Research Article
Volume 1 Issue 6 - 2018
Removal of Phenols from Industrial Wastewaters by Immobilized Pseudomonas Stutzeri A3 Tyrosinase
1Department of Botany and Microbiology, Faculty of Science, Zagazig University, 44519, Zagazig, Egypt
2Head of Animal Health Research Institution (AHRI-ARC), Sharkia, Egypt
2Head of Animal Health Research Institution (AHRI-ARC), Sharkia, Egypt
*Corresponding Author: Fifi M Reda, Department of Botany and Microbiology, Faculty of Science, Zagazig University, 44519, Zagazig, Egypt.
Received: December 22, 2017; Published: January 05, 2018
Abstract
Extracellular tyrosinase produced by Pseudomonas stutzeri A3 (LC192883) was purified to homogeneity with an apparent molecular mass of 45 kDa. The enzyme was purified 6.5 fold with a final specific activity of 45.6 U/mg protein and 43% yield recovery. The purified tyrosinase was immobilized using different solid carriers. The maximum enzyme immobilization yield was observed using Ca-alginate (88.8 %) followed by polyvinyl alcohol (79.8 %). The immobilized enzyme exhibited an improved stability towards pH, temperature and storage time. The Tm value for free enzyme (24.4ºC) was less than immobilized tyrosinase (52.72ºC) assuming thermal stability of the later below 60ºC. The affinity of immobilized enzyme (Km 0.033 mM and Vmax 27.77 U/mg) towards catechol was slightly reduced compared with free enzyme (Km 0.032 mM and Vmax 29.49 U/mg). Thus, it could be deduced that, free enzyme had a high catalytic affinity of catechol, comparing with immobilized enzyme. In addition, immobilized enzyme also showed good operational stability, retained 50% of its activity after reused for 5 cycles to remove tyrosine. The tested immobilized tyrosinase showed its capability of phenol removal at different wastewaters. Our findings supported that enzyme immobilization was preferable to expand the use of tyrosinase based techniques due to its capacity to increase enzyme stability and reusability, and to reduce costs.
Keywords: Tyrosinase; Immobilization; Kinetic properties; Phenol removal; Industrial effluents
Introduction
Tyrosinase is a copper-containing Metallo-protein that is ubiquitously distributed in nature. Polyphenol oxidase is a group of enzymes that mainly exist in two forms; tyrosinase (E.C. 1.14.18.1) and laccase (E.C. 1.10.3.1) which are widely distributed among microorganisms, plants and animals (Mukherjee., et al. 2013). Tyrosinase catalyzes the conversion of L-tyrosine to L-DOPA and melanin (Valipour and Arikan, 2015). Tyrosinase is found to possess excellent capacity for oxidizing phenolic compounds (Balakrishnan and Kalirajan 2015).
In addition to the use of tyrosinase in several industrial application (Duarte., et al. 2012), polyphenol oxidase (PPO) has an important role in the bioremediation of phenolic contaminants from industrial wastewater. These oxidoreductive enzymes remain effective in a wide range of pH and temperature, particularly if they are immobilized on some carriers or matrices. However, high production costs inhibit the widespread use of these enzymes for remediation in industrial scale. Nevertheless, bench and field studies have shown enzymatic wastewater treatment to be feasible options for biodegradation of phenols through biological route (Mukherjee., et al. 2013).
Due to the potential applications of tyrosinase in biotechnology, in particular in bio catalysis and for biosensors, it is desirable to develop a suitable low-cost process for efficient production of this enzyme (Ren., et al. 2013). The optimization of cultural conditions for tyrosinase production is necessary for a successful cultivation process. The key enzyme responsible for biosynthesis of L-dopa is tyrosinase (Ali and Haq 2010). A key enzyme, tyrosinase, catalyzes the first and only rate-limiting steps in melanogenesis (Zaidi., et al. 2014). Melanins are widely used in medicine, pharmacology, cosmetics and other fields. Also, there is a strong consumer demand for melanin as a natural colorant in food and cosmetics, particularly as a component of photo-protective creams and as substitute for synthetic dyes (Dong and Yao 2012).
The term “enzyme immobilization” encompasses a wide range of laboratory and industrial processes aimed at retaining a fully active enzyme on a solid insoluble support (Minteer 2011). Immobilization, not only do multi-enzyme cascade processes become feasible (Jung., et al. 2008) but also, there are several reasons to immobilize an enzyme: first of all, the efficient recovery of the catalyst after the reaction, and its immediate reuse for multiple catalytic cycles. Subsequently, the contamination of reaction products by the catalyst itself is also minimized.
Besides, immobilized enzymes usually feature enhanced specificity, selectivity (Rodrigues., et al. 2013), storage and operational stability (Tran and Balkus 2011) towards various denaturing agents (i.e., extreme pH values, heat, organic solvents), and possibly prevent inhibition (Rodrigues., et al. 2013). In the light of the previous fact, there is an increasing interest for development of the enzyme. So, this study was attempted for the production, purification, characterization and immobilization of Pseudomonas stutzeri tyrosinase in order to investigate its potential for industrial applications.
Material and Methods
Bacterial isolation and screening for tyrosinase production
Bacterial cultures were isolated from five soil samples which collected from rhizosphere zone of potato cultivated soils in Tanta and Zagazig City, Gharbia and Sharkia governorates using standard dilution plate technique (Johnson., et al. 1959).
Bacterial cultures were isolated from five soil samples which collected from rhizosphere zone of potato cultivated soils in Tanta and Zagazig City, Gharbia and Sharkia governorates using standard dilution plate technique (Johnson., et al. 1959).
Pure bacterial isolates were tested for tyrosinase production using production medium (w/v): casein broth hydrolysate (1%), K2HPO4 (0.05%), MgSO4.7H2O (0.025%), L-tyrosine (0.1%), agar (1.5%) (Lelliott., et al. 1966). Melanin production was recorded by the appearance of black or black-brown color around the margin of colonies. The promising isolates were examined for morphological, physiological and biochemical characteristics.
Bacterial identification
The most potent tyrosinase producer isolate was traditionally identified and characterized according to Bergey's Manual of systematic bacteriology (Holt., et al. 1994 and Brenner., et al. 2005). Identification was molecularly confirmed by the analysis of 16S rRNA gene sequence (Altschul., et al. 1997).
The most potent tyrosinase producer isolate was traditionally identified and characterized according to Bergey's Manual of systematic bacteriology (Holt., et al. 1994 and Brenner., et al. 2005). Identification was molecularly confirmed by the analysis of 16S rRNA gene sequence (Altschul., et al. 1997).
Tyrosinase activity assay and Protein determination
Since tyrosinase catalysis two different oxidation reactions, the substrates used to determine its activity were divided into two groups, monophenols and diphenols. A continuous spectrophotometric rate determination method was used to monitor the change of the absorbance due to the transformation of the substrates to products (Espin., et al. 1997). Monophenol L-tyrosine was selected as the basis of the activity assay. This activity assay consists of 1 mM L-tyrosine, 0.1 M pH 6.5 sodium phosphate buffer, and 6 mg/mL tyrosinase reacting at 25ºC and pH 6.5 (Decker 1977). Tyrosinase oxidizes L-tyrosine to L-3, 4- dihydroxyphenylalanine (L-DOPA) which in turn was oxidized to dopaquinone.
Since tyrosinase catalysis two different oxidation reactions, the substrates used to determine its activity were divided into two groups, monophenols and diphenols. A continuous spectrophotometric rate determination method was used to monitor the change of the absorbance due to the transformation of the substrates to products (Espin., et al. 1997). Monophenol L-tyrosine was selected as the basis of the activity assay. This activity assay consists of 1 mM L-tyrosine, 0.1 M pH 6.5 sodium phosphate buffer, and 6 mg/mL tyrosinase reacting at 25ºC and pH 6.5 (Decker 1977). Tyrosinase oxidizes L-tyrosine to L-3, 4- dihydroxyphenylalanine (L-DOPA) which in turn was oxidized to dopaquinone.
Protein content of the free enzyme was estimated on whole cell suspension or crude enzyme preparation by the method of Lowry., et al. (1951). The bound protein of the immobilized enzyme was calculated by the difference between the concentrations of unbound to the initial protein.
