Research Article
Volume 2 Issue 2 - 2018
Arachis hypogaea Yield Analysis in Rhizobial (Bradyrhizobium sp.) Inoculated Post-solarized Soil
1Department of Biological Sciences, Federal University of Agriculture, Nigeria
2Department of Microbiology, Delta State University, Nigeria
2Department of Microbiology, Delta State University, Nigeria
*Corresponding Author: Monday Ubogu, Department of Biological Sciences, Federal University of Agriculture, Nigeria.
Received: December 18, 2017; Published: March 30, 2018
Abstract
In the bid to enhance A. hypogaea yield through cheaper, environmentally friendly agronomical techniques, biofertilization through rhizobial (Bradyrhizobium sp.) inoculation in post-solarized soil was studied after subjecting soil to the following treatments: solarized inoculated, unsolarized uninoculated (control), solarized uninoculated, and unsolarized inoculated with Bradyrhizobium sp. Following these, viable A. hypogaea seeds were planted 14.0 days after the treatments and then monitored for 120 days period to ascertain the influence of treatments on germination, root lengths, plant heights, weights (fresh and dry), pod and nodule numbers. Soil temperature fluctuated between 28.0 and 54.0°C within the 16 days period of solarization.
Seed germination was not affected by the various treatments applied. Nevertheless, pod and nodule numbers, weights (fresh and dry), height and root length growth were affected by treatments (P ˂ 0.05). Weights, pod and nodule numbers were in the order: solarized inoculated ˃ unsolarized inoculated ˃ unsolarized uninoculated ˃ solarized uninoculated. On the other hand, plant height followed the order: solarized inoculated ˃ unsolarized uninoculated ˃ unsolarized inoculated ˃ solarized uninoculated, while root length growth in the order: solarized uninoculated ˃ solarized inoculated ˃ unsolarized inoculated ˃ unsolarized uninoculated. The highest number of pods and nodules which were recorded in solarized inoculated soil were 11.0 and 136.0 per plant respectively, while the lowest pods and nodules which were recorded in solarized uninoculated were 3.0 and 31.0 per plant respectively. Rhizobial inoculation in post-solarized soil enhanced A. hypogaea yield.
Keywords: A. hypogaea; Inoculation; Post-solarized; Rhizobia; Soil; Yield
Introduction
Arachis hypogaea (Groundnut) is the most significant crop next to soybean among legumes which serve as food for livestock and human, supplying useful dietary protein especially where animal protein is non-existence (Redden., et al. 2005 as cited by Grandawa, 2014). Nigeria is one of the major producers of groundnut in Africa with an estimated production level of 172 kilogram per hectare (Grandawa, 2014).
Although, increase in agricultural productivity through inorganic fertilizers application has helped to a greater extent meet the food demand of the ever soaring human population; this practice is bedevilled with increasing high cost of procurement, environmental pollution accompanying over fertilization and its attendant problems. Cheaper and environmentally friendly agronomical techniques and biofertilization are therefore a more preferable option for plant growth enhancement and crop yield.
While soil solarization (cost-effective and environmentally friendly means of controlling soil-borne plant pathogens) has been shown to enhance plant growth (Pokharel, 2011; Chauhan., et al. 1988; Elmore., et al. 1997), this technique is detrimental to rhizobial population in soil vital for legume nodulation and yield (Pokharel, 2011; Linke., et al. 1991; Chauhan., et al. 1988). On the other hand rhizobial inoculation in unsolarized soil faces possible challenges of stiff competition from well adapted soil microflora and microbial grazing phenomenon (Bashan, 1998) which could lead to inoculants failure.
However, the inoculation of rhizobiain a post-solarized soil may help address these challenges highlighted herein. It is on the premise of enhancing the yield of A. hypogaea by re-inoculation of rhizobia (Bradyrhizobium sp.) in a post-solarized soil that this study was embarked upon.
Materials and Methods
Evaluation of soil baseline physicochemical characteristics
Reference data on the physicochemical characteristics of experimental soil were obtained first before soil treatments and plant propagation. Triplicate soil samples were collected and evaluated for total organic carbon, porosity, nitrogen, phosphorus, pH and textural composition engaging the methods of Black (1965), Ezzati., et al. (2012), micro-Kjeldahl procedure (Hesse, 1979), Bray and Kurtz (1971), Black (1965) and the hydrometer technique as stated by Aliyu and Oyeyiola (2011) respectively.
Reference data on the physicochemical characteristics of experimental soil were obtained first before soil treatments and plant propagation. Triplicate soil samples were collected and evaluated for total organic carbon, porosity, nitrogen, phosphorus, pH and textural composition engaging the methods of Black (1965), Ezzati., et al. (2012), micro-Kjeldahl procedure (Hesse, 1979), Bray and Kurtz (1971), Black (1965) and the hydrometer technique as stated by Aliyu and Oyeyiola (2011) respectively.
