 6 million hijras lived in India. In most cases they remain infertile due to genetic mutations during genital development. Several genes SOX9, DAX1, WT1, WNT4 and FGF9 are autosomal genes involve in sex determination and development. SRY gene is a candidate gene involve in sex determination which is located on Y chromosome. Mutation in any of these genes involve in sex differentiations can cause an abnormal genital development. These conditions are commonly called as Disorders of the Sex Determination (DSD). Present article describes involvement of the genes in sex development and causes of DSD in eunuchs.
Keywords: Eunuchs; DSD; Sex determination; SRY; disorders of sex differentiation; genes involve in sex differentiation
Introduction
The English word eunuch is from the Greek eune (bed) and ekhein (to keep), precisely "bed keeper". However, popularly, eunuchs are known as hijra/hijda in Hindi. Eunuchs are the people who are with uncategorized sex, or more specifically a male to female psychosexual (Sindhe, 2012; Sharma, 2012). They are males, but having a female like abnormal external structure and behavior, estimated to be 5 to 6 million hijras lived in India (Sharma, 2012). Since their gender is not specified, eunuchs are categorized into third gender. Though, it is a psychosexual problem, but in almost 99% of cases the problem is originated due to clinical or genetic abnormalities (Sharma, 2012). Decreased production of male sex hormones, abnormal sex organs or abnormal gonad developments are major clinical causes in eunuchs. Chromosomal imbalance, deletion, translocation and sex reverse are genetic factors that might responsibly for gender identity disorder. In India, eunuchs lived a taboo life as they are not accepted in the society and therefore their medical and genetic status is restricted.
The present article describes involvement of genetics in gender identity disorder, specifically in the male. Chromosomal anomalies and gene mutation play a major role in interruption of sex determination. Sex development can be divided into two categories; sex determination and sexual differentiation.
Sex Determination
Sex determination is defined as the developmental decision that directs the bipotential gonad to develop as a testis or an ovary. In mammals, sex determination is genetically controlled depending on a developmental time and gene expression. After the discovery of the sex determining region of chromosome Y (SRY) in 1990 (Berta., et al. 1990; Gubbay., et al. 1990; Sinclair., et al. 1990), research efforts have led to the identification master gene of SRY region. SOX9, DAX1, WT1, WNT4 and FGF9 are autosomal genes involve in sex determination and genital development (She and Yang, 2014). Azoospermia, abnormal external genitalia, hypospadias and gyaenacomastis are several common problems related to sex determination. These disorders related to sex determination are commonly called as disorders of sex development (DSD).
Sex determination is defined as the developmental decision that directs the bipotential gonad to develop as a testis or an ovary. In mammals, sex determination is genetically controlled depending on a developmental time and gene expression. After the discovery of the sex determining region of chromosome Y (SRY) in 1990 (Berta., et al. 1990; Gubbay., et al. 1990; Sinclair., et al. 1990), research efforts have led to the identification master gene of SRY region. SOX9, DAX1, WT1, WNT4 and FGF9 are autosomal genes involve in sex determination and genital development (She and Yang, 2014). Azoospermia, abnormal external genitalia, hypospadias and gyaenacomastis are several common problems related to sex determination. These disorders related to sex determination are commonly called as disorders of sex development (DSD).
The sex of an individual is the unique identity of his or her own on earth. How the sex is determined is still a question for scientists. In mammals a complex genetic mechanism and an outer environmental influence, decides the sex of the fetus. Since the discovery of the Y chromosome, researchers got some direction about the sexual differentiation pathway. Interestingly, after discovery of SRY gene in 1990, the mechanism became more clearly defined.
Sex Differentiation
At early embryonic stage gonads remain undifferentiated; during this stage the fetus is phenotypically female because all the sexes are same in fetus. After 6 to 7 weeks, development of testis begins under the influences of Y-chromosome. Sex differentiation is governed by meiosis by which the genetic basis of sex differentiation is determined. Meiosis is a process in which chromosomes are separated and individual sperm or egg is formed. At later stage it fertilizes to form a zygote (Hughes, 2001). The bipotential gonad is the stage wherein the genital ridge is formed resulting in either testis or ovary formation. Urogenital system is developed from genital ridge or gonadal ridge (Rey and Josso, 2013). (Table 1)
| Age of Conception | Event |
| 32 Days | Gonadal primordia developed, Growth of Wolffian ducts, Primordial germ cell differentiation |
| 37Days | Primordial germ cells reaches gonad ridge, Differentiation of Müllerian ducts |
| 42-50 Days | Somniferous cord differentiation |
| 55-60 Days | Beginning of secretion of AMH, Leyden cell differentiation, Cranial part of Müllerian ducts begins to regress |
| 9th Week | Leydig cells produce testosterone. Beginning of masculinization of urogenital sinus and external genitalia |
| 10th-12th Week | The vaginal cord is formed Primordial follicles appear Seminal vesicles developed |
| 14th Week | Completion of male urethral organogenesis |
Table 1: Embryonic development stages during sex differentiation.
Bipotential gonad & embryogenesis
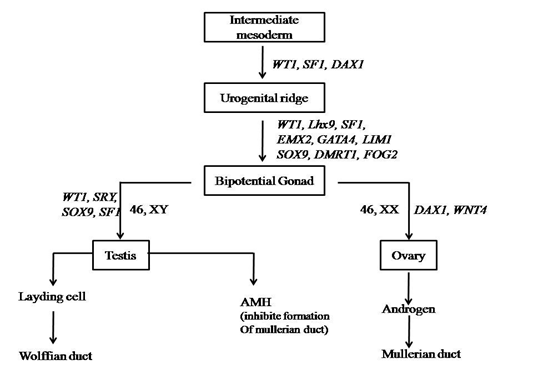
Cells present in genital ridge, or bipotential gonad having capacity to develop in either male or female fate. This type of cells are common in both (Wilhelm., et al. 2007). During the first 6 weeks of embryonic development the gonadal ridge, germ cells, internal ducts, and genitalia are bipotential in both 46, XX and 46, XY embryos. (Figure 1)
Cells present in genital ridge, or bipotential gonad having capacity to develop in either male or female fate. This type of cells are common in both (Wilhelm., et al. 2007). During the first 6 weeks of embryonic development the gonadal ridge, germ cells, internal ducts, and genitalia are bipotential in both 46, XX and 46, XY embryos. (Figure 1)
As shown in figure1, the bipotential gonad represents the leyding cells and sertoli cells. As the SRY expressed, leyding cells undergo development of wolffian duct and finally to male sex. Whereas, sertoli cells produces anti mullerian hormones which inhibit formation of mullerian duct (Whitten, 1982). During the developmental stages genes as like SRY, SOX9, SF1, WT1, GATA4, DMRT1, FOG2, WT4 and EMX2 play an important role to determine sex.
Bipotential gonadal ridge is located medially on the urogenital ridge which can be detected by 5 weeks of gestation (Roucher., et al. 2014). Male sex and reproductive organs like epididymis, vast deference and seminal vehicles are developed from the wolffian duct system, if all the necessary conditions are fulfil. In contrast female reproductive organs are developed from the mullerian duct system, i.e fallopian tube, uterus and vagina (Wilhelm., et al. 2007).