Optimized culture conditions for tyrosinase production
Pseudomonas stutzer A3 was cultivated in tyrosinase production broth medium (Lelliott., et al. 1966). Enzyme production was tested under different cultured conditions; different incubation periods (12-72h); different temperatures (20-55°C); different pH-values (pH 5-11); different carbon sources (glucose, xylose, starch, sucrose, maltose, lactose, and mannitol) and different nitrogen sources (peptone, asparagine, glycine, tyrosine, glutamine, casein, yeast and gelatin) under shaking and static conditions. The culture was harvested and centrifuged at 10,000 rpm for 30 min and the obtained cell free filtrate was used as crude enzyme according to Arikan (2008).
Pseudomonas stutzer A3 was cultivated in tyrosinase production broth medium (Lelliott., et al. 1966). Enzyme production was tested under different cultured conditions; different incubation periods (12-72h); different temperatures (20-55°C); different pH-values (pH 5-11); different carbon sources (glucose, xylose, starch, sucrose, maltose, lactose, and mannitol) and different nitrogen sources (peptone, asparagine, glycine, tyrosine, glutamine, casein, yeast and gelatin) under shaking and static conditions. The culture was harvested and centrifuged at 10,000 rpm for 30 min and the obtained cell free filtrate was used as crude enzyme according to Arikan (2008).
Purification of laccase and determination of its molecular weight
The crude enzyme was prepared from three liters of optimized submerged culture of P. stutzeri A3 growing in L-tyrosinase producing medium. The crude enzyme preparation was subjected to slow addition of 70% ammonium sulfate with stirring at 4ºC. The precipitated protein was collected by centrifugation at 10.000 rpm at 4°C and dissolved in a minimum volume of phosphate buffer (0.01 M, pH 8.0) (Bollag., et al. 1996).
The crude enzyme was prepared from three liters of optimized submerged culture of P. stutzeri A3 growing in L-tyrosinase producing medium. The crude enzyme preparation was subjected to slow addition of 70% ammonium sulfate with stirring at 4ºC. The precipitated protein was collected by centrifugation at 10.000 rpm at 4°C and dissolved in a minimum volume of phosphate buffer (0.01 M, pH 8.0) (Bollag., et al. 1996).
The precipitate dialyzed against the same buffer for 24h at 4°C with continuous stirring and occasional changes of the buffers. The dialyzate was fractionated by ion-exchange chromatography (DEAE-Cellulose) and finally by gel-filtration chromatography (Sephadex G100) (Dhevagi and Poorani 2006). The molecular weight of the purified enzyme was checked by denaturing polyacrylamide gel electrophoresis according to the protocol of Laemmli (1970).
Immobilization of tyrosinase
Different methods of tyrosinase immobilization described by Kumar., et al. (2012) were used during these experiments. The different carriers which used in this study were named as silica gel, Ca-alginate, agar–agar and polyvinyl alcohol (PVA). The activity of the immobilized enzyme was assessed as described previously. Immobilization efficiency (%) was expressed by the specific activity of immobilized L-tyrosinase per specific activity of the soluble enzyme.
Different methods of tyrosinase immobilization described by Kumar., et al. (2012) were used during these experiments. The different carriers which used in this study were named as silica gel, Ca-alginate, agar–agar and polyvinyl alcohol (PVA). The activity of the immobilized enzyme was assessed as described previously. Immobilization efficiency (%) was expressed by the specific activity of immobilized L-tyrosinase per specific activity of the soluble enzyme.
Characterization of free and immobilized tyrosinase
Optimum pH and pH stability
Tyrosinase activity (free or immobilized) was assayed using 1mM tyrosine as substrate in 0.1M sodium phosphate buffer (pH 2.0–9.0) at 40°C. Stability of L-tyrosinase was examined after preincubation of the enzyme for 1h at pH from 5.0-11.0. After adding tyrosine (1mM) the reaction mixture was incubated at 40°C for 40 min. The residual tyrosinase activity was determined for each pH.
Optimum pH and pH stability
Tyrosinase activity (free or immobilized) was assayed using 1mM tyrosine as substrate in 0.1M sodium phosphate buffer (pH 2.0–9.0) at 40°C. Stability of L-tyrosinase was examined after preincubation of the enzyme for 1h at pH from 5.0-11.0. After adding tyrosine (1mM) the reaction mixture was incubated at 40°C for 40 min. The residual tyrosinase activity was determined for each pH.
Optimum temperature and thermal stability
The study was carried out at various temperatures (30º–60°C) and tyrosinase activity was then assayed at the corresponding temperature in standard conditions. The thermal stability of the free and Ca-alginate immobilized enzymes were assessed by pre-incubation of enzyme without substrate at various temperatures (55, 60 and 65ºC) using 0.1M phosphate buffer for different incubation periods (20-150 min). The residual enzyme activity was determined for each temperature. The thermal inactivation rate kr (min) was calculated by the first-order kinetic model (Whitaker, 1972).
The study was carried out at various temperatures (30º–60°C) and tyrosinase activity was then assayed at the corresponding temperature in standard conditions. The thermal stability of the free and Ca-alginate immobilized enzymes were assessed by pre-incubation of enzyme without substrate at various temperatures (55, 60 and 65ºC) using 0.1M phosphate buffer for different incubation periods (20-150 min). The residual enzyme activity was determined for each temperature. The thermal inactivation rate kr (min) was calculated by the first-order kinetic model (Whitaker, 1972).
Substrate specificity
The substrate specificity of free and Ca-alginate immobilized enzymes were determined by measuring activity towards several monohydroxyphenol and dihydroxyphenol compounds like L-tyrosine, catechol, and hydroquinone. The activities of immobilized enzyme for this purpose were measured using solutions of these compounds prepared in 0.1M sodium phosphate buffer at concentrations of 1 to 5 mM for catechol, hydroquinone and L-tyrosine. The enzyme activity was assessed as described above.
The substrate specificity of free and Ca-alginate immobilized enzymes were determined by measuring activity towards several monohydroxyphenol and dihydroxyphenol compounds like L-tyrosine, catechol, and hydroquinone. The activities of immobilized enzyme for this purpose were measured using solutions of these compounds prepared in 0.1M sodium phosphate buffer at concentrations of 1 to 5 mM for catechol, hydroquinone and L-tyrosine. The enzyme activity was assessed as described above.
The kinetic parameters of tyrosinase as Vmax, Km and kcat were estimated using different concentration of L-tyrosine, catechol, and hydroquinone, separately (1, 2, 3, 5, 7, 10 and 20 mM). Michaelis-Menten constant (Km) and maximum velocity (Vmax) were calculated from Line weaver-Burk plot. Catalytic efficiency (kcat) was expressed by the specific activity per mol enzyme.
Storage stability
The stability of free and Ca-alginate immobilized tyrosinase preparations were determined after storing in phosphate buffer (50 mM, pH 8) at -20oC for a predetermined period. Under the same storage conditions, the activities of free and immobilized tyrosinase were assessed as described above after 15, 30, 45, 60, 75 and 90 day.
The stability of free and Ca-alginate immobilized tyrosinase preparations were determined after storing in phosphate buffer (50 mM, pH 8) at -20oC for a predetermined period. Under the same storage conditions, the activities of free and immobilized tyrosinase were assessed as described above after 15, 30, 45, 60, 75 and 90 day.
Reusability
Several oxidative cycles were determined using 1 mM tyrosine in order to assess the operational stability of the immobilized tyrosinase. At the end of each oxidation cycle, the immobilized tyrosinase pellets were washed three times with sodium phosphate buffer and the procedure repeated with a fresh aliquot of substrate, as described by Donato., et al. (2014).
Several oxidative cycles were determined using 1 mM tyrosine in order to assess the operational stability of the immobilized tyrosinase. At the end of each oxidation cycle, the immobilized tyrosinase pellets were washed three times with sodium phosphate buffer and the procedure repeated with a fresh aliquot of substrate, as described by Donato., et al. (2014).
The infra-red (IR) analysis for free and Ca-alginate immobilized enzymes
The infra-red was carried out in Micro Analytical Center of Faculty of Science, Cairo University, Egypt. This analysis technique elucidated the types of functional groups on the surface of the free and Ca-alginate immobilized purified enzymes.
The infra-red was carried out in Micro Analytical Center of Faculty of Science, Cairo University, Egypt. This analysis technique elucidated the types of functional groups on the surface of the free and Ca-alginate immobilized purified enzymes.