Soil solarization treatment
Soil solarization was carried out by adapting the procedure described by Elmore., et al. (1997). Soil used for the propagation of plant was collected within the range of 0-15 cm soil horizon. Four kilogram (4.0 kg) of soil collected per container (12 plastic containers in total with a dimension of 22.0 cm width and 20.0 cm depth). Six of these containers together with soil were solarized for the period of 16.0 days, while the other six remained unsolarized.
Soil solarization was carried out by adapting the procedure described by Elmore., et al. (1997). Soil used for the propagation of plant was collected within the range of 0-15 cm soil horizon. Four kilogram (4.0 kg) of soil collected per container (12 plastic containers in total with a dimension of 22.0 cm width and 20.0 cm depth). Six of these containers together with soil were solarized for the period of 16.0 days, while the other six remained unsolarized.
Soil solarization was embarked upon in mid-November by moisturizing soil and covering it with transparent sheets of polyethylene of 0.025 cm thickness. This was allowed to stay in the open to receive direct solar radiation. Heat escape from soil was prevented by ensuring complete coverage with sheet touching soil surface. Soil daily temperature measurements were taken at 10.00 hours and 16.00 hours using inserted thermometer at 10.0 cm depth.
Rhizobia isolation from A. hypogaea nodules
Rhizobial cells was isolated from root nodules of A. hypogaea adapting the methods of Deka and Azad (2006) and Ben-Gweirif., et al. (2005). Properly formed nodules (matured size and pinkish) were removed from roots and washed properly using tap water, followed by surface sterilization for 2.0 minutes with 70% ethanol. Thereafter, nodules were rinsed in distilled sterile water before engaging sodium hypochlorite (3.5% w/v) for additional surface sterilization of nodule for 2.0 minutes. This was followed by series (thrice) of rinsing using distilled sterile water. Nodules were placed in Mac Cartney bottle and crushed with the addition of few drops of distilled sterilized water.
Rhizobial cells was isolated from root nodules of A. hypogaea adapting the methods of Deka and Azad (2006) and Ben-Gweirif., et al. (2005). Properly formed nodules (matured size and pinkish) were removed from roots and washed properly using tap water, followed by surface sterilization for 2.0 minutes with 70% ethanol. Thereafter, nodules were rinsed in distilled sterile water before engaging sodium hypochlorite (3.5% w/v) for additional surface sterilization of nodule for 2.0 minutes. This was followed by series (thrice) of rinsing using distilled sterile water. Nodules were placed in Mac Cartney bottle and crushed with the addition of few drops of distilled sterilized water.
Wire loop was used to collect loop-full of the crushed nodules. This was streaked on petri dishes containing yeast extract manitol agar (YEMA)incorporated congo red and subsequently incubated at 30.0 ± 2.0°C (room temperature) for 72 hours. Colonies on YEMA plates that were viscous,smooth, musky odour, circular edge, convex and raised, which took up faintly incorporated dye were considered as colonies of rhizobia. These colonies were removed from the plates for further purification and identification before storing in slant of YEMA as stock culture for future use
Identification of Bradyrhizobium sp. isolated from A. hypogaea nodules
Bradyrhizobium sp. isolated from the nodules of A. hypogaea was characterized using Bergey’s Manual of Determinative Bacteriology (John., et al. 1994) on the basis of morphological, cultural and biochemical features of the isolate as well as its intrinsic resistance to tested array of antibiotics.
Bradyrhizobium sp. isolated from the nodules of A. hypogaea was characterized using Bergey’s Manual of Determinative Bacteriology (John., et al. 1994) on the basis of morphological, cultural and biochemical features of the isolate as well as its intrinsic resistance to tested array of antibiotics.
Bradyrhizobium population scale up and the inoculation of unsolarized and solarized soil
Three of the unsolarized and solarized plastic containers with soil were inoculated with the previously isolated bradyrhizobium sp. From the root nodules of A. hypogaea 24 hours after soil solarization activity. Culture of Bradyrhizobium sp. that have been purified was scaled up in a four liter jerry can (sterilized with 70.0% ethanol) containing two liter of yeast extract manitol broth (YEMB) after previous transfer from a 500 ml flask containing 200 ml of YEMB culture of Bradyrhizobium. Aliquot of the culture in test tubes were subsequently centrifuged for five minutes at the rate of 4000 rev/minute to harvest cells. Rhizobial cells that were harvested were transferred into a four liter sterile jerry can and the volume made up to three liter mark using physiological saline so as to obtain a concentration of 8.5 x 108 cfu/ml. Each of the solarized and unsolarized soil plastic containers was then inoculated with a 500 ml physiological saline containing rhizobia at the designated cell concentration. The inoculated cells were then plowed into soil with the aid of a sterile hand towel.