Important Genes Involve In Sex Determination
NR0B1nuclear receptor subfamily 0, group B, member 1
NR0B1located on X chromosome, situated at Xp21.2 (Barbaro., et al. 2009). Four out of eight patients had a XY Disorders of Sexual Development (DSD) with different size of Xp duplication was reported earlier (Larson, 2012). DAX1 is a nuclear receptor protein encoded by NR0B1 gene, hence it is also referred as a DAX1 mutation. NR0B1 specifically expressed in adrenal gland, gonads, hypothalamus and pituitary gland. At the time of testicular development and before the expression of SRY, NR0B1 is expressed in somatic cells (Wilhelm., et al. 2007). Hence, despite the presence of SRY, it can causes female development with increased expression (Baxter and Vilain, 2015). It is believed to be involved in ovarian development and so called as an anti- testis gene (Wilhelm., et al. 2007). Duplication in NR0B1 leads to DSD in male while deletion leads to congenital adrenal hypoplasia in male (Larson., et al. 2012). Anti-testis function of this gene is yet not conformed but the gonad differentiation is based on dosage sensitivity of DAX1 therefore it is called as dosage sensitive gene which leads to female development in normal conditions (Baxter and Vilain, 2015). The condition is initially referred as dosage sensitive sex reversal.
NR0B1located on X chromosome, situated at Xp21.2 (Barbaro., et al. 2009). Four out of eight patients had a XY Disorders of Sexual Development (DSD) with different size of Xp duplication was reported earlier (Larson, 2012). DAX1 is a nuclear receptor protein encoded by NR0B1 gene, hence it is also referred as a DAX1 mutation. NR0B1 specifically expressed in adrenal gland, gonads, hypothalamus and pituitary gland. At the time of testicular development and before the expression of SRY, NR0B1 is expressed in somatic cells (Wilhelm., et al. 2007). Hence, despite the presence of SRY, it can causes female development with increased expression (Baxter and Vilain, 2015). It is believed to be involved in ovarian development and so called as an anti- testis gene (Wilhelm., et al. 2007). Duplication in NR0B1 leads to DSD in male while deletion leads to congenital adrenal hypoplasia in male (Larson., et al. 2012). Anti-testis function of this gene is yet not conformed but the gonad differentiation is based on dosage sensitivity of DAX1 therefore it is called as dosage sensitive gene which leads to female development in normal conditions (Baxter and Vilain, 2015). The condition is initially referred as dosage sensitive sex reversal.
WT1 (Wilms tumor suppressor 1)
WT1 is located on chromosome number 11, mapped on 11p13 region which is expressed during genital development. It is a type of transcriptional factor with zinc figure protein. WT1 has wide spectrum of expression with different isoforms, 2 well known isoforms are + KTS and – KTS. During gonad development + KTS isoforms increases stability of SRY whereas –KTS binds to SRY (Hammes., et al. 2001). Major expression site for WT1 are coelomic epithelia cells, In contrast with other genes, WT1 may not be a major clinical concern for DSD. Deletion or duplication of this gene is associated with complex phenotypic conditions. Wilms’s tumor, aniridia, Denys-Drash syndrome, and Frasier syndrome (Pandian 1900), all of which share features of genitourinary abnormalities, particularly XY gonadal dysgenesis (Larson., et al. 2012). Notably WT1 is associated with wide range of disorders but majorly it is involve with developmental disorders.
WT1 is located on chromosome number 11, mapped on 11p13 region which is expressed during genital development. It is a type of transcriptional factor with zinc figure protein. WT1 has wide spectrum of expression with different isoforms, 2 well known isoforms are + KTS and – KTS. During gonad development + KTS isoforms increases stability of SRY whereas –KTS binds to SRY (Hammes., et al. 2001). Major expression site for WT1 are coelomic epithelia cells, In contrast with other genes, WT1 may not be a major clinical concern for DSD. Deletion or duplication of this gene is associated with complex phenotypic conditions. Wilms’s tumor, aniridia, Denys-Drash syndrome, and Frasier syndrome (Pandian 1900), all of which share features of genitourinary abnormalities, particularly XY gonadal dysgenesis (Larson., et al. 2012). Notably WT1 is associated with wide range of disorders but majorly it is involve with developmental disorders.
NR5A1
It is called as nuclear receptor subfamily 5, group a, member 1, which regulates the expression of other genes. Generally NR5A1 works further with other transcriptional factors. During embryogenesis it expressed in urinogenital ridge, hypothalamus and anterior pituitary gland (Wilhelm., et al. 2007) and promotes or involves in regulating expression of AMH (anti-mullerian hormone) and SOX9 (Baxter and Vilain, 2015). The 13% of NR5A1 mutations is directly involve in 46 XY DSD (Baxter & Vilain, 2015; Sadovsky & Dorn, 2000). Experiments on mice were clearly suggest that lacking of SF1 develops mulllerian duct, while mutant SF1gene, fails to develop adrenal and male gonad (Sadovsky and Dorn, 2000).
It is called as nuclear receptor subfamily 5, group a, member 1, which regulates the expression of other genes. Generally NR5A1 works further with other transcriptional factors. During embryogenesis it expressed in urinogenital ridge, hypothalamus and anterior pituitary gland (Wilhelm., et al. 2007) and promotes or involves in regulating expression of AMH (anti-mullerian hormone) and SOX9 (Baxter and Vilain, 2015). The 13% of NR5A1 mutations is directly involve in 46 XY DSD (Baxter & Vilain, 2015; Sadovsky & Dorn, 2000). Experiments on mice were clearly suggest that lacking of SF1 develops mulllerian duct, while mutant SF1gene, fails to develop adrenal and male gonad (Sadovsky and Dorn, 2000).
Master gene SRY (Sex determine region on the chromosome Y)
Historically it was believed that sex of mammals was decided by Y chromosome. The factor present on Y chromosome is called as Testis Determining Factor; TDF(Graves, 2002; She & Yang, 2014). A region of 35kb located on the short arm of Human Y chromosome is responsible for sex determination and gene which is located within this TDF region is called as SRY (She & Yang, 2014; Drobnič, 2006; Koopman., et al. 2016).
Historically it was believed that sex of mammals was decided by Y chromosome. The factor present on Y chromosome is called as Testis Determining Factor; TDF(Graves, 2002; She & Yang, 2014). A region of 35kb located on the short arm of Human Y chromosome is responsible for sex determination and gene which is located within this TDF region is called as SRY (She & Yang, 2014; Drobnič, 2006; Koopman., et al. 2016).