Removal of phenols from industrial effluents by Ca-alginate immobilized tyrosinase
Six effluent samples (100 mL) were collected separately in sterile bottles from different Factories in Tenth of Ramadan City, Sharkia Governorate, Egypt. The working volume was prepared by adding 2 mL of each effluent sample with different weights of immobilized tyrosinase; 0.1, 0.2 and 0.3g. Then, 3.3 mg. mL-1 of chitosan was added to each sample. Chitosan was added to the reaction mixtures either before initiation or after completion of the reaction, to prevent color generation or to remove color solution. Chitosan solution (0.5% w/v) was prepared by dissolving chitosa in acetic acid 0.5 % (v/v). Reactions were stopped by adding 0.1mL of H3PO4 8.5 % (w/v). Phenolic concentration was analyzed at the beginning and after 20 hours reaction (Bevilaqua., et al. 2002).
Six effluent samples (100 mL) were collected separately in sterile bottles from different Factories in Tenth of Ramadan City, Sharkia Governorate, Egypt. The working volume was prepared by adding 2 mL of each effluent sample with different weights of immobilized tyrosinase; 0.1, 0.2 and 0.3g. Then, 3.3 mg. mL-1 of chitosan was added to each sample. Chitosan was added to the reaction mixtures either before initiation or after completion of the reaction, to prevent color generation or to remove color solution. Chitosan solution (0.5% w/v) was prepared by dissolving chitosa in acetic acid 0.5 % (v/v). Reactions were stopped by adding 0.1mL of H3PO4 8.5 % (w/v). Phenolic concentration was analyzed at the beginning and after 20 hours reaction (Bevilaqua., et al. 2002).
Determination of total phenolic content
The total phenolic content was determined by using Folin-ciocalteu reagent following a slightly modified method of Ainsworth (Ainsworth and Gillespie, 2007). Gallic acid was used as a reference standard for plotting calibration curve. A volume of 0.5 mL of sample was mixed with 2 mL of the Folin-ciocalteu reagent (diluted 1:10 with de-ionized water) and were neutralized with 4 mL of sodium carbonate solution (7.5%, w/v). The reaction mixture was incubated at room temperature for 30 min with intermittent shaking for color development. The absorbance of the resulting blue color was measured at 765 nm using double beam UV-VIS spectrophotometer (UV Analyst-CT 8200). The total phenolic content was determined from the linear equation of a standard curve prepared with gallic acid. The content of total phenolic compounds expressed as mg/mL gallic acid.
The total phenolic content was determined by using Folin-ciocalteu reagent following a slightly modified method of Ainsworth (Ainsworth and Gillespie, 2007). Gallic acid was used as a reference standard for plotting calibration curve. A volume of 0.5 mL of sample was mixed with 2 mL of the Folin-ciocalteu reagent (diluted 1:10 with de-ionized water) and were neutralized with 4 mL of sodium carbonate solution (7.5%, w/v). The reaction mixture was incubated at room temperature for 30 min with intermittent shaking for color development. The absorbance of the resulting blue color was measured at 765 nm using double beam UV-VIS spectrophotometer (UV Analyst-CT 8200). The total phenolic content was determined from the linear equation of a standard curve prepared with gallic acid. The content of total phenolic compounds expressed as mg/mL gallic acid.
Results and Discussion
Screening and identification of Tyrosinase producing bacteria
Tyrosinase enzymes and their genes have previously been characterized from bacteria, fungi, plants and mammals. Bacterial tyrosinases have been reported, of which Streptomyces tyrosinases are the most thoroughly characterized (Selinheimo., et al. 2006). In the present study, forty bacterial isolates were isolated from rhizosphere of potato cultivated soils in Tanta and Zagazig City, Egypt. All bacterial isolates were screened for tyrosinase production using medium containing tyrosine capable of forming melanin. One of all bacterial isolates, isolate no.3, attained the highest melanin production zone and was characterized morphologically and biochemically according to Bergey's key as a member of Pseudomonas genus.
Tyrosinase enzymes and their genes have previously been characterized from bacteria, fungi, plants and mammals. Bacterial tyrosinases have been reported, of which Streptomyces tyrosinases are the most thoroughly characterized (Selinheimo., et al. 2006). In the present study, forty bacterial isolates were isolated from rhizosphere of potato cultivated soils in Tanta and Zagazig City, Egypt. All bacterial isolates were screened for tyrosinase production using medium containing tyrosine capable of forming melanin. One of all bacterial isolates, isolate no.3, attained the highest melanin production zone and was characterized morphologically and biochemically according to Bergey's key as a member of Pseudomonas genus.
The identification of the selected isolate was molecularly confirmed based on 16S rRNA gene sequence. BLAST search indicated that the selected isolate showed 99% identify to Pseudomonas stutzeri and identified as Pseudomonas stutzeri A3 with accession no. LC192883 (Figure 1). Extracellular tyrosinases were previously reported bacteria, there are several reports on tyrosinase from B. thuringiensis strains (Dalfard., et al. 2006). Also, some strains of Streptomyces have extracellular tyrosinases (Claus and Decker 2006).
Optimization of enzyme production by submerged fermentation
Numerous investigations have revealed that the production of tyrosinase by a microorganism in a growth medium is regulated by genetics of the microorganism, the composition of the medium, the growth duration and temperature, pH, the presence of biosynthetic inhibitors, the density of tyrosinase-producing cells and the presence of enzyme inducers (Popa and Bahrim 2011). The present data revealed the maximum activity of extracellular tyrosinase by P. stutzeri A3 achieved after incubation for 48 h at 40°C in production medium adjusted at pH 8 and contained tyrosine as carbon and nitrogen sources under shaking condition (120 rpm) (Data not shown).
Numerous investigations have revealed that the production of tyrosinase by a microorganism in a growth medium is regulated by genetics of the microorganism, the composition of the medium, the growth duration and temperature, pH, the presence of biosynthetic inhibitors, the density of tyrosinase-producing cells and the presence of enzyme inducers (Popa and Bahrim 2011). The present data revealed the maximum activity of extracellular tyrosinase by P. stutzeri A3 achieved after incubation for 48 h at 40°C in production medium adjusted at pH 8 and contained tyrosine as carbon and nitrogen sources under shaking condition (120 rpm) (Data not shown).
These results similarly with Valipour and Arikan (2015) who showed the optimum enzyme activity by Bacillus sp. MV29 after 48h incubation at 40°C at pH 7.0. Also, Roy., et al. (2014) showed that Streptomyces espinosus strain LK4 enzyme activity was found to be optimum at pH 8.0 and 40°C. Furthermore, Majidi and Aksoz (2000) demonstrated that agitation conditions are more favorable for pigment and tyrosinase production more than static conditions. In explanation, agitation showed direct effect on the growth, pigment and tyrosinase biosynthesis of Aspergillus oryzae because agitation affected aeration and mixing of the nutrients in the fermentation medium (El-Batal and Al Tamie 2016).
Purification of tyrosinase
After optimizing the growth and enzyme productivity by P. stutzeri A3, the tyrosinase was purified to apparent homogeneity from the liquid state cultures by gel filtration. Fractional precipitation was carried out initially with 70% ammonium sulphate at 4.0°C (Bollag., et al. 1996). The obtained precipitated protein was suspended immediately (separately), in definite volume of 0.1M sodium phosphate buffer (pH 8.0). From the overall purification profile, the fine specific activity and purity of P. stutzeri A3 tyrosinase were increased to 45.6 Umg-1 and 6.5 fold respectively with 43% yield by Sephadex G100 (Table 1). In this connection, partial purification of thermophilic Bacillus sp. was performed by acetone precipitation and gel filtration chromatography with 35% yield and 1.24 purification fold (Güray 2009). The yield of purified tyrosinase enzyme from Streptomyces espinosus strain LK4 was 31.88% (Roy., et al. 2014).
