Review Article
Volume 1 Issue 2 - 2017
The Great Influenza Pandemic: What Really Happened in 1918?
New York Institute of Medical Research, USA
*Corresponding Author: Dr. Lawrence Broxmeyer MD, New York Institute of Medical Research, New York, USA.
Received: October 29, 2017; Published: November 07, 2017
Abstract
The word Influenza comes from mid–18th century Italian and literally means ‘influence’. Similar to how the coronavirus was once best known to cause the common cold, in 1918 Influenza was felt to be so benign a disease that it was not reportable in the United States. And it was not until 1933 that Influenza was “discovered”. So just what was causing the deaths in 1918? And why did the corona “virus” turn so deadly so quickly? Before we can be certain of anything, these questions need to be answered. But to this point, they have not been answered. In the meantime, during both events there was a deadly pandemic of tuberculosis going on, a pandemic which even in 2020 kills one person every 21 seconds for a total of at least 1.5 to 1.8 million dead in 2018 alone. Yet Robert Koch, TB’s discoverer, was repeatedly forced to call it a virus, to assuage other authorities of his time. And, as we shall soon see, the concept that only a new and virulent strain of a virus can be so infectious and kill so quickly is not only fallacious, but dangerous -and completely at odds with what used to be called the acute form of “galloping consumption” [tuberculosis] which did and still can kill in a matter of hours, days or weeks.
“Viral” influenza was informally first mentioned as being behind America’s 1918 influenza pandemic not by a direct study of the disease in humans, but rather from studies on animal diseases. In 1918, J.S. Koen, a veterinarian, observed a disease killing thousands of pigs which he believed to be the same disease as the now infamous influenza Pandemic of 1918. He felt that it was a virus. It was solely his belief. Yet new evidence and older historical findings bring up the possibility that influenza doesn't originate from a virus –despite the indefatigable efforts, up to the present, of flu enthusiasts to viralize the 1918-19 pandemic. (Burnet F, Clark E. 1942) (Morens DM, Taubenberger JK. 2009). As we shall see, such efforts on the part of viral devotees are nothing new, and began in force with scant evidence in Great Britain during the 1st World War, also known as the Great War.
Introduction
"Viruses should be considered as viruses because viruses are viruses."
– Pioneer virologist André Lwoff in 1957
Of course, the scientific merits and existence of the Influenza virus “type A” (for avian) has been established beyond dispute –or has it? Not according to virologist Stefan Lanka and others. World-renowned German virologist/molecular biologist Lanka, an expert on the documentation of viruses, who studied at the University of Koblenz, and was first to discover a maritime virus [1-3], simply isn’t buying it. This was an old story for Lanka, who knew that scientists were certain that a virus was behind Lyme disease, mycoplasma pneumonia, and Legionnaires’ disease before their respective bacteria were found. With regard to the influenza virus itself, Dr. Lanka had this to say [4]:
“Dr. Jeffery Taubenberger, from whom the allegation of a reconstruction of the 1918 pandemic virus originates, works for the US-American army and has worked for more than 10 years on producing, on the basis of samples from different human corpses, short pieces of gene substance by means of the biochemical multiplication technique PCR. Out of the multitude of produced pieces he has selected those which came closest to the model of the genetic substance of the idea of an influenza virus, and has published these. In no corpse however was a virus seen or isolated or was a piece of gene substance from such isolated. By means of the PCR technique there were produced out of nothing pieces of gene substance whose earlier existence in the corpse could not be demonstrated.”
“If viruses had been present, then these could have been isolated, and out of them their gene substance could have been isolated too; there would have been no necessity for anyone to produce laboriously, by means of PCR technique - with clearly a [deceptive] intention - a patchwork quilt of a model of the genetic substance of the idea of an influenza virus. ... In order to see through this one only has to be able to add up the published length pieces, in order to ascertain that the sum of the lengths of the individual pieces, which supposedly makes up the entire viral gene substance of the purported influenza virus, does not make up the length of the idea of the genome of the influenza virus model.”
“Even simpler it is to ask in what publication you can find the electron microscope photo of this supposedly reconstructed virus. There is no such publication."
And when questioned regarding the electron pictograph of H1N1 that the CDC came up with on their website, virologist Lanka attested to the fact that the H1N1 picture was bogus. He said that he had “written the CDC many times as to who made the H1N1 photos and whether they were scientifically documented as to chemical characteristics and other properties.” There was never any reply. He concluded “If CDC refuses to cite the source of the photos, they are fake. ... In conclusion, without the isolation of the H1N1, there is no H1N1 infecting virus. Even more bizarre is the admission by the US Government’s Food and Drug Administration ... that the ‘test’ approved for premature release to test for H1N1 is not even a proven test. More to the point ―there is no forensic evidence in any of the deaths reported to date that has been presented that proves scientifically that any single death being attributed to H1N1 Swine Flu virus was indeed caused by such a virus.”[4]
Early Pandemic History
At one time, there wasn’t a major research or medical center in the US that did not feel that Pfeiffer’s bacillus, originally called Mycobacterium Influenzae was not behind the Great Pandemic. Even in the UK, Pfeiffer’s bacillus claim to being behind influenza was supported by Klein, one of the founders of British bacteriology. Pfeiffer’s influenza bacillus was indeed considered, by most, to be the cause of influenza until at least 1933. Today, the current name for Pfeiffer’s bacillus is Haemophilus influenzae. Yet the National Center for Biotechnology Information of the NIH (National Institutes of Health), still lists its older designation “Mycobacterium influenzae” [5,6]. Pfeiffer’s Influenza bacillus was therefore surely considered a mycobacterium, just as tuberculosis is today. Pfeiffer’s stained with the same “acid-fast” stain used to stain TB and many of its fungal-like and bacillary forms were quite similar to Mycobacterium tuberculosis. American physician Herbert Wade and colleague Christobal Manalang proved that Pfeiffer’s had filter-passing, viral-like fungal forms, validating Pfeiffer’s bacillus original name: Mycobacterium Influenzae. The “myco” in the word mycobacterium means fungal.
At one time, there wasn’t a major research or medical center in the US that did not feel that Pfeiffer’s bacillus, originally called Mycobacterium Influenzae was not behind the Great Pandemic. Even in the UK, Pfeiffer’s bacillus claim to being behind influenza was supported by Klein, one of the founders of British bacteriology. Pfeiffer’s influenza bacillus was indeed considered, by most, to be the cause of influenza until at least 1933. Today, the current name for Pfeiffer’s bacillus is Haemophilus influenzae. Yet the National Center for Biotechnology Information of the NIH (National Institutes of Health), still lists its older designation “Mycobacterium influenzae” [5,6]. Pfeiffer’s Influenza bacillus was therefore surely considered a mycobacterium, just as tuberculosis is today. Pfeiffer’s stained with the same “acid-fast” stain used to stain TB and many of its fungal-like and bacillary forms were quite similar to Mycobacterium tuberculosis. American physician Herbert Wade and colleague Christobal Manalang proved that Pfeiffer’s had filter-passing, viral-like fungal forms, validating Pfeiffer’s bacillus original name: Mycobacterium Influenzae. The “myco” in the word mycobacterium means fungal.
Before the Great War, Harvard bacteriologist and physician S.B. Wolbach summarized filterable viruses this way: “By filterable viruses, we mean microorganisms which will pass through filters, the pores of which are too small to give passage to ordinary bacteria.” [7]
If Wolbach’s choice of the words “ordinary bacteria” seemed to differentiate these from forms of bacteria that could pass through a filter –that was precisely his intention. Wolbach’s review found four ‘viruses’ which cultivated out bacteria. He also mentioned that historically, the first filterable “virus” discovered by Nocard in 1899, passed through a Chamberland filter but was subsequently cultivated back into bacterial forms, among them, one of a coccoid shape. Unfortunately, such bacterial forms have repeatedly muddied virologist’s quests to find filter-passing “viruses”. Among them, said Wolbach, were those who sought to prove a filter passing virus for dog distemper in a field where most credible evidence pointed towards distemper in dogs as being a bacillary disease. Surely for Torrey and Rank it seemed “certain that if every case [of dog distemper] had been autopsied during the first week of symptoms, this bacillus might have been readily recovered from all” [8]. Indeed for Torrey, Carré’s theory of a filterable virus as essential to canine distemper was not confirmable, not only in his own studies, but those of Kregenow [9], and other workers.