Three of the unsolarized and solarized plastic containers with soil were inoculated with the previously isolated bradyrhizobium sp. From the root nodules of A. hypogaea 24 hours after soil solarization activity. Culture of Bradyrhizobium sp. that have been purified was scaled up in a four liter jerry can (sterilized with 70.0% ethanol) containing two liter of yeast extract manitol broth (YEMB) after previous transfer from a 500 ml flask containing 200 ml of YEMB culture of Bradyrhizobium. Aliquot of the culture in test tubes were subsequently centrifuged for five minutes at the rate of 4000 rev/minute to harvest cells. Rhizobial cells that were harvested were transferred into a four liter sterile jerry can and the volume made up to three liter mark using physiological saline so as to obtain a concentration of 8.5 x 108 cfu/ml. Each of the solarized and unsolarized soil plastic containers was then inoculated with a 500 ml physiological saline containing rhizobia at the designated cell concentration. The inoculated cells were then plowed into soil with the aid of a sterile hand towel.
Assessing seeds of A. hypogaea for viability
Seeds of A. hypogaea purchased from a local market (North Bank Market, Benue State, Nigeria) were initially assessed for viability before propagation. Viability test was carried out by adapting the floatation method of Suma and Srimathi (2014). A. hypogaea seeds were immersed for 12.00 hours in lukewarm water after which floating seeds which are non-viable were discarded and those that remained immersed (which are viable) were preferentially selected for propagation.
Seeds of A. hypogaea purchased from a local market (North Bank Market, Benue State, Nigeria) were initially assessed for viability before propagation. Viability test was carried out by adapting the floatation method of Suma and Srimathi (2014). A. hypogaea seeds were immersed for 12.00 hours in lukewarm water after which floating seeds which are non-viable were discarded and those that remained immersed (which are viable) were preferentially selected for propagation.
Treatment of soil and propagation of plant
Soil meant for the propagation of A. hypogaea was initially subjected to the following treatments: solarized inoculated, unsolarized uninoculated (control), solarized uninoculated and unsolarized inoculated with Bradyrhizobium sp. Each treatment was replicated thricewith soil receiving regular watering before seed propagation.
Soil meant for the propagation of A. hypogaea was initially subjected to the following treatments: solarized inoculated, unsolarized uninoculated (control), solarized uninoculated and unsolarized inoculated with Bradyrhizobium sp. Each treatment was replicated thricewith soil receiving regular watering before seed propagation.
Seed propagation was done two weeks after rhizobial inoculation into soil. Viable seeds were propagated five per container of soil ensuring consistency in depth (2.0 cm deep). Thinning of germinated seeds was done leaving the best growth, two stands per container of soil. Propagated plants were monitored for four months ensuring regular watering (avoiding flooding condition).
Evaluating treatment effect on A. hypogaea germination
The effect of the various treatments on A. hypogaea germination was assessed by observing the earliest time of shoot appearance in each of the treated soils, while percentage germination was evaluated using the formula:
Percentage germination (%) = Number of germinated seeds × 100
Number of propagated seeds
The effect of the various treatments on A. hypogaea germination was assessed by observing the earliest time of shoot appearance in each of the treated soils, while percentage germination was evaluated using the formula:
Percentage germination (%) = Number of germinated seeds × 100
Number of propagated seeds
Evaluating treatment effect on pod and nodule formation in A. hypogaea
Nodule and pod numbers per plant under the influence of the various treatments were evaluated after 4 months of plant propagation. Running tap water at slow speed was used to wash uprooted roots making sure there was no root damage. The number of nodules and pods per plant were then evaluated by enumeration after roots have been air-dried for 30 minutes.
Nodule and pod numbers per plant under the influence of the various treatments were evaluated after 4 months of plant propagation. Running tap water at slow speed was used to wash uprooted roots making sure there was no root damage. The number of nodules and pods per plant were then evaluated by enumeration after roots have been air-dried for 30 minutes.
Evaluating treatment effects on the weights (fresh and dry), root length and height growth of A. hypogaea
The effect of treatments on the weights (fresh and dry), root length and height growth were determined after 4 months of planting. For the evaluation of weights, plant was uprooted with roots washed in slow running tap water to free all adhering soil particles. Fresh weight was then taken after air-drying for 30 minutes, while dry weight taken after heating to constant weight in hot-air oven at 600C with the aid of electronic weighing balance.