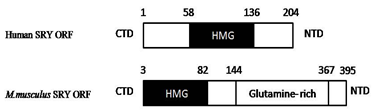
Structure of SRY gene
SRY gene is located on distal, short arm of Y chromosome. As shown in figure.2, it has C terminus and N terminus ends, between both regions, high mobility group (HMG) box is present. This group of genes are known as SOX (SRY related high mobility group) family (She and Yang, 2014). HMG box contains 79 amino acids and interact with (A/T) ACAA (T/A) sequences. HMG box is highly conserved sequences which has DNA binding and DNA bending activities. It binds to the minor groove of DNA and bent it nearby 60 - 85° bend (Ono & Harley, 2013; Good fellow., et al. 1995; Bowles & Koopman, 2001). Excluding HMG box, sequences present on C- terminus and N- terminus region of SRY are poorly conserved within the species and among the species. Although this sequences does not have any clear role in sex determination, several mutations in CTD (C-Terminal Domain) and NTD (N-terminal domain) leads to DSD (Wilhelm., et al. 2007; She & Yang, 2014). Furthermore Transcriptional regulation is a function of HMG box (Correa., et al. 2004). The 20% of XY sex reversal is due to mutations in SRYgene.
Previous studies illustrate that X chromosome based SOX gene is a source of evolution for SRY gene. The HMG box sequences of SOX gene is very much similar to that of SRY which conclude that SRY gene was originated from ancestral SOX gene (Forger., et al. 2004). In almost all mammals (except platypus and echidna), SRY is a master gene which regulates sex determination. It is a male switch gene because adequate expression of SRY decides sex of the fetus. Gain of function and loss of functions studies on mouse implies that difference in SRY expression leads to different level of DSD in mouse and human (Migeon and Wisniewski, 1998). In combination with other genes, SRY regulates the whole mechanism and these genes are called as “SRY and Friend genes”. The WT1, SOX, SF1 and several other genes are associated in sex determination.
Mechanism of SRY regulation
Regulation of SRY starts at intermediate mesoderm, where the first gene WT1 initiates the path of sex determination. As we discussed in previous section +KTS of WT1 can directly binds to promoter sequences of SRYgene and increase the expression level of SRY during male sex determination or male gonad formation (Wu., et al. 2014). In second step GATA4 (GATA binding protein 4) and its co factors like GATA2 (previously called as FOG2) involves in sex determination. Normal differentiation of sertoli cells are regulated by GATA4 and co factors, which indirectly regulates expression of SRY gene (Hacker., et al. 1995). Recently it is clearly known that mutant co-factor FOG2 cannot binds to GATA4. This results in male gonadal dysgenesis and fails to determine specific sex (Lourenço., et al. 2009). Another gene CBX2 (chromobox 2) directly influences SRY expression. Loss of function studies on SRY gene in mice implies that decreased CBX2 results in male to female sex reversal (Biason-Lauber and Schoenle, 2000). At this stage urogenital ridge is formed from intermediate mesoderm. From urinogenital ridge to bipotential gonad, several other target genes of SRY direct sex determination. (Figure3).
Regulation of SRY starts at intermediate mesoderm, where the first gene WT1 initiates the path of sex determination. As we discussed in previous section +KTS of WT1 can directly binds to promoter sequences of SRYgene and increase the expression level of SRY during male sex determination or male gonad formation (Wu., et al. 2014). In second step GATA4 (GATA binding protein 4) and its co factors like GATA2 (previously called as FOG2) involves in sex determination. Normal differentiation of sertoli cells are regulated by GATA4 and co factors, which indirectly regulates expression of SRY gene (Hacker., et al. 1995). Recently it is clearly known that mutant co-factor FOG2 cannot binds to GATA4. This results in male gonadal dysgenesis and fails to determine specific sex (Lourenço., et al. 2009). Another gene CBX2 (chromobox 2) directly influences SRY expression. Loss of function studies on SRY gene in mice implies that decreased CBX2 results in male to female sex reversal (Biason-Lauber and Schoenle, 2000). At this stage urogenital ridge is formed from intermediate mesoderm. From urinogenital ridge to bipotential gonad, several other target genes of SRY direct sex determination. (Figure3).
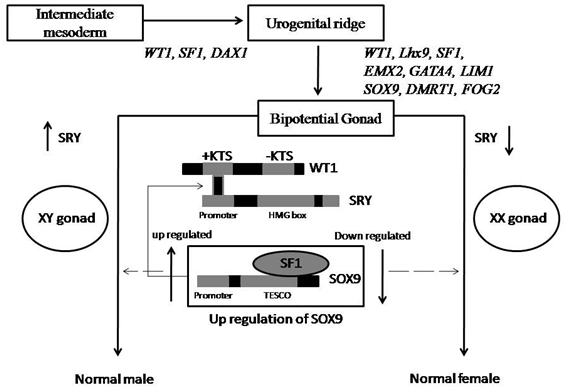
Figure 3: Mechanism of SRY regulation and involvement of other genes in sex determination/ differentiation.
During mammalian sex determination, SOX9 (SOX-box 9) is a direct target gene. It is a best candidate gene in sex determination, second after SRY gene. In bipotential gonad, SOX9 is unregulated in sertoli cell precursors leads to sertoli cell differentiation, immediate after SRY expressed. Functionally down regulated SOX9 causes autosomal sex reversal (Lamb., et al. 2000). SOX9 is too involve in several skeletal malformations such as Campomelic dysplasia (Kwok., et al. 1995; Parma., et al. 2006). TESCO, testis specific enhancer of SOX9 core elements are 1.4kb region which is located 11-13kb upstream to SOX9 and is highly conserved in mammalian gonads (Hacker., et al. 1995). Three putative binding sites present onTESCO allow binding SF1 to TESCO which regulates normal expression of SOX9 in bipotential gonad. This TESCO-SF1 complex binds to SRY resulting to increase or up regulated SOX9 expression in male gonad (Wu., et al. 2014). Several previous studies indicated that during the differentiation of sertoli cells, fibroblast growth factor 9, FGF9 interferes with SOX9 expression leads to male to female sex reversal. Hence it is important that SOX9is up regulated in male and down regulated in female.
SOX 9 (SRY-box 9)
SOX9 is a candidate gene involve in sex determination pathway. Possibly it is a target site for SRY action. It belongs to SOXfamily, named SRY related HMG box. SOX9 is located on long q arm of chromosome no. 17, mapped 17q24.3 - 17q25.1 (Knower and Harley, 2003). Initially, SOX9 is expressed in bipotential genital ridge on lateral side and regulated in sertoli cells, immediately after the expression of SRY (She and Yang, 2014). SRY like HMG domains are encoded by 509 amino acids polypeptide chain which helps SOX9 to bind with SRY (Mcdowall., et al. 1999). SOX9 is a universal transcriptional factor which is due to presence of HMG-domain and helps SRY to bind and bend DNA (Kwok., et al.. 1995; She & Yang, 2014). Loss or gain functional studies indicates that mutations of SOX9 is involve in male to female sex reversal (Lamb., et al. 2000). Several mutations like deletion, duplication and translocation in sequences of SOX9 HMG domain are causes of male to female sex reversal. Studies indicated that beside conserved HMG-Domain, several mutations in ORF (open reading frame) of SOX9 gene is also involve in sex reversal or gonadal failure (Mcdowall., et al. 1999; Knower & Harley, 2003; Kwok., et al. 1995; Shaw., et al. 2015). Sequences 193bp far from the starting transcriptional domain, is candidate target domain involve majorly in sex determination pathway, however the mechanism is still unknown. In vitro studies on mice model (Kanai and Koopman 1999) suggest that induced expression of SOX9 in XX gonad is sufficient to activate SRY, leads to testis development in XX mice gonad (Parma., et al. 2016). Hence it conforms SOX9 is essential for SRY expression.