England’s Big Push to Viralize Influenza
But such information never stopped Walter Fletcher, head of Britain’s Medical Research Council (MRC) from plotting, soon after the Great War to “prove” that the Great Pandemic of 1918 was caused by a filter-passing “virus”. And Fletcher had a specific plan. He would make canine distemper in dogs, which was similar to human influenza, the experimental model for human influenza itself. Find a ‘virus’ and vaccine for canine distemper, predicted Fletcher, and that study would lead to gaining general acceptance that a virus was behind the Great Pandemic. Nor would Fletcher rest until his scheme was realized.
But such information never stopped Walter Fletcher, head of Britain’s Medical Research Council (MRC) from plotting, soon after the Great War to “prove” that the Great Pandemic of 1918 was caused by a filter-passing “virus”. And Fletcher had a specific plan. He would make canine distemper in dogs, which was similar to human influenza, the experimental model for human influenza itself. Find a ‘virus’ and vaccine for canine distemper, predicted Fletcher, and that study would lead to gaining general acceptance that a virus was behind the Great Pandemic. Nor would Fletcher rest until his scheme was realized.
So soon, Walter Morley Fletcher, head of the UK’s MRC, which was originally founded by and for the Royal Commission on Tuberculosis, held a top secret meeting with British pathologists at England’s War Department with the sole intent of scheming to show that the 1918 “influenza” pandemic was the work of a virus. This resulted in sending two research teams to France late in 1918, despite the fact that Fletcher openly admitted that any work towards proving an influenza virus would likely be hindered by “the difficulty of proceeding by sound experimental methods”. And with no specific laboratory test to prove his hypothesized influenza virus, nor the means to show it, Fletcher was not far from wrong. His arsenal: mere symptoms and serologic tests which indirectly said, yes, this pathogen exists, it passes through a filter and it reacts positively in serologic assays. But unfortunately all of this certainly still did not mean it was a “virus”.
Nevertheless, Fletcher and several other zealots fully intended to pull this viral charade off, and had the influence, the money, the research facilities, the publicity and the publication resources to do just that, including an ongoing collaboration with the Rockefeller Institute in the US, which threw money in the form of grants towards Fletcher’s project. And so it was that plans were carefully laid, early on, to viralize the legacy of the Great Pandemic. By the summer of 1922, Fletcher had already recruited virologist Patrick Playfair Laidlaw to develop a project to prove that canine distemper and eventually influenza in man were caused by filter-passing “viral organisms”. And for the better part of a decade, Laidlaw would work with veterinarian G.W. Dunkin on canine distemper. While it was Walter Morley Fletcher’s feeling that if they isolated the infectious agent behind such distemper and eventually created a vaccine for it, there would be no doubt about their historic ‘viral’ study, in fact the filter-passing “virus” that Laidlaw claimed that he had isolated, left behind many doubts. Yet just the fact that Laidlaw had successfully created a vaccine for dogs to prevent the deadly canine distemper made him somewhat of a national hero. And Walter Fletcher was not about to lose the momentum gained in this dog arena, making sure that Laidlaw, along with MRC virologists Smith and Andrewes, quickly pressed the case that a “virus” was also behind influenza and the Pandemic of 1918. So less than a year after Laidlaw’s group started investing their efforts into finding a human influenza virus, they threw together a Lancet paper published on July 8, 1933. What was Walter Fletcher’s rush? It was simply that things in general where not going that well for virology in general.
Virology as a Science Threatened
In the words of respected historian Ton van Helvoort, during the 1930s and 1940s, the concept of Laidlaw, Smith and Andrewes’s "filterable influenza virus" was subjected to such criticism that the very foundations of virology as a science were threatened [10]. And those threats came from many fronts.
In the words of respected historian Ton van Helvoort, during the 1930s and 1940s, the concept of Laidlaw, Smith and Andrewes’s "filterable influenza virus" was subjected to such criticism that the very foundations of virology as a science were threatened [10]. And those threats came from many fronts.
Laidlaw’s entire basis for calling dog distemper and eventually human influenza ‘filter-passing viruses’ was based on three roundly disputable qualities: their filterability, their resistance to culturing on ordinary bacterial culture media and their dependence on living tissue –meaning that they could not grow outside of the animal or human body [11]. Each of these criteria where under constant assault, the latest of which came from Northwestern University bacteriologist Arthur Isaac Kendall [12]. Kendall, similar to Laidlaw in the UK had been picking up favorable press and peer-reviewed status in the United States –but in quite the opposite direction.
Kendall knew that Laidlaw, using these three questionable criteria, was attempting to dismantle the impressive and plentiful evidence that showed that dog distemper was from a bacterial bacillus. Yet oddly, rather than come out and call it a ‘virus’, Laidlaw and Dunkin opted for the description “ultra-microscopic organism” [11], implying a small microbe.
And so, by 1932, Dr. Arthur Kendall, director of Medical Research at Northwestern, and no fan of “so-called” viral influenza, spoke before the Association of American Physicians at Johns Hopkins University. Basically Kendall reminded virologists that his "K Medium", which he used to grow “viruses”, contained nothing living, yet produced filterable bacterial forms. William Henry Welch, the dean of American Medicine, and a survivor of the Great Pandemic, rose to say that: “Kendall’s observations marks a distinct advance in medicine.” [13] The reasons for Welch’s support was obvious. Kendall had executed a blistering well-written paper published in Science entitled Observations upon the filterability of bacteria, including a filterable [bacterial] organism obtained from cases of influenza.
Therefore, in so far as Walter Morley Fletcher was concerned, one year to solidify an influenza viral study was more than enough. Laidlaw and team now had to publish and do so immediately: and the Lancet article “A virus obtained from influenza patients” was the result [14]. Yet, once again the best that Laidlaw and team could offer was that “The evidence strongly suggests that there is a viral element in epidemic influenza, and we believe that the virus is of great importance in the aetiology of the human disease.” However to the news hungry editors at the Lancet, heavily under the influence of the Royal Medical Council, Laidlaws carefully couched word “suggests” did not go quite far enough. Rather Lancet announced that Laidlaw’s study “offered almost conclusive evidence….” [that a filter-passing virus was behind 1918] –which of course it did not.

Figure 2: A poster circa 1918, this one from the Brooklyn Tuberculosis Committee. ‘Consumption’ is the older name for tuberculosis
Despite all of this, Pfeiffer's influenza bacillus, a bacterium, was heavily isolated at one time or another in victims of the 1918-19 pandemic by practically all major research centers in the United States.
A ‘Virus’ by Any Other Name
Although the term ‘virus’ has existed since 1898, the infectious agent it was attempting to describe was so unclear and mysterious that for many decades scientists considered it purely theoretical. Certainly, even by 1917, "influenza" was still not felt to be serious enough to be a reportable disease, and no doctor had to report it to state or local health officials. Most cases were self-limiting and gone in 10 days. Yet the great "influenza" pandemic that swept the world in 1918–19 may have been the most virulent outbreak in history, at least in terms of the swiftness of its devastation. According to some estimates, it killed more than 50 million with estimates as high as 100 million persons around the world, including some 550,000 in the United States— and it did so swiftly, all within two years.
Although the term ‘virus’ has existed since 1898, the infectious agent it was attempting to describe was so unclear and mysterious that for many decades scientists considered it purely theoretical. Certainly, even by 1917, "influenza" was still not felt to be serious enough to be a reportable disease, and no doctor had to report it to state or local health officials. Most cases were self-limiting and gone in 10 days. Yet the great "influenza" pandemic that swept the world in 1918–19 may have been the most virulent outbreak in history, at least in terms of the swiftness of its devastation. According to some estimates, it killed more than 50 million with estimates as high as 100 million persons around the world, including some 550,000 in the United States— and it did so swiftly, all within two years.