After optimizing the growth and enzyme productivity by P. stutzeri A3, the tyrosinase was purified to apparent homogeneity from the liquid state cultures by gel filtration. Fractional precipitation was carried out initially with 70% ammonium sulphate at 4.0°C (Bollag., et al. 1996). The obtained precipitated protein was suspended immediately (separately), in definite volume of 0.1M sodium phosphate buffer (pH 8.0). From the overall purification profile, the fine specific activity and purity of P. stutzeri A3 tyrosinase were increased to 45.6 Umg-1 and 6.5 fold respectively with 43% yield by Sephadex G100 (Table 1). In this connection, partial purification of thermophilic Bacillus sp. was performed by acetone precipitation and gel filtration chromatography with 35% yield and 1.24 purification fold (Güray 2009). The yield of purified tyrosinase enzyme from Streptomyces espinosus strain LK4 was 31.88% (Roy., et al. 2014).
| Purification Steps |
Total Enzyme activity (U) | Total Protein content (mg) | Specific activity Umg-1 | Purification fold |
Yield % |
| Crude enzyme | 95 | 13.5 | 7.0 | 1 | 100 |
| 70% Amm. sulfate | 72 | 5.4 | 13.3 | 1.9 | 75 |
| DEAE-Cellulose | 58 | 2.2 | 26.4 | 3.8 | 61 |
| Sephadex G100 | 41 | 0.9 | 45.6 | 6.5 | 43 |
Table 1: Purification profile of Pseudomonas stutzeri A3 tyrosinase.
Molecular weight of P. stutzeri A3 tyrosinase
The purified homogeneity subunit structure of tyrosinase from culture of P. stutzeri A3 was analyzed using denaturing PAGE. From the profile of SDS-PAGE, a distinct band of 45 kDa for P. stutzeri A3 was appeared (Data not Shown). Similarly, Dalfard., et al. (2006) stated that the molecular weight of Bacillus sp. HR03 purified tyrosinase has 50 kDa.
The purified homogeneity subunit structure of tyrosinase from culture of P. stutzeri A3 was analyzed using denaturing PAGE. From the profile of SDS-PAGE, a distinct band of 45 kDa for P. stutzeri A3 was appeared (Data not Shown). Similarly, Dalfard., et al. (2006) stated that the molecular weight of Bacillus sp. HR03 purified tyrosinase has 50 kDa.
Immobilization of P. stutzeri A3 tyrosinase
The purified of P. stutzeri A3 tyrosinase was immobilized using different solid carriers. The main reason for enzyme immobilization is the anticipated increase in its stability to various deactivating force due to restricted conformational mobility of the molecules following immobilization (Estrada., et al. 1991). High yields of immobilization were defined as the activity ratio of immobilized enzyme to the activity of the free enzyme (Quiroga., et al. 2011).
The purified of P. stutzeri A3 tyrosinase was immobilized using different solid carriers. The main reason for enzyme immobilization is the anticipated increase in its stability to various deactivating force due to restricted conformational mobility of the molecules following immobilization (Estrada., et al. 1991). High yields of immobilization were defined as the activity ratio of immobilized enzyme to the activity of the free enzyme (Quiroga., et al. 2011).
The present work was extended to elucidate the immobilization of P. stutzeri A3 tyrosinase on solid carriers (Table 2). The enzyme activity under all immobilization methods was slightly lower than soluble one. The maximum enzyme immobilization yield was observed using Ca-alginate (88.8 %) followed by polyvinyl alcohol (79.8%). In contrary, the lowest immobilization yield was measured using agar-agar (56.5%). While, the physical adsorption of enzyme via activated silica gel displayed a relative lower activity, comparing to the entrapment methods. Brooks., et al. (2006) showed that crude cell extracts of P. putida F6 expressing tyrosinase activity were immobilized in a calcium alginate matrix with an efficiency of approximately 95%. In this relation, it was found that the maximum tyrosinase adsorption capacity of the Poly (ethylene glycol dimethacrylate-N-Vinyl Imidazole)-Cu2+ beads was observed as 14.04 mg/g at pH 6.5. This could be due to the specific interactions between tyrosinase molecules and Cu2+ ions, as tyrosinase is a copper dependent enzyme (Osman., et al. 2007). Also, they added that, the surface functional groups of tyrosinase (nitrogen and sulfur groups) could easily chelate with the poly (EGDMA-VIM)-Cu2+ complexes therefore, yield substantially high enzyme adsorption. Stabilization of multimeric enzymes was observed after immobilization by entrapment, covalent immobilization and physical adsorption (Fernandez-Lafuente, 2009). Moreover, the alginate matrix preserved the structure of the enzyme after immobilization process and it protected the enzyme from conformational changes caused by effects of the temperature. This suggested that the thermal stability of the immobilized enzyme increases as a consequence of the immobilization within calcium alginate beads (Quiroga., et al. 2011).
| Immobilization method | Carrier | Specific Activity (U mg-1 enzyme) |
Immobilization yield (%) | Enzyme loading (mg enzyme g-1 microbeads) | Activity (U g-1 ̄ˡmicrobeads) |
| Physical adsorption | Silica gel | 32.7 | 71.7 | 1.5 | 49.1 |
| Entrapment | Ca-alginate | 40.5 | 88.8 | 2.4 | 97.2 |
| Agar-Agar | 25.8 | 56.5 | 1.1 | 28.4 | |
| Ionic binding | P.V.A | 36.4 | 79.8 | 1.8 | 65.5 |
| Free enzyme | 45.6 | 100 | - | - |
Table 2: Immobilization of purified Pseudomonas stutzeri A3 tyrosinase.
Biochemical properties of the purified free and immobilized enzyme
Optimal pH and pH stability
The present investigation was extended to study the biochemical properties of free and Ca-alginate immobilized P. stutzeri A3 tyrosinase. The optimum pH and temperature of free or immobilized tyrosinase achieved maximum oxidation of L-tyrosine to o-benzoquinone was pH 8 and 40°C respectively (Data not shown). Besides, in the whole investigated temperature and pH ranges, the curve profile of the immobilized enzyme was broader.
Optimal pH and pH stability
The present investigation was extended to study the biochemical properties of free and Ca-alginate immobilized P. stutzeri A3 tyrosinase. The optimum pH and temperature of free or immobilized tyrosinase achieved maximum oxidation of L-tyrosine to o-benzoquinone was pH 8 and 40°C respectively (Data not shown). Besides, in the whole investigated temperature and pH ranges, the curve profile of the immobilized enzyme was broader.
This is probably due to an increase of thermal stability (Li., et al. 2004) because the immobilization reduces the conformational mobility of the enzyme molecules, thus preserving its tertiary structure (Bayramoğlu and Arica 2008). Moreover, hydrophobic interactions and other secondary interactions of the immobilized enzyme might impair conformational flexibility needing higher temperatures for the enzyme molecule to reorganize and attain a proper conformation in order to keep its reactivity (Munjal and Sawhney 2002).
From the profile of pH stability (Figure 2A), free and immobilized P. stutzeri A3 tyrosinase had maximum structural and catalytic stability at pH range from 7 to 9 and reduction on enzyme activity at pH 5 and pH 11. Thus, the negative effect on enzyme activity at higher and lower pH, may be due to the change in enzyme ionization state, modifying its surface charge or dissociation of subunits. In general immobilized tyrosinase exhibited higher stability than the free counterpart.
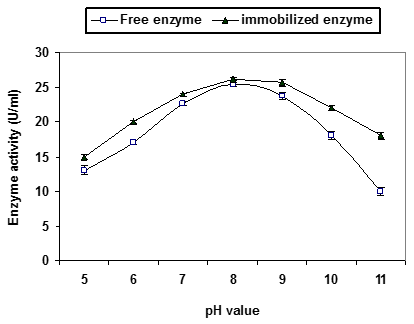
Figure 2A: Characterization of free and immobilized Pseudomonas stutzeri A3 tyrosinase.
(A) pH stability profile. The enzyme was preincubated for 1h at various pH s (5.0 -11.0),
then measuring the residual activity
(A) pH stability profile. The enzyme was preincubated for 1h at various pH s (5.0 -11.0),
then measuring the residual activity
These results could be attributed to the stabilization of the enzyme molecules resulting from their multipoint attachment on the membrane surface (Bayramoğlu and Arica, 2008). Also, Zaidi., et al. (2014) revealed that pH 7.0 was the optimal pH for tyrosinase from A. bisporus using phosphate buffer. Moreover, the lower activity of enzyme at higher temperature, assuming the denaturation of enzyme subunits or unfolding of enzymatic active tertiary structure. Similarly, Donato., et al. (2014) reported that, the influence of temperature on the relative activity of both free and immobilized tyrosinase from mushroom is shown as free and immobilized enzymes exhibited a maximum of activity at 35°C.