Disorders of sex development
DSD is gender related disorders or it is related to sex. But ‘sex’ and ‘gender’ have different definitions and characteristic; biologically determined are ‘sex’ whereas socially created male or female characteristic are ‘gender’ (Achermann and Jameson, 2015). Congenital conditions in which development of chromosome, gonads or physical sexual characters is abnormal, called as DSD (Achermann & Jameson, 2015; Mendonca., et al. 2009). In 2006, International Consensus Conference on Intersex replaced the term ‘inter sex’ with DSD (Disorders of Sex development) (leuan., et al. 2006).
DSD is gender related disorders or it is related to sex. But ‘sex’ and ‘gender’ have different definitions and characteristic; biologically determined are ‘sex’ whereas socially created male or female characteristic are ‘gender’ (Achermann and Jameson, 2015). Congenital conditions in which development of chromosome, gonads or physical sexual characters is abnormal, called as DSD (Achermann & Jameson, 2015; Mendonca., et al. 2009). In 2006, International Consensus Conference on Intersex replaced the term ‘inter sex’ with DSD (Disorders of Sex development) (leuan., et al. 2006).
Studies indicated that DSD involves vast majorities of Disorders. Some DSDs are due to gene mutations and others are because of chromosomal imbalance. Turner syndrome (45 X0) (I, Dourado., et al. 2009; Bernadete & Marqui, 2015; Sybert & Mccauley, 2004), Klinefelter syndrome (47 XXY) (Sybert & Mccauley, 2004; Visootsak & Jr, 2006; Nieschlag, 2013) and chimeric (46 XX/ 46 XY) (Macías-gómez., et al. 2012). DSDs are caused by chromosomal imbalance whereas Frasier syndrome (Guaragna., et al. 2012; Peco-antić., et al. 2013), Denys–Drash syndrome (Kucinskas & Just, 2005; Kabra & Kataria, 1994; Niaudet, 2004) with Wilms’ tumor (Kabra and Kataria 1995), congenital adrenal hyperplasia (White., et al. 2012; Speiser., et al. 2016; Speiser, 2016) and androgen insensitivity (Galani., et al. 2008; Oakes., et al. 2008; Hughes., et al. 2012) are caused by mutations in autosomal or sex genes. Several other DSDs which are very rare, are partial or complete harmaphroditism (Jaubert., et al. 2004; Anton Brogger, 1965), ovotesticulars (Macías-gómez., et al. 2012) and ablasio penis (Migeon and Wisniewski, 1998) micro penis. Summary of DSDs are given in image below:
| Gene | Mutations | Phenotype |
| SRY | c.397C > T, c.380A > T, c.337G > A, c.331C > T, c.326T > C, c.324delA c.320G > A, c.317A > T , c.284G > A, c.283G > C, c.277C > T , c.274A > T c.209G > A, c.203T > C , c.192G > A, c.178G > C, c.53G > A, c.12T > A, c.4C > T c.364_367delGAGA, c.270C > G |
46, XY sex reversal, type 1 46, XY sex reversal, type1, True hermaphrodite |
| SOX3 | 21-BP DUP 33-BP DUP |
Panhypopituitarism X-linked Isolated growth hormones deficiency |
| SOX9 | 136-KB DEL, 577-KB DEL, 240-KB DEL 148-KB DUP, 96-KB TRIPLICATION, 178-KB DUP, 1-BP INS, 1103A 1-BP DEL, 296G 1-BP INS, 783G 4-BP INS, c.472G > A c.736dupC, c.1320C > G 583C-T, c.227C > A, 30-BP DEL, c.462C > G, c.493C > T c.507C > G, c.509C > T c.517A > G, c.1249C > T c.1320C > A c.442G > T, c.522C > G, c.555delG, c.738delG c.1180C > T, c.1262_1278del17 |
46, XY sex reversal 1 46, XX sex reversal 2 Campomelic dysplasia with autosomal sex reversal Camptomelic dysplasia No specified phenotype in DSD |
| RSP01 | (IVS5DS, G-A, +1) EX4 DEL, (1-BP INS, 896G) |
true hermaphroditism 46, XX sex reversal |
| NR5A1 | (8-BP DEL, NT1058) c.1310T > A, c.1210T > G, c.271G > A, c.234G > A, c.151G > T, c.43G > A c.104_105delGCinsAA, c.48C > A, c.34_38delCTGTGinsGACCTGGACCTGT c.764G > T c.691_699delCTGCAGCTG, c. [368G > C;386C > T], c.634G > A, c.392C > T c.275G > A, c.274C > T c.3G > A |
46, XY sex reversal
Adrenal insufficient Premature ovarian failure Spermatogenic failure 46, XY and 46, XX sex reversal, adrenal insufficient 46, XY sex reversal, Premature ovarian failure |
| NR0B1 | DUP in NR0B1 2.2-KB DEL/27-BP INS, (1-BP INS, 430G) 1-BP DEL, 501A, (4-BP DEL, NT1464) (2-BP DEL, 388AG), (1-BP DEL, 1169C) 2-BP DEL, 1610AG, AND 1-BP INS 1-BP DEL, c.1319A > T, c.1316T > G, c.1274G > T, c.1197C > A, c.1183C > T, c.1146G > T, c.1142T > A, c.1138T > G, c.1107G > A, c.890T > C, c.873G > C, c.847C > T, c.813C > G, c.800G > C, c.788T > A, c.704G > A, c.591C > A, c.513G > A, c.273C > A, c.109C > T |
46, XY sex reversal Congenital adrenal hypoplasia |
| WT1 | c.787+15T > A, c.1432+4C > T, c.1378T > C c.1432+5G > A, c.1391A > G, c.1390G > A, c.1385G > C, c.1333C > T, c.1323C > G, c.1301G > A, c.1288C > T, c.1282T > G, c.1193G > A c.1384C > T, c.1372C > T, |
Frasier syndrome Drash syndrome Frasier syndrome, Drash syndrome |
Table 2: Several common pathogenic mutations reported in genes responsible for sex determination and differentiation (the data was derived from the ClinVar database of NCBI).
| Sr. No. | Gene Name | Type of DSD | Reference |
| 1 | WT1 | Frasier syndrome, Denys–Drash syndrome with Wilms’ tumor | (Gao., et al. 2006; Hammes., et al. 2001) |
| 2 | SF1 | Gonadal and adrenal dysgenesis | (Park., et al. 2005; Lin., et al. 2007) |
| 3 | SOX9 | Campomelic dysplasia, male gonadal dysgenesis or XY sex reversal | (Barrionuevo., et al. 2006; Lamb., et al. 2000; Parma., et al. 2016) |
| 4 | DAX1 | Gonadal dysgenesis, congenital adrenal hypoplasia | (Meeks., et al. 2003) |
| 5 | SRY | Gonadal dysgenesis | (Amudha., et al. 2012; Polanco & Koopman, 2007; Rajender., et al. 2006) |
| 6 | GATA4 | Ambiguous external genitalia | (Hu., et al. 2013) |
| 7 | WNT4 | Ambiguous genitalia | (Jordan., et al. 2003; Yao., et al. 2014) |
| 8 | RSP01 | complete XX sex reversal | (Chassot., et al. 2008; Parma., et al. 2016) |
Table 3: Major genes involve in sex determination and its association with DSD.