The effect of treatments on the weights (fresh and dry), root length and height growth were determined after 4 months of planting. For the evaluation of weights, plant was uprooted with roots washed in slow running tap water to free all adhering soil particles. Fresh weight was then taken after air-drying for 30 minutes, while dry weight taken after heating to constant weight in hot-air oven at 600C with the aid of electronic weighing balance.
On the other hand, treatment effect on plant height growth was evaluated by measuring plant from it base to the tip of the tallest leaf, and root length measured from base to the tip of the longest root with the aid of a calibrated meter rule.
Analysis of data
Numerical data obtained from the study were evaluated employing statistical tools such dispersion, measure of central tendency, analysis of variance and Student’s t-test (P ˂ 0.05).
Numerical data obtained from the study were evaluated employing statistical tools such dispersion, measure of central tendency, analysis of variance and Student’s t-test (P ˂ 0.05).
Results
Baseline physicochemical characteristics of soil
Baseline data from this study showed that the soil employed for the propagation of A. hypogaea has a neutral pH with fair amount of nitrogen content and porosity sufficient for A. hypogae cultivation. Based on its textural constituents the soil may describe as loamy sand (Table 1).
Baseline data from this study showed that the soil employed for the propagation of A. hypogaea has a neutral pH with fair amount of nitrogen content and porosity sufficient for A. hypogae cultivation. Based on its textural constituents the soil may describe as loamy sand (Table 1).
Solarized soil temperature
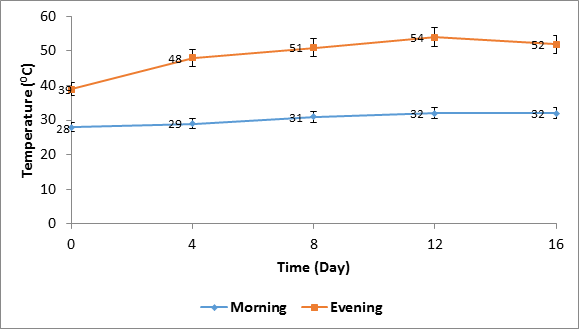
Temperature of solarized soil varied between 28 and 32°C in the morning hours (10.00 hours), and 39 and 54°C in the evening hours (16.00 hours) within the solarization period. Comparatively, soil temperatures were consistently higher in the evening than morning hours. (Figure 1).
Temperature of solarized soil varied between 28 and 32°C in the morning hours (10.00 hours), and 39 and 54°C in the evening hours (16.00 hours) within the solarization period. Comparatively, soil temperatures were consistently higher in the evening than morning hours. (Figure 1).
Treatments effects on A. hypogaea germination
In this study, not minding the fact that solarized inoculated treatment recorded the fastest germination time of 3.7 days, while unsolarized uninoculated recorded the most delayed germination (6.0 days); the differences in the germination time among the array of treatments were not statistically significant (P ˂ 0.05). Similarly, all the various soil treatments witnessed a 100% germination of propagated A. hypogae (Table 2).
In this study, not minding the fact that solarized inoculated treatment recorded the fastest germination time of 3.7 days, while unsolarized uninoculated recorded the most delayed germination (6.0 days); the differences in the germination time among the array of treatments were not statistically significant (P ˂ 0.05). Similarly, all the various soil treatments witnessed a 100% germination of propagated A. hypogae (Table 2).
Treatments effects on nodule and pod formation in A. hypogae
The number of nodules and pods per A. hypogaea plant were significantly affected by the applied treatments (P ˂ 0.05) (Table 3). While the lowest number of nodules and pods (31.0 and 3.0 per plant respectively) were recorded in solarized uninoculated soil, the highest number of nodules and pods (136.0 and 11.0 per plant respectively) were recorded in solarized inoculated soil.
The number of nodules and pods per A. hypogaea plant were significantly affected by the applied treatments (P ˂ 0.05) (Table 3). While the lowest number of nodules and pods (31.0 and 3.0 per plant respectively) were recorded in solarized uninoculated soil, the highest number of nodules and pods (136.0 and 11.0 per plant respectively) were recorded in solarized inoculated soil.
Treatments effects on the weights (fresh and dry), height and root length growth in A. hypogaea
Fresh and dry weights of the plant were significantly affected by the various soil treatments (P ˂ 0.05) (Table 4). The highest fresh and dry weights were recorded in Solarized inoculated (20.3 and 7.6g respectively), while the lowest was in solarized uninoculated treatment (10.0 and 3.0 g respectively).
Fresh and dry weights of the plant were significantly affected by the various soil treatments (P ˂ 0.05) (Table 4). The highest fresh and dry weights were recorded in Solarized inoculated (20.3 and 7.6g respectively), while the lowest was in solarized uninoculated treatment (10.0 and 3.0 g respectively).