Optimal temperature and thermal stability
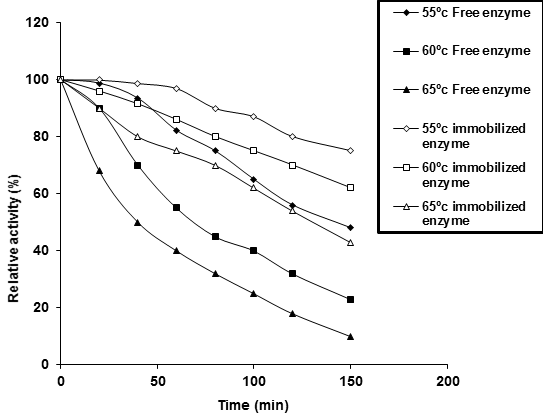
From the profile of thermal stability, the enzyme half-life times (T1/2) of Ca-alginate immobilized P. stutzeri A3 tyrosinase (4.93, 3.29 and 2.18h) were more than the free one (2.37, 1.40 and 0.96h) at incubation temperatures 55, 60 and 65°C, respectively (Figure 2B). From the data, an obvious acquired thermal stability of free and immobilized tyrosinase was thermal denaturation rate (kr). Its value for immobilized enzyme (2.37 x 10-3 min-1) was less than free tyrosinase (4.81 x 10-3 min-1) at 60°C.
From the profile of thermal stability, the enzyme half-life times (T1/2) of Ca-alginate immobilized P. stutzeri A3 tyrosinase (4.93, 3.29 and 2.18h) were more than the free one (2.37, 1.40 and 0.96h) at incubation temperatures 55, 60 and 65°C, respectively (Figure 2B). From the data, an obvious acquired thermal stability of free and immobilized tyrosinase was thermal denaturation rate (kr). Its value for immobilized enzyme (2.37 x 10-3 min-1) was less than free tyrosinase (4.81 x 10-3 min-1) at 60°C.
After incubation of enzyme in different temperature (55, 60 and 65ºC) at various periods (20-150min), the residual activity was determined by the standard assay method (Free enzyme: 55°C: y = -0.3828x + 104.59, 60°C: y = -0.5265x + 94.39, 65°C: y = -0.5446x + 81.675; Immobilized enzyme: 55°C: y = -0.1823x + 103.96, 60°C: y = -0.2581x + 100.95, 65°C: y = -0.3646x + 97.727);
As well as, the acquired structural has stabilized effect by immobilization to the free tyrosinase was clearly revealed from the half-life temperature (Tm). The Tm value was expressed by the degree of temperature, where the enzyme retains about half of its initial activity for 60 minutes of pre-heating, without substrate. The Tm value for free P. stutzeri A3 tyrosinase (24.4°C) was less than the immobilized one (52.72°C) assuming thermal stability of later (Figure 2C).
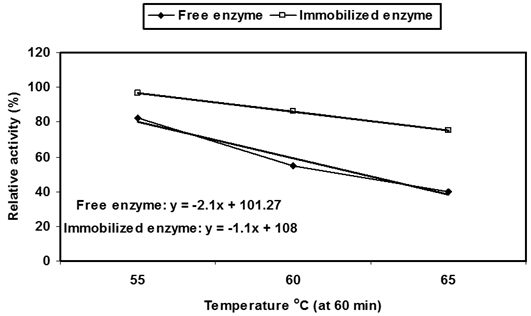
Figure 2C: Thermal inactivation profile.
Tm is temperature degree at which the enzyme retains half of its initial activity at 60 min.
Tm is temperature degree at which the enzyme retains half of its initial activity at 60 min.
Similarly, Arica., et al. (2004) reported that, at 50°C, the free and the spacer-arm attached immobilized tyrosinase from mushroom retained their activity to a level of 47 and 77% respectively during 120 min incubation period. At 55°C, the free and the immobilized enzymes retained their activity to a level of 23 and 61%, respectively. Immobilized tyrosinase was inactivated at a much slower rate than that of the native form. The half-life values (T1/2) of the free and immobilized enzyme were 113 min and 261 min, respectively at 50°C, also (kr) 6.29 x 103 min-1 and 2.18 x 103 min-1, respectively at 50°C
So, the thermo stability of immobilized tyrosinase may be increased considerably because of covalent immobilization. While, McMahon., et al. (2007) reported that the tyrosinase from P. putida F6 was most stable at 30ºC but above this temperature stability decreased dramatically. Longer half-life was reported, e.g. 2h at 50°C for P. sanguineus tyrosinase. While, the half-life for immobilized tyrosinase from mushroom, using a membrane bioreactor in a recycle mode at 35ºC, was 17h (Donato., et al. 2014).
Substrate specificity and kinetic properties of free and immobilized P. stutzeri A3 tyrosinase
The kinetic parameters of free and immobilized tyrosinase for P. stutzeri A3 as Vmax, Km and kcat were estimated using different concentrations of L-tyrosine, hydroquinone and catechol, separately (1-5 mM). From Line weaver-Burk plots, the maximum affinity of the free and immobilized tyrosinase was for catechol followed by hydroquinone and L-tyrosine. The affinity of immobilized enzyme (Km 0.033 mM and Vmax 27.77 U/mg) towards catechol was slightly reduced compared with free enzyme (Km 0.032 mM and Vmax 29.49 U/mg) (Table 3).
The kinetic parameters of free and immobilized tyrosinase for P. stutzeri A3 as Vmax, Km and kcat were estimated using different concentrations of L-tyrosine, hydroquinone and catechol, separately (1-5 mM). From Line weaver-Burk plots, the maximum affinity of the free and immobilized tyrosinase was for catechol followed by hydroquinone and L-tyrosine. The affinity of immobilized enzyme (Km 0.033 mM and Vmax 27.77 U/mg) towards catechol was slightly reduced compared with free enzyme (Km 0.032 mM and Vmax 29.49 U/mg) (Table 3).
| Substrate | Free enzyme | Immobilized enzyme | ||||
| Vmax (U/mg) | Km (mM) |
kcat (min-1) |
Vmax (U/mg) |
Km (mM) |
kcat (min-1) |
|
| Tyrosine | 25.38 ± 0.38 | 0.040 ± 0.008 | 0.564 ± 0.004 | 24.15 ± 0.57 | 0.038 ± 0.01 | 0.536 ± 0.09 |
| Hydroquinone | 26.95 ± 0.20 | 0.041 ± 0.005 | 0.599 ± 0.005 | 25.25 ± 0.14 | 0.040 ± 0.01 | 0.561 ± 0.1 |
| Catechol | 29.49 ± 0.40 | 0.032 ± 0.002 | 0.655 ± 0.02 | 27.77 ± 0.56 | 0.033 ± 0.01 | 0.617 ± 0.08 |
Table 3: Kinetic parameters of substrate specificity of free and Ca-alginate immobilized Pseudomonas stutzeri A3 tyrosinase.
The kinetic parameters were determined by incubation of the enzyme (45.6 U/mg protein) in sodium phosphate buffer (pH 8) with various concentrations of substrate (1, 2, 3, 5, 7, 10 and 20 mM) under the standard assay conditions, then measuring the activity of the enzyme. Maximum velocity (Vmax) was expressed by activity of enzyme in μmol of dopaquinone compounds formed per minute per mg protein enzyme. Km is the substrate concentration (mM) at half of maximum velocity. Kcat is the maximum velocity of the enzyme per mol per min.
Also, it was found that the highest catalytic efficiency of free and immobilized enzymes was for catechol followed by hydroquinone and L-tyrosine. The catalytic efficiency of immobilized enzyme (kcat 0.617 min-1) toward catechol was slightly reduced upon immobilization, comparing to free enzyme (kcat 0.655 min-1). Thus, it could be deduced that, free enzyme had a high catalytic efficiency of catechol, comparing to immobilized enzyme.
These results are in agreement with Donato., et al. (2014) reported that the immobilized tyrosinase from mushroom exhibited a lower Km (reduction of 27% with respect to that the free one), as Km = 1.56 mM and 2.10 mM for the immobilized and free enzyme, respectively. So, results indicate a better affinity of the substrate towards the immobilized tyrosinase compared to the free one. The Km value of A. paeoniifolius was found to be 3.6 mM and Vmax value was determined to be 0.1 s-1 (Balakrishnan and Kalirajan 2015).