By 2000, Dr Andrew Noymer and Michel Garenne, demographers from the University of California, Berkeley, thought they knew just why this discrepancy existed, reporting convincing statistics showing that undetected tuberculosis (TB) may have been the real killer in the 1918 flu epidemic. Aware of more recent attempts to isolate the "influenza virus" from human cadavers and their specimens in the US, Noymer and Garenne concluded: "Frustratingly, these findings have not answered the question why the 1918 virus was so virulent, nor do they offer an explanation for the unusual age profile of deaths."[16] Pearl from Johns Hopkin’s , who lived through the Great Pandemic, in 1919 concluded much the same, showing that there was a definite relationship between the explosiveness and fatality of that killer pandemic in 40 American cities and the existing death rates of pulmonary tuberculosis and related disease [17].
In Nishiura’s study not only was TB shown to be associated with influenza death, but there was no influenza death among controls without tuberculosis. Nishiura concluded: “Should a highly fatal influenza pandemic occur in the future, testing the role of TB in characterizing the risk of death would be extremely useful in minimizing the disaster…” [18].

Figure 4: Another poster, circa 1918. New York’s Shuyler Center had just loaned their tuberculosis staff to the American Red Cross to help organize and direct a three months emergency campaign against influenza in New York, New Jersey, and Connecticut at a time when they could least afford to do so. Five new tuberculosis hospitals and eight new TB dispensaries were scheduled to open in 1918 to meet the upsurge of that disease
More recently, in 1999, Fredj Tekaia, of the Institute Pasteur, Paris, and colleagues, looking for "overall gene similarities as signatures of common ancestry", found similar genetic profiles and sequencing for Pfeiffer's bacillus (Mycobacterium influenzae) and Mycobacterium tuberculosis, lumping them together in the same "well-defined group". [19] Tekaia’s diagrammatic genomic tree shows the two organisms directly next to one another. [See figure below] This reopened the historical argument as to whether Pfeiffer’s bacillus and tuberculosis were related.
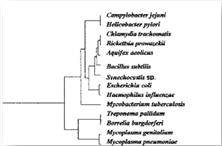
Figure 5: The genomic tree of Fredj Tekaia, of the Institut Pasteur, Paris,1999. Notice how close Haemophilus influenzae (Pfeiffer’s bacillus) and M. tuberculosis are placed ― directly next to one another
In 1933, the very year that British MRC virologists Smith, Andrewes and Laidlaw claimed that they had discovered the virus behind human influenza "virus", Stobie, in the British Medical Journal, still was objecting that rather than being a virus, the real nature of "influenza" could well be a form of Mycobacterium tuberculosis[20].
Stobie’s view simply reflected the active, vigorous, yet historically suppressed debate that had been raging in medical journals for decades. He mentions cases of tuberculosis complicated by influenza which together exhibited “a sinister type of disease which rarely responded to treatment." Enter "galloping consumption", the most devastating form of tuberculosis, then called "consumption" – a galloping tuberculosis that could kill in hours to days, the mere memory of which brought terror to the faces of those who had witnessed it.
The Reichsgesundheitsamt, Berlin, March 1882
Although it has always perplexed doctors, scientists and historians alike as to why so many microbes were involved during the deadly 1918 pandemic, the explanation was carefully laid out decades before by German physician/microbiologist Robert Koch, the discoverer of tuberculosis. From the onset, Koch concluded [21] that other microorganisms secondarily infected and thus shared in the destructive work of the tubercle bacilli. Gaffky, Pansini, Cornet, Spengler, Schabad, Ortner and Flick, among others, verified this [22]. Various organisms, all of which reappeared to confuse scientists in 1918, were assigned a share in the clinical picture of tuberculosis –among them the streptococcus, staphylococcus, pneumococcus and even the variably acid-fast influenza bacillus itself. The pyogenic, pus-forming cocci were more generally suspected than other bacteria of complicating tuberculosis. In the British Medical Journal of 28 July 1900, the following editorial appeared, dealing with the role of streptococci in tuberculosis: "It is a remarkable fact that…the bulk of the disturbing and dangerous features of tuberculosis are not due to the tubercle bacillus, but to streptococci and other pyogenic organisms." The pneumococci, Staphylococci and methicillin-resistant staph (MRSA) are other pyogenic pathogens that subsequently have been documented.
Although it has always perplexed doctors, scientists and historians alike as to why so many microbes were involved during the deadly 1918 pandemic, the explanation was carefully laid out decades before by German physician/microbiologist Robert Koch, the discoverer of tuberculosis. From the onset, Koch concluded [21] that other microorganisms secondarily infected and thus shared in the destructive work of the tubercle bacilli. Gaffky, Pansini, Cornet, Spengler, Schabad, Ortner and Flick, among others, verified this [22]. Various organisms, all of which reappeared to confuse scientists in 1918, were assigned a share in the clinical picture of tuberculosis –among them the streptococcus, staphylococcus, pneumococcus and even the variably acid-fast influenza bacillus itself. The pyogenic, pus-forming cocci were more generally suspected than other bacteria of complicating tuberculosis. In the British Medical Journal of 28 July 1900, the following editorial appeared, dealing with the role of streptococci in tuberculosis: "It is a remarkable fact that…the bulk of the disturbing and dangerous features of tuberculosis are not due to the tubercle bacillus, but to streptococci and other pyogenic organisms." The pneumococci, Staphylococci and methicillin-resistant staph (MRSA) are other pyogenic pathogens that subsequently have been documented.
Pandemic scientist Oswald Avery of Rockefeller research explained the principle behind this phenomena six years before the death and destruction now known as the Great Pandemic. Secondary infection by pus forming bacteria such as streptococcus and pneumococcus are “particularly common in those diseases [such as pulmonary tuberculosis] in which tissue destruction and ulceration have already occurred as a result of primary infection” This was because with tuberculosis, “the initial focus offers a ready portal of entry and a suitable nidus for secondary invaders, since local resistance is already diminished and the natural protective forces of the body are exhausted by the original [tubercular] infection.”[23] Thus did microorganisms normally part of the regular flora of the mouth and mucous secretions turn deadly.
The British Medical Journal’s editorial dealing with the destructive and predominant role of streptococci in tuberculosis was further confirmed when Pandemic physician Edward Rosenow reported his findings that green-producing Streptococcocus viridians were more constantly found and more destructive than any other organism associated with influenza [24].
Yet few were suggesting that Rosenow’s green streptococci, which often normally inhabit buccal secretions, were behind the origin of the Pandemic of 1918.
British pathologist William Crofton chafed at the ridiculous notion that in influenza, Pfeiffer's bacillus and only Pfeiffer’s bacillus alone should be found in pure cultures [25]. He asked whether the typhoid bacillus was ever found in pure culture.
Obuchow Hospital, St Petersburg, Russia, 1890 “In relation to the question of the effect of influenza upon tuberculosis, it should be pointed out that in many cases in which pulmonary tuberculosis has been thought to have followed an attack of influenza, it is altogether probable that the supposed attack of influenza was, in reality, a manifestation of an existing tuberculous infection; for tuberculoprotein, whether absorbed from spreading lesion or injected into the body, can cause constitutional symptoms (fever, malaise, headache, joint pains, anorexia, prostration) quite like those of influenza. The writer has seen attacks closely simulating influenza occur in healthy, tuberculin-positive laboratory workers as a result of the accidental inhalation of the vapor of boiling tuberculin." [72]
―Arnold Rich, MD, Head of Pathology, Johns Hopkins, 1946
In 1890, a fierce "influenza” struck worldwide, killing approximately a million people. Occurring at the end of the 19th century, this second most severe influenza ever to hit the world occurred at a time when there was fear that tuberculosis would destroy the civilization of Western Europe –as it steadily moved east to ravage Eastern Europe just in time for the “flu” pandemic of 1889-1890.