Similarly, plant height and root length growth also varied with soil treatments (P ˂ 0.05) (Figure 2). While the highest height growth occurred in solarized inoculated (48.5 cm), the lowest occurred in solarized uninoculated soil (22.1 cm). In contrast to the height growth; the longest root length occurred in solarized uninoculated (43.7 cm) and the shortest in usolarized uninoculated soil (24.3 cm). However, while the root length growth in solarized inoculated did not differ significantly from solarized uninoculated, that of unsolarized uninoculated was also statistically the same with unsolarized inoculated (P ˂ 0.05).
| Soil Properties | Values |
| Texture | |
| Sand (%) | 73.0 ± 1.0 |
| Silt (%) | 18.0 ± 2.0 |
| Clay (%) | 9.0 ± 1.0 |
| pH | 7.2 ± 0.2 |
| Water Holding Capacity (%) | 71.0 ± 1.7 |
| Nitrogen (%) | 0.08 ± 0.01 |
| Phosphorus (mg/kg) | 0.7 ± 0.17 |
| Organic Carbon (%) | 1.5 ± 0.1 |
| Organic Matter (%) | 1.6 ± 0.3 |
Table 1: Baseline physicochemical properties of experimental soil.
| Treatment | Germination Time (Days) | % Germination |
| Unsolarized uninoculated | 6.0 ± 1.0a | 100.0 |
| Unsolarized inoculated | 4.3 ± 0.6a | 100.0 |
| Solarized uninoculated | 4.2 ± 0.8a | 100.0 |
| Solarized inoculated | 3.7 ± 0.6a | 100.0 |
Table 2: Influence of different treatments on germination in A. hypogaea.
Key: *Values with the same superscript alphabet in the same column did not differ significantly (P ˂ 0.05).
Key: *Values with the same superscript alphabet in the same column did not differ significantly (P ˂ 0.05).
| Treatment | Nodules/plant | Pods/plant |
| Unsolarized uninoculated | 36.0 ± 7.8a | 5.3 ± 0.6a |
| Unsolarized inoculated | 70.3 ± 1.5b | 8.3 ± 1.5b |
| Solarized uninoculated | 31.0 ± 6.1c | 3.0 ± 0.0c |
| Solarized inoculated | 136.0 ± 17.8d | 11.0 ± 1.0d |
Table 3: Influence of different treatments on nodule and pod formation in A. hypogaea.
Key: *Values with the same superscript alphabet in the same column did not differ significantly (P ˂ 0.05).
Key: *Values with the same superscript alphabet in the same column did not differ significantly (P ˂ 0.05).
| Treatment | Fresh weight (g) | Dry weight (g) |
| Unsolarized uninoculated | 12.7 ± 2.1a | 3.8 ± 0.6a |
| Unsolarized inoculated | 15.7 ± 1.5b | 5.2 ± 0.4b |
| Solarized uninoculated | 10.0 ± 2.0a | 3.0 ± 0.6a |
| Solarized inoculated | 20.3 ± 2.1c | 7.6 ± 1.2c |
Table 4: Influence of treatments on fresh and dry weights of A. hypogaea.
Key: *Values with the same superscript alphabet in the same column did not differ significantly (P ˂ 0.05).
Key: *Values with the same superscript alphabet in the same column did not differ significantly (P ˂ 0.05).
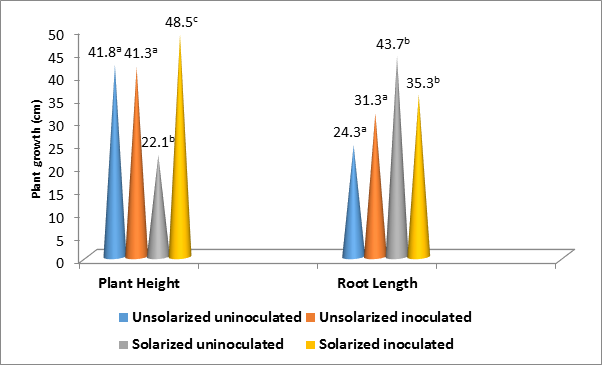
Figure 2: Influence of treatments on the height and root length growth in A. hypogaea.
*Key: Values with the same superscript alphabet for the same growth parameter did not differ significantly (P ˂ 0.05)
*Key: Values with the same superscript alphabet for the same growth parameter did not differ significantly (P ˂ 0.05)
Discussion
Soil with a pH of 5.3 to 7.3, moderate organic matter, light coloured, sandy loam, sandy loam, loamy sand texture, with moderate water holding capacity and very low level of clay are deemed best for the growth of A. hypogaea (Nyambok., et al. 2011; Ajeigbe., et al. 2014; Okello., et al. 2014). Though the degree of tolerance to acidic pH depend on species; legume and rhizobial cells are generally sensitive to pH less than 5.0 as a result of magnesium and aluminum induced toxicity, which result in impaired nodulation and symbiotic nitrogen fixation (Lowendorf., et al. 1981; Munns., et al. 1981; Grahamp et. al. 1994; Mohammadi., et al. 2012), the overall consequent of these is a reduction in plant biomass and yield (Rice., et al. 2000). The soil engaged in this study typified texturally a loamy sand soil, having a moderate water holding capacity with a neutral pH (7.2). In line with the aforementioned reports, the baseline properties suggest that the soil is considerably adequate for the propagation of A. hypogaea.