Numerical chromosomal DSD
Turner Syndrome (TS)
In 1938, Turner described a group of anomalies with short stature. Later on streak gonads was observed in this particular group of individuals which is called as Turner Syndrome. It is observed in 1 out of 2500 individual with female karyotype ((Bernadete and Marqui, 2015) and is a most common type of enuploidy. 45 XO karyotype was first observed by Ford., et al. in 1959.
Turner Syndrome (TS)
In 1938, Turner described a group of anomalies with short stature. Later on streak gonads was observed in this particular group of individuals which is called as Turner Syndrome. It is observed in 1 out of 2500 individual with female karyotype ((Bernadete and Marqui, 2015) and is a most common type of enuploidy. 45 XO karyotype was first observed by Ford., et al. in 1959.
TSare not inherited. It is arising due to error in cell division; a process called non-disjunction is results in abnormal numbers of chromosome (Zhong and Layman, 2012). Clinical sign includes lymphedema of hands and feet, webbed neck, short stature, gonadal dysgenesis, primary amenorrhea, infertility, shield chest and cardiac-renal anomalies are most commonly reported (Gonzalez and Witchel, 2012). Some females with TS born with heart defect which is life threatening. Genetically TS is a monosomy in which one X chromosome is absent or altered. Approximately 50% to 60% females (I., et al. 2009) with TS does not have one X chromosome and the remaining TS have altered X chromosome as like dicentric, deletion of short arm or ring chromosome. In some cases mosaic X chromosome is reported which is known as mosaic TS. Genital ambiguities are frequent in females with TS. The ovarian failure at early age and infertility is common among turner syndrome (Bernadete and Marqui, 2015).
Klinefelter syndrome (KS)
Klinefelture syndrome is a condition in which one extra X chromosome is observed in males and this condition is first reported by Harry Klinefelter in 1942. The rarest condition in this group is 49/XXXXY and, are the group of klinefelter syndrome (Visootsak and Graham, 2006). KS is occurred in 500 to 1000 in 1 newborn. However 49/XXXXY in rarest among this (Nieschlag, 2013; Amory & Bremner., et al. 2000).
Klinefelture syndrome is a condition in which one extra X chromosome is observed in males and this condition is first reported by Harry Klinefelter in 1942. The rarest condition in this group is 49/XXXXY and, are the group of klinefelter syndrome (Visootsak and Graham, 2006). KS is occurred in 500 to 1000 in 1 newborn. However 49/XXXXY in rarest among this (Nieschlag, 2013; Amory & Bremner., et al. 2000).
KS is originated due to error in cell division in germ cells and nondisjuction of chromosome. Hence KS is not inherited as like other genetic condition (Amory., et al. 2000). Small testis or testicular failure is common clinical sign. Other characteristics are low testosterone level, eunuchoidism, azoospermia, reduced facial and body hair, gynecomastia, breast enlargement, hypospedia and micro penis (Visootsak and Graham, 2006; Amory., et al. 2000). Chances of breast cancer and developmental disabilities are high in this group of individuals. Speech and language problems are mildly observed (Amory, et al. 2000).
Structural chromosomal abnormality
Del (Yp), Del (Yq), Dic (Yp), r (Y), mar (Y), iY (p10), r (X) and mar (X) are common structural abnormalities. Gonadoblastoma, azoospermia, infertility and streak gonads are commonly observed phenotypes of structural rearrangement (Shabsovich and Tirado, 2014).
Del (Yp), Del (Yq), Dic (Yp), r (Y), mar (Y), iY (p10), r (X) and mar (X) are common structural abnormalities. Gonadoblastoma, azoospermia, infertility and streak gonads are commonly observed phenotypes of structural rearrangement (Shabsovich and Tirado, 2014).
Other chromosomal abnormality
Sex reversal
Visible sex or phenotypic sex does not correlate with the observed sex is called as sex reversal (Wilhelm., et al. 2007). In simple words, XX males are phenotypically male (physically looks like male) but genotypically female. It is a condition in which a phenotipically normal male has a female genotype (de la Chapelle, 1972). This condition is called as testicular DSD and was first reported by la cheppele in 1964. It is a type of rare genetic condition with a frequency of 1:25,000 male new born (Rajender., et al. 2006). During the process of meiosis, several TDFgene regions transfers to X chromosome through illegitimate recombination between X and Y chromosome. This process is responsible for XX sex reversal. The pseudo autosomal regions (PAR1 and PAR2) are short regions of homology between the mammalian X and Y chromosomes. The PAR behaves like an autosome and recombine during meiosis. Thus genes in this region are inherited in an autosomal rather than a strictly sex-linked fashion. When some of gene of TDF and SRY are also crossover with PAR that causes sex reversal (Wilhelm., et al. 2007). Several parts ofSRY gene or complete SRY gene is translocated on X chromosome (Alves., et al. 2010). Patient with 46 XX sex reversal +SRYhas completely normal male external genitalia but they are azoospermic (de la Chapelle, 1972). Several cases of sex reversal with –SRY was also observed. Absence ofSRY in XX sex reversal leads to abnormal testis development and partial hermaphrodites (Rajender., et al. 2006; Abusheikha., et al. 2001).
Sex reversal
Visible sex or phenotypic sex does not correlate with the observed sex is called as sex reversal (Wilhelm., et al. 2007). In simple words, XX males are phenotypically male (physically looks like male) but genotypically female. It is a condition in which a phenotipically normal male has a female genotype (de la Chapelle, 1972). This condition is called as testicular DSD and was first reported by la cheppele in 1964. It is a type of rare genetic condition with a frequency of 1:25,000 male new born (Rajender., et al. 2006). During the process of meiosis, several TDFgene regions transfers to X chromosome through illegitimate recombination between X and Y chromosome. This process is responsible for XX sex reversal. The pseudo autosomal regions (PAR1 and PAR2) are short regions of homology between the mammalian X and Y chromosomes. The PAR behaves like an autosome and recombine during meiosis. Thus genes in this region are inherited in an autosomal rather than a strictly sex-linked fashion. When some of gene of TDF and SRY are also crossover with PAR that causes sex reversal (Wilhelm., et al. 2007). Several parts ofSRY gene or complete SRY gene is translocated on X chromosome (Alves., et al. 2010). Patient with 46 XX sex reversal +SRYhas completely normal male external genitalia but they are azoospermic (de la Chapelle, 1972). Several cases of sex reversal with –SRY was also observed. Absence ofSRY in XX sex reversal leads to abnormal testis development and partial hermaphrodites (Rajender., et al. 2006; Abusheikha., et al. 2001).