Of all the forms of "influenza" known in 1890, none was more dreaded nor struck more terror into the hearts of victims and their families than that described by Wiltschur as "galloping consumption" [26]. An attending physician at the Obuchow Hospital, St. Petersburg, Wiltschur, astounded by the number of TB patients coming down with influenza, documented what happened when influenza punctuated previous dormant or active cases of tuberculosis: "The [influenza] patients were, for the most part, still well nourished", chronicled Wiltschur in Obuchow. This mirrored the flu pandemic of 1918, where healthy young soldiers were suddenly decimated by disease. Wiltschur continued: "Cyanosis of the face and extremities was a frequent occurrence." Patients exhibited severe difficulty in breathing (dyspnea), an extremely high temperature not characteristic of the flu, pulmonary hemorrhages and a rapid progression of lung disease, "with death occurring in many instances unexpectedly and suddenly.” Wiltschur was witnessing the acute blood-borne (miliary) form of virulent galloping tuberculosis, a disease which according to McCall Anderson [27], then Professor of Clinical Medicine at the University of Glascow, could kill within a few days even without influenza. It began with high fever and pneumonia in one or both lungs. And even when this was not the case, acute “galloping” tuberculosis was usually rapidly fatal. To San Francisco pathologist Albert Abrams, a physician originally from Heidelberg, in galloping consumption “usually the entire lung becomes affected and the condition is not unlike that of an acute pneumonia.” [28] Why these findings, including the well-known high fatality of "galloping consumption" with its high fever, profuse hemorrhaging, brownish spots or splotches on the face, strawberry tongue and typhoid-like symptoms, all documented so clearly in and after the pandemic of 1890, were ignored by the historians, scientists and practitioners of 1918 and beyond, defies comprehension.
Historian/researcher René Dubos, of the Rockefeller Institute for Medical Research, assured his readers that "galloping consumption" was not an isolated, but a frequent diagnosis in the 19th century [29]. Despite persistent myths to the contrary, in the early phase of any new TB epidemic from a new and virulent strain, tuberculosis manifests itself as an acute disease and only much later as the chronic pulmonary tuberculosis that we know in today’s Western world. An example of this was found in the high mortality during the 1918 "influenza" pandemic, when African Americans were brought to fight in France during World War I, large numbers of them dying from the accelerated tubercular "galloping consumption" of yesteryear. But was it only this specific group that was affected circa 1918? Thomas Mays’ study of galloping consumptive cases in America were Caucasian. Mays [30], an infectious disease specialist at Philadelphia polyclinic, during the Pandemic of 1890, spoke of such “acute” tuberculosis formerly called “galloping consumption” which not only “invariably” resulted in death but depending upon its intensity, could kill young people in their prime within two or three days –although the majority of cases lived 11 days to five weeks, and could even endure for three or four months.
Just listening to and percussing the chest, which the US Army’s medical department would heavily rely upon in assessing new recruits during the upcoming Great War, would not be satisfactory, said Mays. The sounds heard simply presented as a bronchitis. Furthermore, in an acute tubercular involvement, pleurisy, an inflammation of the lining of the lung, was frequent and extensive. To Thomas Mays, it was the degree of fever that was a reliable index of the degree and intensity of galloping tubercular consumption, similar to the extremely high fevers often recorded during both of the large ‘flu” pandemics. Such sprinting tuberculosis was usually blood borne (or miliary), seeding the blood, and could cause the symptoms such as a splitting headache –if it had time to reach the serous coverings of the brain before death. Also nose bleeds (epistaxis) could occur, and sometimes hemoptysis (the spitting up of blood tinged sputum), as the ceaseless cough took its toll and marked difficulty of breathing arose. Indeed acute tuberculosis could have many hemorrhagic manifestations. And cyanosis, the blue to dark blue discoloration of the skin and mucous membranes was never far away. All told, Mays’ description of the signs and symptoms of “galloping” tubercular consumption remain a version in miniature of the 1918 Great Pandemic –a disease both acute and fatal, with signs and symptoms so non-specific that a proper diagnosis was near impossible to make.
College of Medicine and Surgery, Philippines, 1919

Figure 7: Influenza. University of the Philippines pre WW II, where Wade and Manalang conducted their landmark study
Pivotal to an understanding of how and why what was once called Mycobacterium Influenzae or Pfeiffer’s bacillus was both mistaken for a filterable virus, misclassified, and at the same time often elusive to bacteriologists is found in Wade and Manalang’s work, which revealed mycobacterial-like fungal forms in Pfeiffer’s bacillus.
In the beginning of 1920, an important study [31] appeared in The Rockefeller Institute’s Journal of Experimental Medicine. Physician Herbert Windsor Wade, an American investigator working out of the Department of Pathology and Bacteriology at the University of the Philippines College of Medicine and Surgery, doubted that a filter-passing virus had anything to do with the then current influenza pandemic. And Wade, with Filipino national Dr. Christobal Manalang, proved this in 1919 in their university laboratory.
When history’s deadliest influenza pandemic began, researchers around the world began to search for Pfeiffer’s bacillus in patients, hoping to develop antisera and vaccines that would protect against infection. In many patients, but not all, Pfeiffer’s was found. Failures to isolate Pfeiffer’s bacillus, at one time also known as Bacillus influenzae (now known as Haemophilus influenzae) were largely chalked up to inadequate technique, as this bacteria was notoriously difficult to culture. Wade and Manalang’s study not only verified such difficulty in isolating Pfeiffer’s bacillary forms, but exposed fungal forms, some of them filterable, and that were not being counted as pathogenic, even by Pfeiffer himself. Pfeiffer would eventually correct his thoughts: true Pfeiffer’s influenza bacillus was not just a bacillus. It also had pathogenic mycobacterial fungus-like forms. But such an admission came only later.
Yet through it all, Pfeiffer still insisted that his organism "had the best claim to serious consideration as the primary etiologic agent [cause] for influenza, and that its only competition was a “vague, unidentified filterable virus". [32] Had Pfeiffer studied and understood Wade and Manalang's laboratory evidence, his comment would have been quite different. Pfeiffer's bacillus itself could have easily been mistaken for that “unidentified filterable influenza virus".
Wade remained a voice of scientific reason throughout the 1918 influenza pandemic, during which he personally experienced a scourge in which, depending upon which province he visited, from 40 to 95 per cent of Filipinos had contracted the disease. In a country ravaged by TB, at least 70,000–90,000 Filipinos had already died, as had 20 million in TB-ridden India. Wade knew what it was like to come into a Philippine village where there were not enough living to bury the dead. Also, being far from the United States, he was not subject to the relentless censorship of the Wilson administration, both against civilians and scientists of the US Army Medical Corps itself. If he saw tubercular mycobacterial forms similar to those of TB in Pfeiffer's influenza bacillus, he could and would report them without fear of being accused of fueling the flames of hysteria. And in documenting such viral appearing, tuberculosis-like fungal forms in Pfeiffer's bacillus, that is exactly what Wade did.
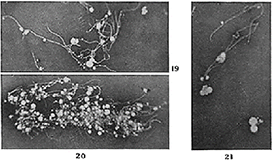
Figure 9: Wade and Manalang. Growth from Pfeiffer’s bacilli. Fungal branching was witnessed in all 3 strains of rigorously purified Pfeiffer’s bacilli used in their experiment. Clusters of mycobacterial-like fungal growth are shown in their Figs. 19 thru 21. The long, more or less frequently branching filaments and the numbers of laterally formed fungal conidial bodies (spores) are particularly striking
Wade's media grew out fungal forms and its fungal spores went through a filter. They were therefore "filterable", but they were not "viruses".
In the words of UK microbiologist Milton Wainwright, Wade and Manalang had now committed "the ultimate pleomorphist heresy"[33], documenting that a bacterium or mycobacterium could have more than one form in its life cycle.