Soil temperature under the influence of solarization fluctuated in both morning and evening hours within the 16.0 days of treatment with higher temperatures in the evening than morning. Previous report by Chauhan., et al. (1988), corroborate this result. The highest temperature attained at the depth of 10 cm in both morning and evening was 39 and 54°C respectively. These temperatures are sufficient to achieve effective solarization within the period of exposure. Elmore., et al. (1997) reported that solarization of soil at temperature of 53°C at depth of 10 cm in greenhouse treatment was sufficient to decimate the population of soil microorganisms within 16 days. Similarly, Sonku and Nomura (1987) maintained that temperature sustenance at 40°C on a daily basis for 10 successive days is what is required to kill soil-borne microorganisms including pathogens.
Notwithstanding the fact that the time and percentage germination in A. hypogaea were not statistically different among the various treatments applied to soil, it is pertinent to point out that Solarized inoculated recorded the fastest germination time followed by Solarized uninoculated soil. This seemingly infinitesimal speed in germination observed may be attributed to solarization effect and not the consequence of rhizobial inoculation. Accelerated germination has been reported to occur in plant due to the influence of solarization (Stapletonand Devay, 1986; Jat., et al. 2014) but not as a result of the inoculation of rhizobia (Argaw, 2012; Ndlovu, 2015), since it is irrelevant to germination (Ndlovu, 2015) as the fixation of nitrogen starts around 14-35 after infection by rhizobial cells (Dupont., et al. 2012).
This study indicate there were significant differences in the number of nodules and pods formed in A. hpogaea as a result of the various treatments applied to soil. All the soils inoculated with Bradyrhizobiumsp. Recorded higher yield of nodules and pods than the uninoculated, with Solarized inoculated producing the highest yield while, solarized uninoculated producing the least. These findings are in consonant with the reports of previous workers. Although, Ahmed., et al. (2006), in their own studies maintained that while nodulation was enhanced in Vigna radiata (Mungbean) in soil inoculated with Rhizobuim over uninoculated, their yield did not differ significantly. However, in a similar study, Megueni., et al. (2006), observed enhanced nodule and seed yield in soybean planted in solarized inoculated soil with Rhizobuim over solarized uninoculated, unsolarized uninoculated and unsolarized inoculated soils with Rhizobium. In the same vein, Habete and Buraka (2016); Nambiar (1985), reported a higher level of nodulation and pod formation in two varieties of P. vulgaris; and A. hypogaea respectively in soil inoculated with Rhizobium over the uninoculated. Furthermore, Yusif., et al. (2016), also recorded higher number of effective nodules in A. hypogaea inoculated with Rhizobium in contrast to uninoculated.
Increasing compactable rhizobia inoculum size in soil has been shown to increase yield in leguminous plants (Rice, 1975). Nevertheless, microbial soil inoculants are faced with several difficulties such as ability to effectively out-compete indigenous soil microflora that are well adapted to the environment and repel protozoan grazing (Bashan, 1998).
Soil solarisation may help in overcoming these challenges as resident microflora in solarised soil are either killed or weakened resulting in their diminished competitive ability. The increased nodulation and pod formation recorded in A. hypogaea in the solarized inoculated soil over the rest treatments in this study may therefore be attributed to the favourable environment that allowed the survival of a critical number of Bradyrhizobiumsp. and their proliferation upon their re-inoculation in the post-solarized soil.
A. hypogaea weights (fresh and dry) measurement revealed that these growth parameters varied with the applied treatments. Solarized inoculated soil produced a higher plant biomass than the rest treatments. This improved plant biomass in the solarized inoculated soil over the rest treatments may once again be ascribed to increased availability and utilization of fixed nitrogen consequent upon the proliferation of inoculated Bradyrhizobiumsp. and increased nodulation efficiency in a less competitive solarized soil. Rhizobium is a plant growth promoting rhizobacteria (Ahmad and Khan, 2010) and has been shown to enhance plant dry matter yield (Thakur and Panwar, 1995). While investigating A. hypogaea response to exogenous inoculation of some stains of Bradyrhizobium, Baba- Moussa., et al. (2014), reported that strain STM 5945 produced a higher aerial and root dry biomass than the uninoculated control. Similarly, Yusif., et al. (2016), also observed a higher root and shoot dry weights in A. hypogaea inoculated with Rhizobium than uninoculated control.