In general, the Km of an immobilized enzyme is different from that of the free enzyme due to diffusional limitations, steric effects and ionic strength (Arica., et al. 2000). An increase in Km after immobilization indicates that the immobilized enzymes have an apparent lower affinity for its substrate than the free enzyme. Also, the decrease in the reaction rate might be attributed to: (i) a limited accessibility of the substrate molecules to the active sites of the enzyme, and (ii) the interaction of the enzyme with the functional groups on the surface of beads or large areas of contact between enzyme and support (Quiroga., et al. 2011). Also, this may be caused by the support steric hindrance of the active site, by the loss of enzyme flexibility necessary for substrate binding, or by diffusional resistance to substrate transport (Sahin., et al. 2005).
Storage stability
One of the most important parameters to be considered in enzyme immobilization is storage stability. The stability of the free and the immobilized P. stutzeri A3 tyrosinase preparations was determined after the preparations were stored in phosphate buffer (50 mM, pH 8) at -20°C for a predetermined period. Under the same storage conditions, the relative stability of the immobilized tyrosinase preparations decreased slower than that of the free tyrosinase with increasing the storage periods at -20°C (Figure 3).
One of the most important parameters to be considered in enzyme immobilization is storage stability. The stability of the free and the immobilized P. stutzeri A3 tyrosinase preparations was determined after the preparations were stored in phosphate buffer (50 mM, pH 8) at -20°C for a predetermined period. Under the same storage conditions, the relative stability of the immobilized tyrosinase preparations decreased slower than that of the free tyrosinase with increasing the storage periods at -20°C (Figure 3).
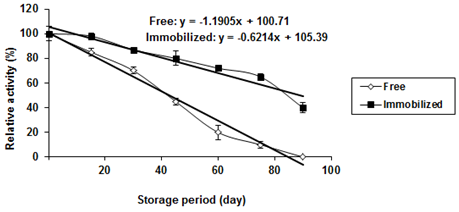
Figure 3: Storage stability of free and Ca-alginate immobilized Pseudomonas
stutzeri A3 tyrosinase (Free enzyme: T1/2 = 42.5 d, Immobilized enzyme: T1/2 = 89d).
Also, it was observed that, the free tyrosinase loss its activity after 90 day, while, the immobilized tyrosinase maintained 40% of its activity after 90 day. Thus, the stored immobilized tyrosinase was more stable than the free one. These results are in agreement with Arica., et al. (2004) who reported that, the free enzyme from mushroom lost all its activity within 4 weeks and the immobilized tyrosinase preserved about 36% of its initial activity during a two months storage period. While, both free and immobilized mushroom tyrosinase were stored at 4°C and activity measurements were performed after 2 months.
After this period, free the enzyme lost about 60% of its initial activity, while the immobilized one lost about 15% (Donato., et al. 2014). It could be maintained here that, the high storage stability of the tyrosinase may be due to a protective microenvironment supplied by hydrogel carrier (alginate) (Quiroga., et al. 2011).
Operational stability of Ca-alginate immobilized tyrosinase
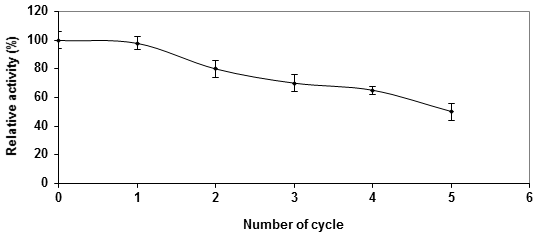
Continuous activity of Ca-alginate immobilized P. stutzeri A3 tyrosinase was assessed for continuous elimination of tyrosine for five successive reactions under standard conditions. It was found that, the activity of immobilized enzyme retained about 80% and 50% by the second and fifth catalytic cycle respectively (Figure 4). Arica., et al. (2004) showed that, during the initial 24h, continuous operation the immobilized mushroom tyrosinase preserved all of its initial activity. After this period, a small decrease in enzyme activity was observed with time. After 40h, the immobilized enzyme lost about 3% of its initial activity, this would be possibly due to the inactivation of tyrosinase upon use.
Continuous activity of Ca-alginate immobilized P. stutzeri A3 tyrosinase was assessed for continuous elimination of tyrosine for five successive reactions under standard conditions. It was found that, the activity of immobilized enzyme retained about 80% and 50% by the second and fifth catalytic cycle respectively (Figure 4). Arica., et al. (2004) showed that, during the initial 24h, continuous operation the immobilized mushroom tyrosinase preserved all of its initial activity. After this period, a small decrease in enzyme activity was observed with time. After 40h, the immobilized enzyme lost about 3% of its initial activity, this would be possibly due to the inactivation of tyrosinase upon use.
One of the problems in continuous enzyme reactions is the operational stability of the enzyme immobilized on the support (Arica., et al. 2004). In addition, the residence time while working in a continuous operation mode is lower than that of the recycle operation mode and thus the suicide inactivation of tyrosinase was reduced (Ramsden., et al. 2010). Moreover, Quiroga., et al. (2011) proved that the immobilized enzyme had important features for uses in continuous processes where they found that after 20 cycles, 78% of the enzyme activity was maintained indicating a good operational stability of the immobilized enzyme.
FTIR analysis for free and immobilized enzyme
The biomass of free and immobilized P. stutzeri A3 tyrosinase were subjected to IR analysis. It was appeared that, the bands in the spectra of the free and immobilized tyrosinase enzymes were assigned and the shift of the wave numbers or the intensity of the peaks for immobilized tyrosinase enzyme indicating to upload enzyme on Ca-alginate. Where, increasing in the molecular weight of the product changed the properties of vibration motions of groups.
The biomass of free and immobilized P. stutzeri A3 tyrosinase were subjected to IR analysis. It was appeared that, the bands in the spectra of the free and immobilized tyrosinase enzymes were assigned and the shift of the wave numbers or the intensity of the peaks for immobilized tyrosinase enzyme indicating to upload enzyme on Ca-alginate. Where, increasing in the molecular weight of the product changed the properties of vibration motions of groups.
Also, it was appeared that, strong broad bands at 3446 cm-1 and 3423 cm-1 for υ (O-H) - phenolic group, strong band 1635 cm-1 and medium band 1631 cm-1 for υ (C = N) and υ (C = O)groups, medium band 1441 cm-1 and strong 1438 cm-1 for υ (C=C) of benzene ring, bond stretch for the band 1101 cm-1, 1132 cm-1 and 1060 cm-1, bend - phenyl for the bands 871 cm-1 and 875 cm-1 and bond stretch for the band 612 cm-1, 538 cm-1 and 526 cm-1 (Figure 5).
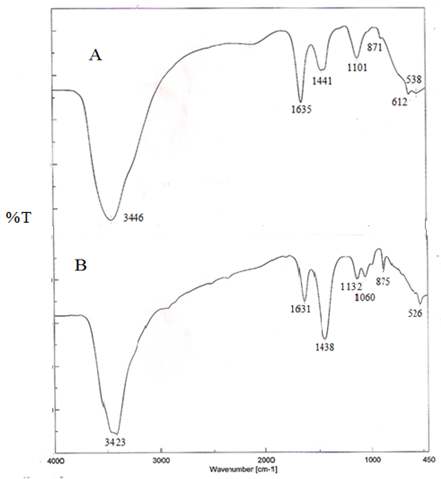
Figure 5: Infrared spectral analysis of free and Ca-alginate immobilized Pseudomonas
stutzeri A3 tyrosinase; A- Free tyrosinase, B- Immobilized tyrosinase.
In this connection, the FT-IR spectrum of purified extracted melanin from Aspergillus oryzae showed the intense broad band at 3447.98 cm−1 corresponds to the OH groups of polymeric structure, the band at 1628.59 and 1114.56 associated with primary amine NH and primary amine CN stretch vibrations of melanin respectively. This band is typical of a conjugated quinoid structure and is believed to be important for the identification of melanin.