Back in the UK Meanwhile in the UK, a special panel on the ‘Bacteriology of Influenza’, was chaired by pathologist Major W. B. Leishman, MB, RAMC. Walter Morley Fletcher had worked closely with William Boog Leishman, who became an Advisor on Pathology to Britain’s War Office. Leishman of all people realized the implication of Wade’s study in the Philippines. In 1902 Leishman had published a review on microbes similar to Wade’s acid-fast TB-like organism [34]. And when William Boog Leishman edited the Medical Services Pathology for the History of the Great War based on official documents [35], the work of Majors Rhea and Malloch in that book stuck with him in which Pfeiffer’s was “found to be by far and away the predominating organism of the sputum”, and how the cut surfaces of autopsied pandemic pneumonias resembled “the cut surface of the lung in many cases to what is seen in acute miliary tuberculosis.” Leishman wondered how many in the meeting had considered the acid-fast properties of Pfeiffer’s cell-wall-deficient forms during the pandemic: how they stained like TB, and how they looked like viruses.
Meanwhile, in the US, it was no accident that Wade and Manalang's paper was published by The Rockefeller Institute. Simon Flexner, then director at the Institute, once followed similar acid-fast fungal forms, associating them with tuberculosis. And he found this similar correlation with the influenza bacillus fascinating. Ironically, Flexner called his filterable fungal forms inside TB "pseudotuberculosis", just as Pfeiffer had labeled them “pseudoinfluenza” [36].
Simon Flexner was a key figure in the 1918 pandemic, heavily influencing Rockefeller research. He also had full status in the close-knit band of major US research operatives during the Pandemic. Why had he not taken the cue, following his own editorial instincts and run with Wade's study further? Wade had found that Pfeiffer's mycobacterial fungal forms could appear as filterable viruses. Even a 1918 British Medical Journal editorial (1918:2:665) had supported this as a key factor in the mystery of the Great Pandemic.
Actually, one researcher had carefully listened to Wade. But since that researcher wasn't a member of the US scientific hierarchy, he would be ignored. His name: Dr. Victor Conrad von Unruh.
Medical Reserve Corps, NYC, 1917
Physician/researcher Victor Conrad von Unruh was born in 1868 in Dolowitz, Germany, at a time when German medical research and science were unrivalled. He immigrated to the US, and by 1917 he had received the commission of Captain in the Medical Reserve Corps, New York City. The pandemic of 1918 was about to hit hard. Von Unruh's "A Comparative Study of the Acid Fast Bacilli" had appeared in 1916, two years prior to the killing fields of 1918 [37].
Physician/researcher Victor Conrad von Unruh was born in 1868 in Dolowitz, Germany, at a time when German medical research and science were unrivalled. He immigrated to the US, and by 1917 he had received the commission of Captain in the Medical Reserve Corps, New York City. The pandemic of 1918 was about to hit hard. Von Unruh's "A Comparative Study of the Acid Fast Bacilli" had appeared in 1916, two years prior to the killing fields of 1918 [37].
Because Pfeiffer's bacillus could stain acid-fast, von Unruh, like others, had been evaluating what medical texts such as Stengel's [38] referred to as Mycobacterium influenzae, also known as Bacillus influenzae. There, the entire influenza group was thought to be caused by this acid-fast mycobacterial bacillus, which was similar to the tubercle bacillus. Both microbes had fowl, swine and human forms. Von Unruh never saw the need to look for a "filterable virus" or "influenza" in the thousands of hogs that died abruptly with flu-like symptoms just before the pandemic, as did virologist Richard Shope. Why should he?
Shope, the American virologist credited with the first isolation of the influenza "virus", seemed oblivious to the fact that more than 60 per cent of hogs circa 1918 were tubercular from fowl TB (Mycobacterium avium), a fact that gave hog breeders such concern that large-scale efforts were under way to rid farms and chicken flocks of avian tuberculosis. The situation had become so grave in hogs and cattle that by 1917, one year before the most destructive pandemic ever, The Cooperative State–Federal Tuberculosis Eradication Program, administered by the US Department of Agriculture and the Animal and Plant Health Inspection Service (APHIS), had to be instituted. For in 1917, it was estimated that 25 per cent of deaths from tuberculosis in adult humans were caused by animal tuberculosis. [39] Nor did the fact that swine freely infected humans and vice versa faze von Unruh. Swine were a mycobacterial laboratory; although they held primarily fowl tuberculosis, they could also acquire bovine and human forms and freely infect people with whatever combined virulent mycobacterial strains resulted.
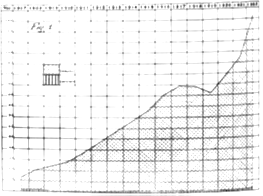
Figure 12: Retentions and Condemnations On Account of Tuberculosis, per 1,000 Head of Swine Slaughtered Between 1907 and 1922. (Proceedings of the Twenty-Eighth Annual meeting of the United States Live Stock Sanitary Association. Chicago, December 3-5, 1924 p.102)
In "A Comparative Study of the Acid Fast Bacilli", von Unruh stressed that the many cases of influenza he had investigated contained both the resting (dormant) form of TB and the influenza bacillus. Although Pfeiffer had likewise documented chronic colonization with his bacillus in TB patients, von Unruh saw this, and the fact that they were both mycobacteria as more suggestive of "a common ancestry or origin". Von Unruh wrote: "We have in influenza the fever, malaise, loss of weight, invasion by the organism of the same anatomical structures as in tuberculosis; we have chronic cases of bronchitis in which the influenza bacillus is constantly present; and lastly, we know that typical tuberculosis often follows an attack, however mild, of influenza." [37]
Such tubercular infection could in turn lead to other secondary bacterial infections. Noymer and Garenne's statement that tuberculosis was behind the many deaths in the 1918 pandemic was specifically based upon the well-known concept that the secondary bacterial infections that cropped up in 1918 were common in TB-infected lungs. Noymer and Garenne: "It is highly plausible that TB infection laid the ground for the massive secondary bacterial pneumonias that killed the victims of the flu in 1918." [16]
Medical Reserve Corps, New York City, July 1918
Victor von Unruh continued to see many reasons to pin a common ancestry onto the two organisms. Both the influenza bacillus and the quiescent TB formed Much's granules. Much's granules, named after their discoverer, Hans Much, passed through filters –then a major criterion for diagnosing a virus. Furthermore, both the influenza bacillus and TB were mycobacteria, with the branched fungal forms characteristic of the mycobacteria. And both could stain with "acid-fast" mycobacterial stains. Von Unruh wrote: "Therefore my conclusion is that the influenza bacillus is merely a weaker or dwarfed form of the real tubercle bacillus, a strain that in this case failed of better development because of a higher degree of resistance in the host. In both tuberculosis and influenza we deal with the self-same organism that in tuberculosis is fully developed, while in influenza it lacks development. In other words, we are dealing with a difference in degree only, but not in kind." [37]
Victor von Unruh continued to see many reasons to pin a common ancestry onto the two organisms. Both the influenza bacillus and the quiescent TB formed Much's granules. Much's granules, named after their discoverer, Hans Much, passed through filters –then a major criterion for diagnosing a virus. Furthermore, both the influenza bacillus and TB were mycobacteria, with the branched fungal forms characteristic of the mycobacteria. And both could stain with "acid-fast" mycobacterial stains. Von Unruh wrote: "Therefore my conclusion is that the influenza bacillus is merely a weaker or dwarfed form of the real tubercle bacillus, a strain that in this case failed of better development because of a higher degree of resistance in the host. In both tuberculosis and influenza we deal with the self-same organism that in tuberculosis is fully developed, while in influenza it lacks development. In other words, we are dealing with a difference in degree only, but not in kind." [37]
Von Unruh's distinction is suggestive of a similar, well-acknowledged comparison between the damaging effects that Mycobacterium tuberculosis has inflicted on man for centuries and the better resistance that humans with a healthy immune system have had against bird or fowl tuberculosis (Mycobacterium avium), found in swine as well. Did types of fowl tuberculosis in the form of Mycobacterium (Haemophilus) influenzae suis from pigs combine additively with latent human tuberculosis to cause the deadly galloping consumption of 1918–19?