The various treatments resulted in differential height and root growth in A. hypogaea. While the highest height was registered in solarized inoculated soil, solarized uninoculated which recorded the lowest height, also had the longest root growth which was however statistically the same with solarized inoculated soil. The enhanced height growth in A. hypogaea in the solarized inoculated soil could be attributed at least in part to Bradyrhizobium sp. inoculation. Enormous body of unsubtle evidence supporting this submission are well documented. Shaheen and Rahmatullah (1994), Basu., et al. (2006), Baba- Moussa., et al. (2014) and Yusif., et al. (2016), have all reported previously that increased height growth in A. hypogaea occurred following rhizobia inoculations as compared to uninoculated controls. The increased root length growth in the solarized inoculated and solarized uninoculated over the rest treatments may be credited to solarization effect, a common denominator separating the two other treatments in this case. Soil solarization has been reported to stimulate plant root growth. Root growth stimulation have been attributed to increased dissolution and availability of nutrients such as Mg2+, NH4+, K+, NO3+, Ca2+ and fulvic acid during thermal breakdown of soil organic matter (Elmore., et al. 1997; Pokharel, 2011; Chauhan., et al. 1988).
Conclusion
It is evidently clear from this study that the yield of A. hypogaea an important dietary legume to man and animal can be improved through the inoculation of Bradyrhizobium sp. (a symbiotic nitrogen fixing bacteria) in a post-solarized soil.
References
- Ahmed ZI., et al. “Effect of Rhizobium inoculation on growth and noduleformation of green gram”. International Journal of Agriculture and Biology2 (2006): 235-237.
- Ahemad M and Khan MS. “Comparative toxicity of selected insecticides to pea plants and growth promotion in response to insecticide-tolerant and plant growth promoting Rhizobium leguminosarum”. Crop Protection29.4 (2010): 325-329.
- Aliyu MB and Oyeyiola GP. “Rhizosphere Bacterial Flora of Groundnut (Arachis hypogeae)”. Advances in Environmental Biology 5.10 (2011): 3196-3202.
- Ajeigbe HA., et al. “Farmer’s Guide to Groundnut Production in Nigeria”. International Crop Research Institute for Semi-Arid Tropics (2014).
- Argaw A. “Evaluation of Co-inoculation of Bradyrhizobium japonicum and Phosphate Solubilizing Pseudomonas spp. Effect on Soybean (Glycine max L. Merr.) in Assossa Area”. Journal of Agricultural Science and Technology 14.1 (2012): 213-224.
- Basu M and Bhadoria PBS. “Performance of groundnut (Arachis hypogaea Linn) under nitrogen fixing and phosphorus solubilizing microbial inoculants with different levels of cobalt in alluvial soils of eastern India”. Agronomical Research 6.1 (2008): 15-25.
- Bashan Y. “Inoculant of plant-growth promoting bacteria for use in agriculture”. Biotechnological Advances 16.4 (1998): 720-729.
- Ben-Gweirif SF., et al. “Effect of some pesticides on different isolates of Rhizobium Leguminosarum in Libya”. Proc Symp New Trends in Science (2005): 20-24.
- Black CA. “Methods of soil analysis”. Agronomy Series no. 9, ASA, Madison, Wiscoson (1965).
- Bray RH and Kurtz LT. “Determination of total organic and available phosphorus in soils”. Soil Science 59.1 (1979): 39-45.
- Chauhan YS., et al. “Effects of Soil Solarization on Pigeonpeaand Chickpea”. Research Bulletin no. 11. International Crops Research Institute for the Semi-Arid Tropics (1988).
- Deka AK and Azad P. “Isolation of Rhizobium Strains: Cultural and Biochemical Characteristics”. Legume Research29.3 (2006):209-212.
- Dupont, L., et al. “The Legume Root Nodule: From Symbiotic Nitrogen Fixation to Senescence”. Tetsuji Nagata ISBN 4 (2012): 978-953-51-0144.
- Elmore CL., et al. “Soil solarization, a nonpesticidal method for controlling diseases, nematodes, and weeds”. University of California, division of agriculture and natural resources publication 21377(1997).
- Ezzati S., et al. “Recovery of soil bulk density, porosity and rutting from ground skidding over a 20-year period after timber harvesting in Iran”. Silva Fennica 46.4 (2012): 521-538.
- Graham PH., et al. “Acid pH tolerance in strains of Rhizobium and Bradyrhizobium, and initial studieson the basis for acid tolerance of Rhizobium tropici UMR1899l”. Canadian Journal of Microbiology 40.3 (1994): 198-207.