The band at 1447.72 cm−1 is assigned to aliphatic methylene scissoring of C-H groups and the band around 2026.83 arises from the carbonyl stretching vibrations. The absorption peak observed at 1071.2 cm−1 was attributed to aromatic ring CH stretching. Bands below 700 cm−1 (685.7, 635.43 and 566 cm−1) ascribed to alkene C-H substitution in the melanin pigment (El-Batal and Al Tamie, 2016). Similarly, FTIR spectrum of sodium alginate showed various distinct peaks of alginate: hydroxyl at 3426cm−1, carbonyl at 1639cm−1, and carboxyl and carboxylate at about 1000–1500cm−1.
Crosslinking of alginate by Ca2+ is confirmed by a decrease in the wave number of the carbonyl peak from 1639 to 1619cm−1 and an increase in the value of wave number of hydroxyl peak (from 3426 to 3447cm−1) (Quiroga., et al. 2011). These shifts account for likely interactions between the enzyme and alginate matrix, which could influence the behavior of the immobilized enzyme (Sahin., et al. 2005).
Application of immobilized enzyme
The enzymatic polishing of phenolic effluent was the aim of this experiment. Phenol removal catalyzed by Ca-alginate immobilized P. stutzeri A3 tyrosinase was initially tested after adding 2 mL of different wastewaters separately with different weights of immobilized tyrosinase. From obtained results in (Table 4), the highest phenolic content was observed in sample 6 (72 mg/L) followed by sample 3 (29 mg/L) and sample 1 (17 mg/L).
The enzymatic polishing of phenolic effluent was the aim of this experiment. Phenol removal catalyzed by Ca-alginate immobilized P. stutzeri A3 tyrosinase was initially tested after adding 2 mL of different wastewaters separately with different weights of immobilized tyrosinase. From obtained results in (Table 4), the highest phenolic content was observed in sample 6 (72 mg/L) followed by sample 3 (29 mg/L) and sample 1 (17 mg/L).
| Immobilized enzyme (g) | Tyrosinase activity (U/mg) | Phenolic concentration (mg/L) | |||||||||||
| Effluent sample | |||||||||||||
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 | ||||||||
| before | after | before | after | before | after | before | after | before | after | before | after | ||
| 0.1 | 4.05 ± 0.028 | 17 ± 0.57* | 6 ± 0.28* | 2 ± 0.28* | - | 29 ± 1.15* | 8 ± 0.57* | 5 ± 0.03 | - | 4 ± 0.57 | - | 72 ± 1.15* | 3 ± 0.28 |
| 0.3 | 12.15 ± 0.08 | 17 ± 0.57* | 2 ± 0.17* | 2 ± 0.28* | - | 29 ± 1.15* | 1.1 ± 0.05* | 5 ± 0.03* | - | 4 ± 0.57* | - | 72 ± 1.15* | 0.1 ± 0.05* |
| 0.5 | 20.25 ± 0.14 | 17 ± 0.57* | 6 ± 0.23* | 2 ± 0.28* | - | 29 ± 1.15* | 2 ± 0.28* | 5 ± 0.03* | - | 4 ± 0.57* | - | 72 ± 1.15* | 0.4 ± 0.05* |
Table 4: Polishing of phenols from industrial effluents by Ca-alginate immobilized tyrosinase.
*: The mean difference is significant at the 0.05 level.
Before: before treatment of effluent with Ca-alginate immobilized tyrosinase
After: after treatment of effluent with Ca-alginate immobilized tyrosinase.
Sample 1: Vegetable factory, Sample 2: Glass house factory, Sample 3: Factory of medication syrup,
Sample 4: Ampoules drugs factory, Sample 5: Pharmaceutical Factory of antibiotic,
Sample 6: Dyes factory, all factories located in Tenth of Ramadan City, Sharkia Governorate, Egypt.
*: The mean difference is significant at the 0.05 level.
Before: before treatment of effluent with Ca-alginate immobilized tyrosinase
After: after treatment of effluent with Ca-alginate immobilized tyrosinase.
Sample 1: Vegetable factory, Sample 2: Glass house factory, Sample 3: Factory of medication syrup,
Sample 4: Ampoules drugs factory, Sample 5: Pharmaceutical Factory of antibiotic,
Sample 6: Dyes factory, all factories located in Tenth of Ramadan City, Sharkia Governorate, Egypt.
So, after treatment of samples 6, 3 and 1 by immobilized tyrosinase, the phenol concentration significantly decreased to 0.1, 1.1 and 2 mg/L, respectively. The best result was achieved using 0.3g of immobilized tyrosinase. Similarly, Roy., et al. (2014) reported that, the tyrosinase enzyme from Streptomyces espinosus strain LK4 was immobilized in sodium alginate which was applied to remove phenolic compounds from water.
Enzymatic polishing processes have several potential advantages over conventional biological treatments. Firstly, they do not need an acclimatization period. Secondly, they suffer less from charge shocks and toxic compounds than microorganisms do. Finally, they are highly specific and do not generate undesired side products (Bevilaqua., et al. 2002).
Phenoloxidases include laccases and tyrosinases have the advantage that they can react with molecular oxygen without the need for externally- supplied co-substrates, which leads to lower costs. Further, when tyrosinases oxidize phenols and other aromatic compounds in wastewaters, typically the oxidized product will polymerize to insoluble compounds that can be removed by filtration or precipitation (Chiacchierini., et al. 2004).
A number of different matrices have been reported for the immobilization of PPO that includes SiO2- alginate hybrid (Abadulla., et al. 2000), calcium and copper alginate and polyamide membrane for (Peralta-Zamora., et al. 2003), cinnamoylated derivatives coated glass beads (Khan and Husain, 2007), chitosan beads (Shao., et al. 2007). These immobilized PPO have been applied for treatment and/or removal of aqueous phenolic contaminants with better performance and reusability with lesser cost in comparison to free enzyme (Mukherjee., et al. 2013).
Conclusion
The immobilization on Ca-alginate improved the activity and stability of tyrosinase. The use of immobilized enzyme lower production costs as these can be readily separated from reaction mixture and it was used repeatedly (for 5 cycles) and continuously. These results indicate that the Ca-alginate is able to provide a protective microenvironment for the enzyme. Moreover, immobilized P. stutzeri A3 tyrosinase can act as a promising technique for phenol removal from wastewater along with maintaining high stability of the enzyme. Hence, it can be concluded that the tyrosinase enzyme from Pseudomonas stutzeri A3 can be potently use in industries in order to remove phenol from wastewater.
Conflict of interest
There is no conflict of interests regarding the publication of this paper.
There is no conflict of interests regarding the publication of this paper.
References
- Abadulla E., et al. “Enzymatic decolorization of textile dyeing effluents”. Textile Research Journal 70.5 (2000): 409–414.
- Ainsworth EA and Gillespie KM. “Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent”. Nature Protocols 2.4 (2007): 875-877.
- Ali S and Haq I “Production of 3,4-dihydroxy L-phenylalanine by a newly isolated Aspergillus niger and parameter significance analysis by Plackett-Burman design”.BMC Biotechnology 10 (2010): 1-8.
- Altschul SF., et al. “Gapped BLAST and PSI-BLAST: a new generation of protein database search programs”. Nucleic Acids Research 25.17 (1997): 3389-3402.
- Arica MY., et al. “Characterisation of tyrosinase immobilised onto spacer-arm attached glycidyl methacrylate-based reactive micro beads”. Process Biochemistry 39.12 (2004): 2007–2017.
- Arica MY., et al. “Invertase immobilised on spacer-arm attached poly (hydroxyethylmethacrylate) membrane: Preparation and properties”. Journal of Applied Polymer Science 75.14 (2000):1685–92.
- Arikan B. “Highly thermo stable, thermophilic, alkaline, SDS and chelator resistant amylase from a thermophilic Bacillus sp. isolate A3-15”. Bioresource Technology 99.8 (2008): 3071-3076.
- Balakrishnan GS and Kalirajan J. “Characterization of Tyrosinase enzyme from the tubers of Amorphophallus paeoniifolius (Dennst.) Nicolson, (Araceae)”. International Journal of Pharmacognosy and Phytochemical Research 7.3 (2015): 585-589.
- Bayramoğlu G and Arica MY “Preparation of poly (glycidylmethacrylate–methylmethacrylate) magnetic beads: application in lipase immobilization”. Journal of Molecular Catalysis B: Enzymatic 55.1-2 (2008): 76–83.
- Bevilaqua JV, Cammarota MC, Freire DMG, Sant'Anna Jr GL “Phenol removal through combined biological and enzymatic treatments”. Brazilian Journal of Chemical Engineering 19.2 (2002): 151 -158.