Victor von Unruh's findings could have been taken lightly, were it not for similar thoughts published in the more prestigious medical journals of his time.
US Department of Agriculture, Washington, DC, 1917:
It had to be more than a coincidence that by the autumn of 1918 thousands of Midwest pigs died, seemingly from the same flu-like illness and in the same Haskell County location in which the worst human pandemic in history, which would kill between 20 and 100 million people was about to begin. The small towns that peppered Haskell County lay west of Dodge City in rich farmland dense with hogs and chickens.
US Inspector and veterinarian J.S. Koen, much to the chagrin of the pork industry, insisted that not only was he seeing pigs get the same ‘‘flu-like’’ illness as human victims, but that he had actually seen the two species transfect one another. Koen, for lack of another term, and with no evidence other than a hunch, quickly called this unknown disease in pigs “swine influenza’’, even as it killed pig after pig.
Koen knew better.
That thousands of pigs died in the Autumn of 1918 was problematical in that bird or fowl TB also called Avian tuberculosis or Mycobacterium avium routinely infects birds as well as hogs and sometimes cattle –but could, under the right conditions also infect man. And to complete this picture, Mycobacterium tuberculosis, although primarily affecting humans, could also be transmitted to hogs and cattle. So tuberculosis struck at will and affected all warm blooded vertebrates, its “atypical strains” other than human tuberculosis apparently getting into humans through the respiratory tract [40]. Also, unknown at this time, but pertinent, since Kansas sits squarely in America’s ‘‘dustbowl’’, were the results of a European experiment wherein guinea pigs exposed to organisms like Avian tuberculosis got little or no lung disease. However, when these mycobacteria were placed in dust aerosols, guinea pigs came down with progressive, fatal lung disease, not unlike what was occurring at the inception of the pandemic of 1918 [41].
Furthermore, although many blindly insist that infection with fowl tuberculosis (M. avium) requires some ‘‘defect’’ in the human immune system, that defect could be as simple as dust tying up the body’s defenses. Certainly a previous tubercular infection, common in 1918, with or without accompanying ‘‘chronic bronchitis’’ would be, in many cases, more than enough to qualify a person as a ‘‘compromised host’’, unleashing an animal or soil non-tubercular superimposed mycobacterial infection. Expert Rosenzweig found a surprising number of such cases in younger adults free of coexisting disease [42]. Rosenzweig isolated a case of Fowl tuberculosis (M. avium-intercellulare) in human lungs [43], commenting, that, although the average case of fowl TB in humans was thought to involve host compromise, that otherwise healthy hosts could also be affected in which severe and progressive disease would and did occur. Mycobacterium kansasii and certain forms of M. avium are the commonest forms of Non-Tubercular Mycobacteria (NTM) in human lungs. And hemoptysis, the coughing up of blood so common in 1918 pandemic victims, is more common in Mycobacterium kansasii induced disease [44]. But why, besides the fact that M. avium and M. kansasii were in abundance, should everything have begun in Kansas?
Epidemiologically, although the present pandemic of human TB began in 16th century England, it reached its peak in 1780 as a result of industrial revolution and the spread of cities, which allowed person-to-person spread. It then traveled rapidly to other large western European cities reaching a second peak in the early 1800s. It was only by the mid and later 1800s that North and South American waves spiked. Likewise, peak mortality rates for New England in the US came first and it was only with industrial development that the US epidemic traveled to the Midwest years later and finally the West [40].
So by 1918, it could be said, in so far as tuberculosis was concerned, that the world was a super-saturated sponge ready to ignite and that among its most vulnerable parts was the very Midwest where the 1918 unknown pandemic hit, in rural Haskell country, Kansas, in the midst of a natural pig slaughter, a few hundred miles from Camp Funston, today Fort Riley.
As the situation worsened, in 1917, the US Bureau of Animal Industry sent out inspectors to conduct an educational campaign on the dangers of bovine and hog tuberculosis. By 1918, the increase in hog tuberculosis had become all too noticeable.
Although Influenza virus type A was subsequently baptized “the avian influenza” or “the bird flu virus”, it had been known for 20 years previous to the Great Pandemic of 1918 that swine were susceptible to poultry tuberculosis just as it was general knowledge that since 1907 there had been an enormous increase in tuberculous farmyard poultry in large sections of the country, Kansas and Nebraska included. Comparative pathologist Herbert Fox, maintained that not only were primates like man the most susceptible to TB, but it was these same primates, including man, that were the most susceptible of all mammals, and perhaps all animals to tuberculosis [45] –a disease which could present as a rapid, progressive tubercular inflammation. Speaking at the Twenty-Eighth Annual Meeting of the US Livestock Sanitary Association, Fox maintained that infection from one species to another was already well known in 1918, including human tuberculosis found in certain birds, and that there was both bovine “and human TB in species no one thought they would be found in.”
Pigs had involuntarily become the living laboratory thru which three of the main types of tuberculosis (human, cow and fowl) could mutate through the genetic exchange by their viral mycobacteriophages, much in the same fashion as has been attributed to the ‘‘influenza virus’’. The stage was set for disaster.
Ground Zero: Haskell Country, Kansas: Autumn of 1918:
America had already entered World War 1. And though consensus has it that the lethal epidemic of 1918 began in Camp Funston, Kansas as early as January, physician Dr. Loring Miner of Haskell Country, situated 300 miles southwest of Funston’s perimeter, was already knee-deep in the medical struggle of his life. Miner, an alumni of Ohio University’s School of Medicine, had decided to become a county doctor in rural America. Loring liked to drink. Yet In the first thirty years of his life in Haskell, Miner’s excellent clinical skills had solidified his reputation as a physician to the point where most of his patients agreed that they would rather have a Loring Miner, occasionally drunk, than another doctor fully sober. But soon, a string of deadly events was about to happen that would sober-up even Loring Miner. Suddenly frantic townspeople were hunting him down at all hours of the day and night, reporting that members of their family would wake up one day feeling fine, have a severe headache by noon, be bedridden by late afternoon, with many of them lying dead by the following morning. The situation was bizarre. A febrile disease of unknown origin, progressing to fulminant pneumonia, was cutting down dozens of Haskell’s strongest and most robust men and women, directly in front of Dr. Loring Miner.
By 1918, Haskell County was one of the poorest areas in America. The spirit of invention and change that had swept America for decades, bringing with it the railroad, the telegraph, and now electricity, had mostly passed Haskell by. Its inhabitants, mostly farmers, grew grain, and raised poultry, hogs, and cattle. Its dominant building material was sod –topsoil held together by the roots of foliage. Even the Haskell post office, built in 1918, was sod.
So you might just say that Haskell was just a place where people, hogs, poultry, earthen sod houses, cattle, and manure melded into one.
Miner would try to see to it that this small rural epidemic got national attention, through the April 1918 issue of what today is called The Morbidity and Mortality Weekly Report [46]. He noted that the disease appeared to be associated with farms. If it was influenza, which at that time wasn’t considered lethal enough to be a reportable disease, it wasn’t acting like any influenza Miner had ever seen, killing the strong indiscriminately.