The reason for sex reversal is Y bearing DNA fragment were present in all cases. In contrast, Ucan., et al. 2013 reported that during recombination 2 case had same DNA fragment and one case had different DNA fragment transferred from Y containing DNA of TDF (Uçan., et al. 2013). Similar study was performed by Wu., et al. (2014) on 5 unrelated male patients. Although external genitalia were normal, SRY gene region was present on the tip of Xp. FSH/LH level was high with respect to low testosterone level. Testis were developed normally but AZF fragments were not present on SRYgene so the patient was found sterile (Wu., et al. 2014). However, 46 XX sex reversal could not only be influenced by SRY, several other genes which are involved in gonadal differentiation such as SOX9, DAX1, WT1, WANT4and RSPO1 were found responsible (Wu., et al. 2014). Therefore, sex determination in XX males depends on amount of X chromosome inactivation and amount of Y chromosome which is transferred to X.
Molecular gene mutation in DSD
Frasier syndrome (FS, OMIM # 136680)
George Fraser in 1962 described this condition. Phenotypic conditions are same as Denys-Drash syndrome (Peco-antić., et al. 2013). Abnormalities of ear, nose, syndactyly with abnormal genital profile are common in most cases. Mutations in WT1 gene are responsible for Frasier condition (Guaragna., et al. 2012).
Frasier syndrome (FS, OMIM # 136680)
George Fraser in 1962 described this condition. Phenotypic conditions are same as Denys-Drash syndrome (Peco-antić., et al. 2013). Abnormalities of ear, nose, syndactyly with abnormal genital profile are common in most cases. Mutations in WT1 gene are responsible for Frasier condition (Guaragna., et al. 2012).
Denys-Drash syndrome (DDS, OMIM # 194080)
Denys., et al. reported syndromic condition associated with ambiguous genetalia (Kabra and Kataria, 1995) and later after three years Drash., et al. conforms the condition. Rarely 150 cases reported word wide till date. The syndrome is associated with ambiguous external genitalia. However most males are normal but ambiguity is observed in females (Mueller, 1994). Within a few months after birth, kidney disease is observed; Glomerulosclerosis (Niaudet, 2004) is also common. Preliminary, Nephropathy is observed that leads to renal failure. Wilms tumor is also associated in some of rare conditions (Heathcott., et al. 2002). Individual with Denys-Drash syndrome remains infertile. In almost all cases pathogenic mutations are inherited in autosomal dominant manner (Niaudet, 2004).
Denys., et al. reported syndromic condition associated with ambiguous genetalia (Kabra and Kataria, 1995) and later after three years Drash., et al. conforms the condition. Rarely 150 cases reported word wide till date. The syndrome is associated with ambiguous external genitalia. However most males are normal but ambiguity is observed in females (Mueller, 1994). Within a few months after birth, kidney disease is observed; Glomerulosclerosis (Niaudet, 2004) is also common. Preliminary, Nephropathy is observed that leads to renal failure. Wilms tumor is also associated in some of rare conditions (Heathcott., et al. 2002). Individual with Denys-Drash syndrome remains infertile. In almost all cases pathogenic mutations are inherited in autosomal dominant manner (Niaudet, 2004).
Transcriptional factor WT1 is involve in development of kidney and gonads (Hammes., et al. 2001). As reported earlier, WT1 plays important role in genital development. Hence mutation in WT1 gene affects the activity of other genes (SRY, SOX9andNR0B1) that accelerates abnormal genitalia and renal failure.
Congenital Adrenal Hyperplasia (CAH, OMIM # 201910)
Adrenal insufficient leads to CAH, in which adrenal gland fails to produce vital hormones for development (Speiser, 2015). Aberrant level of testosterone and androgen leads to abnormal external genitalia. CAH is recessive condition (White., et al. 2012) which occurs 1 in 10,000 to 1 in 20,000 worldwide (Speiser., et al. 2010). Aberrant sex hormones, under developed reproductive tissue, chrptorchidism and infertility are usually observed. CYP21A2 gene is responsible for classical type of CAH in which aberrant characters notable in female too (White., et al. 2012). In contrast NR0B1 gene involve in congenital X-linked adrenal hyperplasia (Larson., et al. 2012).
Adrenal insufficient leads to CAH, in which adrenal gland fails to produce vital hormones for development (Speiser, 2015). Aberrant level of testosterone and androgen leads to abnormal external genitalia. CAH is recessive condition (White., et al. 2012) which occurs 1 in 10,000 to 1 in 20,000 worldwide (Speiser., et al. 2010). Aberrant sex hormones, under developed reproductive tissue, chrptorchidism and infertility are usually observed. CYP21A2 gene is responsible for classical type of CAH in which aberrant characters notable in female too (White., et al. 2012). In contrast NR0B1 gene involve in congenital X-linked adrenal hyperplasia (Larson., et al. 2012).
NR0B1 gene is located on X chromosome and important factor during sex determination. Mutation in NR0B1 results in abnormal DAX1 protein production and that impaired the process of sex development (Barbaro., et al. 2009).
Androgen insensitivity syndrome (AIS, OMIM # 300068)
During AIS body is unable to respond to sex hormones androgens. In case of complete androgen insensitivity syndrome, true hermaphrodite (both male and female phenotype) condition is notable (Hughes., et al. 2012). It may occurs 1 in 99,000 genetic male individuals (Oakes., et al. 2008).
During AIS body is unable to respond to sex hormones androgens. In case of complete androgen insensitivity syndrome, true hermaphrodite (both male and female phenotype) condition is notable (Hughes., et al. 2012). It may occurs 1 in 99,000 genetic male individuals (Oakes., et al. 2008).
Androgen receptor (AR) gene is located on X chromosome and inherited as autosomal recessive condition (Oakes., et al. 2008). Androgen Receptor gene encodes a protein which helps cells to recognize androgen hormones. Mutations in AR gene results in low level of testosterone (Galani., et al. 2008). Hence male sexual characters are endured under-developed. Depending upon the level of androgens, individual became ovotesticular to partial hermaphrodite or true hermaphrodite.
Campomelic dysplasia (CD, OMIM # 114290)
Campomelic dyplasia, affects the growth and development of skeleton and reproductive system (Karaer., et al. 2014). Mutations inSOX9 influence the developmental process during sex determination. The faulty protein formed, is inadequate to normal development. As previously reported, SOX9 have major role in SRY mechanism which ultimately decides the sex of fetus (Lamb., et al. 1999). Hence any mutation in SOX9 gene eventually causes DSD.
Campomelic dyplasia, affects the growth and development of skeleton and reproductive system (Karaer., et al. 2014). Mutations inSOX9 influence the developmental process during sex determination. The faulty protein formed, is inadequate to normal development. As previously reported, SOX9 have major role in SRY mechanism which ultimately decides the sex of fetus (Lamb., et al. 1999). Hence any mutation in SOX9 gene eventually causes DSD.
It is prevalent 1 in 20, 0000 with autosomal dominant inheritance pattern (Karaer., et al. 2014). Affected fetus born with club feet and long abnormal legs, Curved bones, dislocated hips, abnormal neck and shoulder bones are commonly observed (Gimovsky., et al. 2008; Mattos., et al. 2015). Prevalence of CD is still uncertain.
Precisely SRY and related genes are responsible for DSD. However genetic basis of eunuchism is still unknown. Definite physical examinations are proven that in most cases eunuchs have abnormal external genitalia and which is due to mutations in several genes, during early developmental stage. Social status of hijras remains controversial in India. So the genetic studies are restricted. Eunuchs (Hijras) are intersex but still their intersex status is not defined due to lack of research.