- Bollag DM., et al. Protein Methods 2nd. Wiley Liss, New York (1996):110-139.
- Brenner DJ., et al. “Bergy's manual of systematic bacteriology”. Springer 2 (2005):
- Brooks SJ., et al. “Tyrosol to hydroxytyrosol biotransformation by immobilised cell extracts of Pseudomonas putida F6”. Enzyme and Microbial Technology 39.2 (2006): 191–196.
- ChiacchieriniE., et al. “Bioremediation of food industry effluents: Recent applications of free and immobilised polyphenoloxidases”. Food Science and Technology International 10.6(2004): 373-382.
- Claus H and Decker H. “Bacterial tyrosinases”. Systematic and Applied Microbiology 29.1 (2006): 3-14.
- Dalfard AB., et al. “Isolation and biochemical characterization of laccase and tyrosinase activities in a novel melanogenic soil bacterium”. Enzyme and Microbial Technology 39.7 (2006): 1409-1416.
- Decker AL. “Polyphenol oxidase”. Worthington Biochemical Corporation (1977): 39-40.
- Dhevagi P and Poorani E. “Isolation and characterization of L-asparaginase from marine actinomycetes”. Indian Journal of Biotechnology 5 (2006): 514-520.
- Donato L., et al. “Kinetic study of tyrosinase immobilized on polymeric membrane”. Journal of Membrane Science 454 (2014): 346–350.
- Dong CH and Yao YJ. “Isolation, characterization of melanin derived from Ophiocordyceps sinensis, an entomogenous fungus endemic to the Tibetan Plateau”. Journal of Bioscience and Bioengineering 113.4 (2012): 474-479.
- Duarte LT., et al. “Production and characterization of tyrosinase activity in Pycnoporus sanguineuscct-4518 crude extract”. Brazilian Journal of Microbiology 43.1 (2012): 21-29.
- El-Batal AI., et al. “Optimization of melanin production by Aspergillus oryzae and incorporation into silver nanoparticles”. Der Pharmacia Lettre 8.2 (2016): 315-333.
- Espin JC., et al. “Monophenolase activity of polyphenol oxidase from Haas avocado”. Journal of Agricultural and Food Chemistry45.4 (1997): 1091- 1096.
- Estrada P., et al. “Characterisation and optimization of immobilized polyphenoloxidase in low-water organic solvents”. Biotechnology and Applied Biochemistry 14 (1991): 12-20.
- Fernandez-Lafuente R. “Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation”. Enzyme and Microbial Technology 45.6-7 (2009): 405–418.
- Güray MZ. “Partial purification and characterization of polyphenol oxidase from thermophilic Bacillus sp.” Graduate School of Engineering and Sci Izmir Institute of Technology (2009):
- Holt JG., et al. “Bergey’s Manual of Determinative Bacteriology”. 9th Ed. Williams & Wilkins, Baltimore, MS, USA (1994):
- Johnson LE., et al. “Methods for studying soil Microflora-plant diseases relationship”. Burgess Publishing Company in Minneapolis USA (1959):
- Jung D., et al. “Oxidation of indole using chloroperoxidase and glucose oxidase immobilized on sba-15 as tandem biocatalyst”. Microporous and Mesoporous Materials 113 (2008): 523–529.
- Khan AA and Husain Q. “Decolorization and removal of textile and non-textile dyes from polluted wastewater and dyeing effluent by using potato (Solanum tuberosum) soluble and immobilized polyphenol oxidase”. Bioresource Technology 98.5 (2007): 1012–1019.
- Kumar K., et al. “Plant asparaginase-based asparagine biosensor for leukemia”. Artificial Cells, Nanomedicine, and Biotechnology 41.3 (2013): 184-188.
- Laemmli UK. “Cleavage of structural proteins during the assembly of the head of bacteriophage T4”. Nature 227 (1970): 680-685.
- Lelliott RA., et al. “A determinative scheme for the fluorescent plant pathogenic pseudomonas”. Journal of Applied Microbiology 29.3 (1966): 470–489.
- Li S., et al. “Use of chemically modified PMMA microspheres for enzyme immobilization”. Bio Systems 77.1-3 (2004): 25–32.
- Lowry OH., et al. “Protein measurement with Folin phenol reagent”. The Journal of Biological Chemistry 193.1 (1951): 265-275.
- Majidi D., et al. International Journal of Science & Technology 1.2 (2000): 40-47.
- McMahon AM., et al. “Biochemical characterisation of the coexisting tyrosinase and laccase in the soil bacterium Pseudomonas putida F6”. Enzyme and Microbial Technology 40.5 (2007):1435-1441.
- Minteer SD. “Enzyme stabilization and immobilization. In Methods and Protocols. Series: Methods in Molecular Biology”. A product of Humana Press New York, NY, USA (2011):
- Mukherjee S., et al. “Potential use of polypheol oxidases (PPO) in the bioremediation of phenolic contaminants containing industrial wastewater”. Reviews in Environmental Science and Bio/Technology 12.1 (2013): 61–73.
- Munjal N. and Sawhney SK. “Stability and properties of mushroom tyrosinase entrapped in alginate, polyacrylamide and gelatine gels”. Enzyme and Microbial Technology 30 (2002): 613-619.
- Osman B., et al. “Tyrosinase Immobilization on Cu2+ Chelated Poly (ethylene glycol dimethacrylate-N-Vinyl Imidazole) Beads”. Hacettepe Journal of Biology and Chemistry 35.3 (2007): 233-241.
- Peralta-Zamora P., et al. “Decolorization of reactive dyes by immobilized laccase”. Applied Catalysis B: Environmental 42.2 (2003): 131–144.
- Popa C and Bahrim G. “Streptomyces tyrosinase: production and practical applications.“Dunărea de Jos” University – Galaţi”. Innovative Romanian Food Biotechnology 8 (2011): 1-7.
- Quiroga E., et al. “Performance improvement of araujiain, a cystein phytoprotease, by immobilization within calcium alginate beads”. Process Biochemistry 46.4 (2011): 1029-1034.
- Ramsden CA and Patrick Riley A. “Mechanistic studies of tyrosinase suicide inactivation”. ARKIVOC (2010): 260–274.
- Ren Q., et al. “High level production of tyrosinase in recombinant Escherichia coli”. BMC Biotechnology 13 (2013): 13-18.
- Rodrigues RC., et al. “Modifying enzyme activity and selectivity by immobilization”. Chemical Society Reviews 42.15 (2013): 6290-6307.
- Roy S., et al. “Isolation and characterization of tyrosinase produced by marine actinobacteria and its application in the removal of phenol from aqueous environment”. Frontiers in Biology 9.4 (2014): 306-316.
- Sahin F., et al. “A novel matrix for the immobilization of acetyl cholinesterase”. International Journal of Biological Macromolecules 37.3 (2005): 148-153.
- Selinheimo E., et al. “Production and characterization of a secreted, C-terminally processed tyrosinase from the filamentous fungus Trichoderma reesei”. The FEBS Journal 273.18 (2006): 4322-4335.
- Shao J., et al. “Immobilization of polyphenol oxidase on chitosan-SiO2 gel for removal of aqueous phenol”. Biotechnology Letters 29.6 (2007): 901-905.
- Tran DN and Balkus KJ Perspective of recent progress in immobilization of enzymes. ACS Catalysis 1.8 (2011): 956–968.
- Valipour E and Arikan B. “Optimization of Tyrosinase Enzyme Production from Native Bacillus sp. MV29 Isolate”.Journal of Applied Biological Sciences 9.2 (2015): 77-82.
- Whitaker JR. “Principles of enzymology for food sciences”. In Enzyme inhibitors New York: Marcel Decker (1972): 225-282.
- Zaidi KU., et al. “Microbial Tyrosinases: Promising Enzymes for Pharmaceutical, Food Bioprocessing, and Environmental Industry”. Biochemistry Research International 2014 (2014): 1-16.
Citation:
Fifi M Reda., et al. “Removal of Phenols from Industrial Wastewaters by Immobilized Pseudomonas Stutzeri A3 Tyrosinase”.
Clinical Biotechnology and Microbiology 1.6 (2018): 242-256.
Copyright: © 2018 Fifi M Reda., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.