Miner’s report was the warning, but went unheeded. So while the Haskell epidemic ended as abruptly as it had begun, soon disease spread to Army barracks in nearby Funston. Many of Haskell counties young farmers had been incorporated into Camp Funston. Lt. Colonel Warren T. Vaughan, a physician of the Department of Preventive Medicine and Hygiene at Harvard Medical School and now on the Army’s Medical Board, indicated that the epidemic at Funston began on March 5th 1918, offering: “The Board decided that the disease should be called influenza, but our only basis for such decision were the clinical symptoms and the contagious character.” Ibid p.16 And that “The difficulty in making a decision in the presence of an epidemic is very similar to that of deciding whether the epidemics of former times were in each case influenza.” [47]
Camp Funston, Kansas, Spring of 1918:

Figure 16: Camp Funston, Kansas, 1918. Because the country was at war, farm boys from isolated communities such as Haskell County were on the move. During the spring, a soldier from Haskell County probably brought this new and virulent form of influenza to Camp Funston, a military camp in Kansas
“During the 1918 epidemic we saw men in the army camps who passed through an attack of influenza-pneumonia and died within a few weeks from tuberculous pneumonia or miliary [blood-borne] tuberculosis. These men had previously been so free from signs of their tuberculosis, as to be accepted for military service as healthy individuals.” Ibid p. 220
-Lt. Colonel Warren T. Vaughan, MD
Whatever the agent responsible for influenza, concluded Vaughan, whether viral or bacterial, many individuals with pulmonary tuberculosis did get influenza, and that this disease, having been contracted, in many cases hastened fatal termination of both the tuberculous process and the patient.
Fort Riley, Kansas was a sprawling establishment housing 26,000 men and encompassing an entire camp, Camp Funston. Soldiers there often complained about the inhospitable weather: bone-chilling winters and sweltering summers. Squeezed between these extremes were the blinding dust storms. Within the camp, thousands of horses, hogs, mules and chickens produced in excess of a stifling nine tons of manure each month. And the accepted method for its disposal was to burn it, even against driving wind. State Veterinarian W.J. Butler would report at the 28th Meeting of the United States Live Stock Sanitary Association: “I consider contaminated manure and stagnant water the most important factors in the spread and propagation of tuberculosis” [48]. And so on Saturday, the 9th of March, 1918, a threatening black sky forecast the coming of a major dust storm. When this storm combined with the ashes of over 9 tons of burning manure, a stinking, stinging yellow haze resulted. The sun was said to have gone black in Kansas that day. Two days later, on March 11th, company cook Albert Gitchell reported to the Funston infirmary saying he had “a bad cold”. Among his symptoms were a headache, a sore throat, muscle aches, chills and fever. He also reported cleaning pig pens on March 4th, one week before feeling sick. Gitchell would never recover from this, his last illness. And by noon of March 14th, a hundred men joined him at the Army infirmary he had walked into. Within a month 1,000 men were sick and approximately 50 dead [49]. Camp Funston was having a deadly epidemic.
These deaths were highly unusual, but nothing like what would return in the fall, when the disease would come back with a vengeance, seeming to gain strength through human passage. Camp Funston in March, Camp Devens in September, then across the country and the world, leaving an estimated 20-100 million dead globally, at least 600,000 to a million of them American, in the span of less than two years –the most destructive plague that man had ever witnessed.
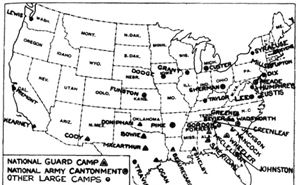
Figure 17: Army training camps in the U.S. in 1918, Camp Funston sitting in the middle of the Country. Source: War Department (US). Annual report, 1919. Washington: Government Printing Office; 1920. p. 1519
Kansas might have been situated in the middle of the United States, but it also found itself a state with a fairly high incidence of TB, sandwiched between states with some of the highest incidence of tuberculosis in the United States [Figure 19]:
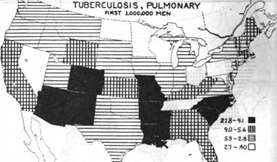
Figure 19: Map of the United States, circa 1918, showing by States the varying incidence of pulmonary tuberculosis as found at mobilization camps in the first million men drafted. The highest incidence is in the blacked-out and darkened-grid designated States, most of them Southern. Note that Kansas, were Fort Funston was located and the Influenza epidemic initially broke out, is squarely sandwiched between some of the States, marked in solid black, with the highest incidence of tuberculosis in the US
And if one took into account the states from which Funston drew its personnel [See Figure 21] –its catchment area, then the highest incidence of TB found in US army mobilization camps for the first million men in WWI was far and away within the circumference from which Camp Funston’s recruits were drawn.
In his post-mortem of the Great Pandemic, in The Medical Department of the United States Army in the World War, Major Milton W. Hall, M.D spoke of inflammatory diseases of the respiratory tract [50] freely using the word “influenza” to explain what happened in 1918 while at the same time admitting that the cause of such influenza was entirely unknown. Pneumonia is what killed in 1918. But pneumonia from what?
Hall addresses these deadly “atypical” pneumonias, saying this:
“The high incidence of pneumonia was largely confined to camps which were made up of men from the states of the south and southeastern part of the country, States whose men had been shown by Civil War figures to be far more susceptible to the respiratory diseases in serious form than those in the north and west.” [50]
Clearly, in 1918 at the time of the mobilization of the first one million men, there existed a specific disease predominantly in the “south and southeastern” parts of the United States –and that disease was pulmonary tuberculosis. [See Map Figure 19, above] Furthermore, in the US in 1918, death through tuberculosis was, in the age group under 45, at an all-time high, targeting the very same age range that would suffer the most deaths in the Great Pandemic [51,52]. Similarly, by 1918, tubercular deaths had spiked around the world, including such places as Japan and Australia.
In 1905, Flick had already concluded that one-fifth of all tubercular patients gave previous histories of pneumonia [53], something which Baum and Amberson pursued in their discussion of how non-tuberculous pneumonias could and did often complicate the pneumonia of pulmonary tuberculosis [54]. Within a year of Major Milton Hall’s pandemic monograph for Surgeon General Ireland, Rist pointed to how lobar and other pneumonias, so frequently calculated statistically as being aside and apart from tuberculosis, were frequently being mistaken for the sudden onset of lung tuberculosis with its lobar or lobular localization [55]. In almost 50 per cent of 300 consecutive admissions, Rist mentioned, there was an acute onset of an “atypical” tubercular pneumonia, for which Rist provided the following description:
“. . . it has the appearance of an acute pulmonary or pleuropulmonary episode. Chills and fever initiate it, the fever being generally high. Pain in the sides, coughing, and expectoration are always present. The sputum may be rusty as in ordinary lobar pneumonia; dullness or flatness, tubular breathing, and crepitant rales.” [55]
Rist’s ‘atypical’ tubercular pneumonia was the same term used by Major Milton W. Hall, M.D for his atypical ‘influenza’ pneumonia.
While Rist pointed out the many cases of pulmonary tuberculosis mistaken for lobar and other pneumonias, Harvard’s physician and epidemiologist Lt Colonel WT Vaughan spoke of the two clinical scenarios he found most prevalent during 1918: First, “a clinical picture which closely simulates ordinary lobar pneumonia”. Second, an illness which “is most suggestive of acute tubercular infection, and it is only by repeated examination of the expectoration that the clinician can satisfy himself he is not really over-looking a case of acute pulmonary tuberculosis.” [47]. The problem was, that quick-killing acute galloping tubercular consumption often did not yield any positive sputum or “expectoration” evidence.
Farber and Clarke reported 100 cases which were admitted to a general hospital for non-tuberculous pneumonia, which were found to be of a tubercular cause [56].
Office of the Surgeon General of the United States, Washington DC, October, 1918
“In the differential diagnosis influenza, acute miliary tuberculosis, sepsis, and malarial fevers must be differentiated.” [80]
–Maj. Gen. Merritte Weber Ireland, US Army, Surgeon General during the later stage of the Great Pandemic of 1918.
Thus did physician and pandemic of 1918 Surgeon General Merritte Weber Ireland, come to admit, seven years after the censor-ridden Woodrow Wilson administration, that during the Pandemic of 1918 it was difficult, if not impossible, to differentiate “influenza” from acute miliary tuberculosis, both of which often began with similar flu-like symptoms.