References
- Abusheikha N., et al. “XX Males without SRY Gene and with Infertility.” Human reproduction16.4 (2001): 717-718.
- Achermann., et al. “Disorders of Sex Development.” Harrison’s Principles of Internal Medicine (2015): 2349-2357.
- Alves., et al. “46, XX Male - Testicular Disorder of Sexual Differentiation (DSD): Hormonal, Molecular and Cytogenetic Studies.” Arquivos brasileiros de endocrinologia e metabologia 54.8 (2010.): 685-89.
- Amory., et al. “Klinefelter’ S Syndrome.” lancet 356 (2000): 333-335.
- Amudha S., et al. “SRY (sex Determining Regions in Y) Basis of Sex Reversal in XY Females.” International Journal of Human Genetics 12.2 (2012): 99-103.
- Barbaro Michela., et al. “Characterization of Deletions at 9p Affecting the Candidate Regions for Sex Reversal and Deletion 9p Syndrome by MLPA.” European journal of human genetics 17.11 (2009): 1439-1447.
- Barrionuevo Francisco., et al. “Homozygous Inactivation of Sox9 Causes Complete XY Sex Reversal in Mice 1.” biology of reproduction 74 (2006):195-201.
- Baxter., et al. “HHS Public Access.” Annual Review of Genomics and Human Genetics 14 (2015): 371-392.
- Bernadete., et al. “Turner Syndrome and Genetic Polymorphism : A Systematic Review.” Revista Paulista de Pediatria (English Edition) 33.3 (2015): 363-370.
- Biason-Lauber and E J Schoenle. “Apparently Normal Ovarian Differentiation in a Prepubertal Girl with Transcriptionally Inactive Steroidogenic Factor 1 (NR5A1/SF-1) and Adrenocortical Insufficiency.” American journal of human genetics 67.6 (2000): 1563-1568.
- Bowles J and P Koopman. “New Clues to the Puzzle of Mammalian Sex Determination.” Genome Biology 2.9 (2001):1025.1-1025.4.
- Chassot., et al. “Activation of ??-Catenin Signaling by Rspo1 Controls Differentiation of the Mammalian Ovary.” Human Molecular Genetics 17.9 (2008): 1264-1277.
- Correa., et al. “A Microdeletion in the Ligand Binding Domain of Human Steroidogenic Factor 1 Causes XY Sex Reversal without Adrenal Insufficiency.” Journal of Clinical Endocrinology and Metabolism 89.4 (2004): 1767-1772.
- Drobnič Katja. “A New Primer Set in a SRY Gene for Sex Identification.” International Congress Series 1288 (2006):268–270.
- Forger., et al. “Deletion of Bax Eliminates Sex Differences in the Mouse Forebrain.” Proceedings of the National Academy of Sciences of the United States of America 101.37 (2004):13666-13671.
- Galani., et al. 2008. “Androgen Insensitivity Syndrome : Clinical Features and Molecular Defects.” Hormones 7.3 (2008): 217-229.
- Gao., et al. “The Wilms Tumor Gene, Wt1, Is Required for Sox9 Expression and Maintenance of Tubular Architecture.” PNAS 103.32 (2006): 11987-11992.
- Gimovsky M., et al. “Campomelic Dysplasia: Case Report and Review.” Journal of perinatology 28 (2008): 71-73.
- Gonzalez., et al. “The Patient with Turner Syndrome: Puberty and Medical Managment Concerns.” Fertility and Sterility 98.4 (2012):780-786.
- Graves., et al. “Evolution of the Testis-Determining Gene--the Rise and Fall of SRY.” Novartis Foundation symposium 244 (2002): 86-97.
- Guaragna., et al. “Frasier Syndrome: Four New Cases with Unusual Presentations.” Arq Bras Endocrinol Metab 56.8 (2012): 525-532.
- Hacker A., et al. “Expression of Sry, the Mouse Sex-Determining Gene.” Development 121.6 (1995): 1603-1614.
- Hammes., et al. “Two Splice Variants of the Wilms’ Tumor 1 Gene Have Distinct Functions during Sex Determination and Nephron Formation.” Cell 106.3 (2001): 319-329.
- Heathcott., et al. “A Review of the Phenotypic Variation due to the Denys-Drash Syndrome-Associated Germline WT1 Mutation R362X.” Human mutation 19.4 (2002): 462.
- Yueh-Chiang Hu., et al. “Gata4 Is Required for Formation of the Genital Ridge in Mice.” PLOS Genetics 9.7 (2013): 1-12.
- Hughes and Ieuan A. “Min review : Sex Differentiation.” 142.8 (2001): 3281-3287.
- Hughes and Ieuan A. “Androgen Insensitivity Syndrome.” The Lancet 380 (2012): 1419-1428.
- I Lilian., et al. “Y chromosome in Turner Syndrome : Review of the Literature.” Sao Paulo Med Journal 2009 127.6 (2009.): 373-378.
- Jaubert F., et al. “Hermaphroditism Pathology.” RJME - Romanian Journal of Morphology and Embryology (2004): 41-51.
- Jordan., et al. “Wnt4 Overexpression Disrupts Normal Testicular Vasculature and Inhibits Testosterone Synthesis by Repressing Steroidogenic Factor 1 ͞ -Catenin Synergy.” PNAS Genetics 100.19 (2003): 10866-10871.
- Kabra M and A Kataria. “Denys-Drash Syndrome.” Indian Pediatrics 32 (1995):1310-1313.
- Kanai., et al. “Structural and Functional Characterization of the Mouse Sox9 Promoter : Implications for Campomelic Dysplasia.” Human Molecular Genetics 8.4 (1999): 691-693.
- Karaer., et al. “Case Report A Case of Campomelic Dysplasia in Whom a New Mutation Was Found in the SOX9 Gene.” Turkish archives of pediatrics 49 (2014):154-156.
- Knower and Vins Kelly Harley. “Turning on the Male - SRY, SOX9 and Sex Determination in Mammals” Cytogenetic and Genome Research 101 (2003): 185-198.
- Koopman., et al. “Of Sex and Determination: Marking 25 Years of Randy, the Sex-Reversed Mouse.” Development 143.10 (2016): 1633-1637.
- Ku Laimutis and Walter Just. “KLINIKINIAI ATVEJAI.” 41.5 (2005): 132-134.
- Kwok., et al. “Mutations in SOX9, the Gene Responsible for Campomelic Dysplasia and Autosomal Sex Reversal.” The American Journal of Human Genetics 57 (1995): 1028-1036.
- De la Chapelle A. “Analytic Review: Nature and Origin of Males with XX Sex Chromosomes.” American journal of human genetics 24.1 (1972): 71–105.
- Lamb., et al. “Autosomal XX Sex Reversal Caused by Duplication of SOX9 Autosomal XX Sex Reversal Caused by Duplication of SOX9.” American journal of medical genetics 87 (1999): 349-353.
- Lamb., et al. “Autosomal XX Sex Reversal Caused by Duplication of SOX9.” American Journal of Medical Genetics 87 (2000): 349-353.