- Grandawa MM. “Characterisation of physico-chemical properties of Arachis hypogaea L. shells (groundnut) as environmental remediation”. International Conference on Chemical, Biological, and Environmental Sciences (2014): 12-13.
- Habete Aand Buraka T. “Effect of Rhizobium inoculation and nitrogen fertilization on nodulation and yield response of common bean (Phaseolus vulgaries L.) at Boloso Sore, Southern Ethiopia”. Journal of Biology, Agriculture and Healthcare6.13 (2016): 72-75.
- Hesse PR. “A textbook of soil chemical analysis”. John Murray, London (1971).
- Jat RK., et al. “Importance of plastics in horticulture”. Popular Kheti 2.1 (2014): 92-94.
- John GH., et al. “Bergey's Manual of Determinative Bacteriology”. 9thedn. the Williams and Wilkins Company Baltimore (1994).
- Linke KH., et al. “Effect of soil solarization on the yield of food legumes on pest control”. In: DeVay J, Stapleton JJ. And Elemore C E. (Eds.). Proceedings of the 1st International Conference on Soil Solarization (1991).
- Lowendorf HS., et al. “Survival of Rhizobium in acid Soils”. Applied and Environmental Microbiology 42.6 (1881): 951-957.
- Megueni., et al. “Response of soybean (Glycine max L.) to soil solarization and rhizobial field inoculation at Dang Ngaoundre, Cameroon”. Asian Journal of Plant Science 5.5 (2006): 832-837.
- Mohammadi K., et al. “Effective factors on biological nitrogen fixation”. African Journal of Agricultural Research 7.12 (2012): 1782-1788.
- Munns DN., et al. “Soil acidity tolerance of symbiotic and nitrogen-fertilized soybeans”. Agronomy Journal 73.3 (1981): 407-410.
- Nambiar PTC. “Response of groundnut (Arachis hypogaea L.) To Rhizobium inoculation in the field: problems and prospects”. MIRCEN Journal 1.4 (1985): 293-309.
- Ndlovu TJ. “Effect of Rhizobium phaseoli inoculation and phosphorus application on nodulation, growth and yield components of two drybean (phaseolus vulgaris) cultivars”. Faculty of Science and Agriculture University of Limpopo, South Africa (2015).
- Nyambok D., et al. “Good agronomic practices for groundnut in Western Kenya”. Training manual for Trainers (2011).
- Okello DK., et al. “Groundnuts seed production manual for Uganda”. National Agricultural Research Organisation Entebbe (2015).
- Pokharel R. “Soil Solarization, an alternative to soil fumigants”. Colorado State University, Western Colorado Research Center (2011).
- Rice WA. “Effect of CaCO3 and inoculum level on nodulation and growth of alfalfa in an acid soil”. Canadian Journal of Soil Science 55.3 (1975): 245-250.
- Rice WA., et al. “Rhizobial inoculants formulations and soil pH influence on field pea nodulation and nitrogen fixation”. Canadian Journal of Soil Science80.3 (2000): 395-400.
- Shaheen A and Rahmatullah. “Differential Growth, Nodulation and Nitrogen Fixation Efficiency of Groundnut Cultivars Inoculated with Different Rhizobium Strains”. Journal of Agronomy and Crop Science 173.5 (1994): 289-292.
- Sonku Y and Nomura Y. “Control against the source of first infection of causal agent to vegetables under plastic house 4. Inoculum potential of the plants residues infected by eggplant bacteria wilt and evolution of solar disinfection to control of infected plant residues”. Kyushu Agricultural Research 49 (1987): 122.
- Stapleton JJ and Devay JE. “Soil solarization: a non-chemical approach for management of plant pathogens and pests”. Crop Protection 5.3 (1986): 190-198.
- Suma N and Srimathi P. “Influence of water floatation Technique on seedling quality characteristics of Sesamum indicum”. IOSR Journal of Agriculture and Veterinary Science 7.8 (2014): 51-53.
- Tharkur AK and Panwar JDS. “Effect of Rhizobium VAM interactions on growth and yield in mungbean (Vigna radiata L.) Under field conditions”. Indian Journal Plant Pathology 38 (1995): 62-65.
- Yusif SA., et al. “Effects of Biochar and Rhizobium inoculation on nodulation and growth of groundnut in Sokoto State, Nigeria”. Journal of Applied Life Sciences International9.2 (2016): 1-9.
Citation:
Monday Ubogu., et al. “Arachis hypogaea Yield Analysis in Rhizobial (Bradyrhizobium sp.) Inoculated Post-solarized Soil”.
Clinical Biotechnology and Microbiology 2.2 (2018): 331-339.
Copyright: © 2018 Monday Ubogu., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.