- Larson., et al. “Disorders of Sex Development: Clinically Relevant Genes Involved in Gonadal Differentiation.” Discovery Medicine 14.78 (2012): 301–309.
- Leuan A Hughes., et al. “Consensus Statement on Management of Intersex Disorders.” Journal of Pediatric urology (2006).
- Lin Lin., et al. “Heterozygous Missense Mutations in Steroidogenic Factor 1(sf1/ Ad4BP , NR5A1 ) Are Associated with 46 , XY Disorders of Sex Development with Normal Adrenal Function.” Journal of Clinical Endocrinology and Metabolism 92.3 (2007): 991-999.
- Lourenço., et al. “Mutations in NR5A1 Associated with Ovarian Insufficiency.” The New England journal of medicine 360.12 (2009): 1200-1210.
- Macías-gómez., et al. “46, XX Ovotesticular Disorder in a Mexican Patient with Beckwith – Wiedemann Syndrome : A Case Report.” Journal of Medical case report 301.6 (2012): 4-7.
- Mattos Eduardo P., et al. “Clinical and Molecular Characterization of a Brazilian Cohort of Campomelic Dysplasia Patients, and Identification of Seven New SOX9 Mutations.” Genetics and Molecular Biology 38 (2015): 14-20.
- Mcdowall., et al. “Functional and Structural Studies of Wild Type SOX9 and Mutations Causing Campomelic Dysplasia.” The journal of biological chemistry 274.34 (1999): 24023-24030.
- Mcdowall., et al. “Functional and Structural Studies of Wild Type SOX9 and Mutations Causing Campomelic Dysplasia” (1999).
- Meeks., et al. “Dax1 Regulates Testis Cord Organization during Gonadal Differentiation.” Development 130 (2003): 1029-1036.
- Mendonca., et al. “46, XY Disorders of Sex Development (DSD).” clinical Endocrinology 70 (2009): 173-87.
- Migeon., et al. “Sexual Differentiation: From Genes to Gender.” Hormone Research 50.5 (1998): 245-51.
- Mosaicism., et al. “The Human Y Chromosome and The Etiology of True with The Report of A Case With XX / XY.” (1964).
- Mueller RF. “The Denys-Drash Syndrome.” Journal of Medical Genetics31 (1994): 471-77.
- Niaudet Patrick. “Denys-Drash Syndrome.” Orphanet Journal of Rare Diseases (2004): 1-3.
- Niaudet Patrick “Denys-Drash Syndrome.” (2004): 1-3.
- Nieschlag and Eberhard. “Klinefelter Syndrome.” Archive of "Deutsches Ärzteblatt International110.3 (2013): 347-54.
- Oakes., et al. “Mini-Reviews Complete Androgen Insensitivity Syndrome — A Review.” Journal of Pediatric and Adolescent Gynecology 21.6 (2008): 305-10.
- Ono., et al. “Disorders of Sex Development: New Genes, New Concepts.” Nature reviews. Endocrinology 9.2 (2013.): 79-91.
- Pandian TJ. “Sex Determination and Differentiation in Teleosts.” Aquaculture and Biotechnology. 157-18 (1900): 367-379.
- Park., et al. “Nuclear Receptors Sf1 and Dax1 Function Cooperatively to Mediate Somatic Cell Differentiation during Testis Development.” Development and Disease 132 (2005): 2415-2423.
- Parma., et al. “R-spondin1 Is Essential in Sex Determination, Skin Differentiation and Malignancy.” Nature Genetics 38.11 (2016): 1304-1309.
- Peco-antić., et al. “Proteinuria in Frasier Syndrome.” Serbian Archives of Medicine141.9 (2013): 685–88.
- Polanco., et al. “Sry and the Hesitant Beginnings of Male Development.” Developmental Biology 302 (2007): 13-24.
- Rajender Singh., et al. “SRY -Negative 46, XX Male with Normal Genitals, Complete Masculinization and Infertility.” 12.6 (2006.): 341-346.
- Rey Rodolfo and Nathalie Josso. “Sexual Differentiation the Bipotential Gonad.” Organogenesis (2013).
- Sadovsky Y and C Dorn. “Function of Steroidogenic Factor 1 during Development and Differentiation of the Reproductive System.” Journal of Reproduction and Fertility 5.3 (2000): 136-142.
- Shabsovich., et al. “Genes, Chromosomes, and Disorders of Sex Development : An Update.” The journal of association of genetic technologists 40.4 (2014): 124-130.
- Sharma Preeti. “Historical Background and Legal Status of Third Gender in Indian Society.” IJRESS 2.12 (2012): 64-71.
- Shaw Marie A., et al. “Sex Reversal.” (July 2016).
- She Zhen and Wan Xi Yang. “Molecular Mechanisms Involved in Mammalian Primary Sex Determination.” Journal of Molecular Endocrinology 53.1 (2014): R21-37.
- Sindhe Ujwal Sunil. “Gender Justice and Status of Eunuch.” International Journal of Humanities and Social Science Invention 4 (2012): 1-6.
- Speiser., et al. “Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency : An Endocrine Society Clinical Practice Guideline Morbidities of Congenital Adrenal”. The Journal of Clinical Endocrinology & Metabolism 95(2010): 4133-4160.
- Speiser, Phyllis W. “Congenital Adrenal Hyperplasia.” F1000 Research (2015): 1-4.
- Sybert., et al. “Turner’s Syndrome.” The new England Journal of Medicine (2004): 1227-38.
- Uçan., et al. “46, XX Male Syndrome.” Turkish Journal of Endocrinology and Metabolism 17.2 (2013): 46-48.
- Visootsak Jeannie and Jr John M. Graham. “Klinefelter Syndrome and Other Sex Chromosomal Aneuploidies.” Orphanet journal of rare disease 1.42 (2006): 1-5.
- White., et al. “Congenital Adrenal Hyperplasia Due to 21 Hydroxylase De Fi Ciency : From Birth to Adulthood.” Seminars in Reproductive Medicine 30.212 (2012): 400-409.
- Whitten WK. “Sex Differentiation.” Nature 736 (1982): 784-785.
- Wilhelm., et al. “Sex Determination and Gonadal Development in Mammals.” Physiological reviews 87.1 (2007): 1-28.
- Wu Qiu-Yue., et al. “Clinical, Molecular and Cytogenetic Analysis of 46, XX Testicular Disorder of Sex Development with SRY-Positive.” BMC urology 14.1 (2014): 70.
- Yao Humphrey H C., et al. “Follistatin Operates Downstream of WNT4 in Mammalian Ovary Organogenesis.” Developmental Dynamics230.2 (2014): 210-15.
- Zhong, Quincy and Lawrence C Layman. “Genetic Considerations in the Patient with Turner Syndrome 45, X with or without Mosaicism.” Fertility and Sterility98.4 (2012): 775-779.
- Zhou., et al. “Genetic of Gonadal Determination Genetic of Gonadal Determination.” (2014).
Citation:
RK Patel., et al. “Genetics of Eunuchs: A Review”. Clinical Biotechnology and Microbiology 2.5 (2018): 472-484.
Copyright: © 2018 RK Patel., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.