Ireland directed physician and Colonel George Bushnell to elaborate further in Chapter Three of The Medical Department of the United States Army in the World War, regarding how to “eliminate” tuberculosis from the army. Colonel Bushnell, constantly referring to a “tuberculosis virus”, long after Koch had discovered his tubercle bacilli, was realistic, if not brutally frank. Bushnell:
“What may be called the modern view is based upon the well-established fact that practically every civilized adult has come into contact with the tubercle bacillus and has thereby acquired what in a sense is a tuberculous infection. Evidence of chronic tuberculous changes is found in some cases of pulmonary tuberculosis with acutely fatal termination. That they are not found in all such cases which occur in civilized man is largely accounted for by the difficulties of the search. Even Nigeli in his classical investigations which finally resulted in finding tuberculous changes present in 97 to 98 per cent of autopsies, at the beginning found only 40 per cent.” Ibid [57], pp.173,188
American Surgeon General from October 4th 1918 to May 31st 1931, Merritte Weber Ireland knew what baptism by fire was all about. And his didn’t begin in October, 1918. Having obtained his M.D. degree Ireland immediately joined the Army’s medical service and was commissioned Assistant Surgeon from Indiana on May 4th 1891. He was in the Philippines during the Filipino-American war, where some of the greatest atrocities, on both sides, were committed in the history of warfare. And while there, Ireland saw direct field action, participating in a dozen engagements in the provinces of Cavite, Camarines, and Albay in southern Luzon.
Once back in the US, Ireland made the rounds through the Army’s fortifications, including a stint at Fort Funston, Kansas under Major John Von R. Hoff. While at Funston, Ireland took direct charge of the first company for instruction of the Hospital Corps organized by Captain Hoff. During WW 1, Funston’s main purpose was to train soldiers drafted in Midwestern states to fight overseas.
Surgeon General Ireland, as Gorgas before him, both knew that the army had a tubercular problem on its hands. They also knew that it could only get worse. War and tuberculosis simply fed off of one another. As early as 1915, the tubercular death rate had not only increased in all countries at war, but even in some, like the United States, that had not yet taken direct action in the conflict. And as the global conflict mushroomed, it became more and more obvious that war was increasingly and commonly spawning deadly forms of tuberculosis with a rapid course and without any tendency towards healing. Such acute, “galloping” forms would be extremely difficult to diagnose, with little time to evolve into the chest x-ray and other evidence that doctors relied upon.
At the time S. Adolphus Knopf estimated that 176,091 in the US army of 1918 were either already tubercular or were strongly predisposed to developing or contracting the disease [58]. And so as soon as war was declared Surgeon General Gorgas, and then Ireland after him , moved to expand a single 500 bed TB facility at Fort Bayard, New Mexico into a 10,000 bed chain of tubercular hospitals around the country. Never had any nation, in so short a time, constructed, equipped and made operational such an efficient chain of TB hospitals for its military personnel. Nor was there a wasted bed.
Fort Funston was in Kansas, a state named after the Kansa Native American tribe, Kansa often said to mean “people of the wind” or “people of the south wind” –originally was a Dakota Sioux word. Pandemic physician Fishberg seemed aware that the inhalation of dust is a strong predisposing factor in the instigation of tubercular lung disease, lowering the resisting powers of the invaded lung, and preparing the soil for the deposit of tubercular bacilli to thrive on inflamed, dust damaged tissue [59].
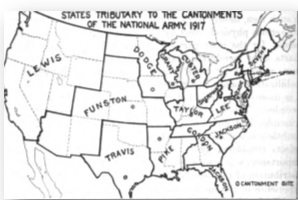
Figure 21: Map of the United States showing the catchment areas of each of the 16 National Army Cantonments, 1917. Camp Funston’s area included many from the American Plains Indians
Merritte Weber Ireland knew that the War Department looked at American Indians as its secret weapon –a group with little fear of the enemy and an almost inborn ability when it came to scouting, reconnaissance, the ability to plan accurate and unexpected attacks –and an uncanny marksmanship.
Thousands of American Indian from various tribes had been moved to the area that is now Kansas from the eastern United States and Great Lakes area. The Indian Removal Act of 1830 resulted in the settlement of more than 10,000 American Indians to what is now Kansas. And by the time of the Great Pandemic, four American Indian tribes still retained reservations there.
Camp Funston sat just off of Fort Riley on the banks of the Republican River. When war became imminent, Congress quickly established a draft, and more than 4,600 Selective Service draft boards screened 10 million men to find the strongest and most fit among them, including 12,500 American Indians, some from the Lakota Sioux [60]. What is known is that at least one multi-tribal group of Indians enlisted at Lawrence, Kansas, from the Haskell Indian School and trained at Funston.
The American Indians, once removed from their native habitat and shoved onto reservations, where no strangers to killer epidemics. Ireland had witnessed some of these himself. Just five years before the Great Pandemic of 1918, almost three thousand Sioux where made prisoners of war and forced to move into barracks, where a people to whom tuberculosis had been rare in their native habitat turned, inside their barracks and reservations, into a tubercular inferno of death in its most acute, “galloping” form, reaching a mortality by 1913 ten times higher than anything that Europe had seen in the worst of its nineteenth-century epidemics.
Nor was it just the Sioux or just those in barracks. What happened to the Sioux was being seen in Indian reservations across the country, including those of the Navaho. In fact it was on such reservations that Indian tubercular mortality rate reached the highest rate anywhere at any time [61]. The problem was that some of the children from these Indian reservations where in Fort Funston now, where unlike the treatment given to other minorities, they were purposely being integrated into regular white military units in a strategy to assimilate them into American culture, and many of these Indian inductees were now at Fort Funston.

Figure 22: American Indian Student battalion at Haskell Institute in Kansas, 1920s. Many from Haskell served with great distinction during the Great War after being trained at Fort Funston, Kansas
As recruits entered Camp Funston through the post’s main gates they had to display their identification and were then directed towards a long line to be examined by camp doctors.
Because of their sheer number and the burden placed on the Medical Corps, the process was of necessity so fast that some men took their mandatory oath of loyalty before the physician had completed his examination, leaving half-dressed young soldiers to pledge their allegiance to the country [62]. There was much emphasis in the Medical Corp towards simply detecting TB through auscultation of the chest with a stethoscope. And as is the case today, the tuberculin skin test could and often was negative even with active tuberculosis.
Bureau of Laboratories, 1918
Something was eating at physician/researcher/editor William Park during the carnage of 1918. Park, heading the state-of-the-art Bureau of Laboratories in New York City, was relied upon by both the US Government Health Corps and other major research centers across the country. Along with collaborators, including bacteriologist Anna Williams, Park co-authored the important teaching text, Pathogenic Microorganisms [63].
At first, Park blamed Pfeiffer's bacillus for the 1918 pandemic and reported it to the US Army Health Corps. But flustered by the secondary infections and lack of consistency in other labs in isolating Pfeiffer's, Park flip-flopped, cautioning against attributing the pandemic solely to Pfeiffer's. By the same token, Park felt uncomfortable with the muzzling of the media and medical corps by the Wilson administration regarding the ferocity of the 1918 American epidemic in general –that is, until it was too late to deny its viciousness. So although Pfeiffer's influenza bacillus, also known as Mycobacterium influenzae, was no longer at the top of his choices for the causal agent of the 1918 pandemic, it was its association with another mycobacterium, tuberculosis, which bothered Park the most. He had just re-read Flick's account of what preceded both Great Pandemics.
By 1888, physician Lawrence Flick, citing US census reports leading into the two greatest world "influenza" pandemics in history, reported that out of every 1,000,000 deaths, 242,842 males and 302,046 females died of tuberculosis. This was for all nationalities and colors. Specific subsets within these statistics revealed that among African Americans, every million deaths represented 248,179 males and 326,973 females having died of tuberculosis. Among people of Irish parentage, 309,507 males and 375,636 females died of TB for every million deaths. And among people of German parentage, its victims numbered 249,498 males and 254,958 females for every one million deaths [64].
Although Park had seen these statistics before, their effect was not lost on him: "Consumptives [people with tuberculosis] frequently carry influenza bacilli [Pfeiffer's bacilli] for years and are particularly susceptible to attacks of influenza” [63].
In 1918, with "flu" victims dropping all around them, John B. Hawes, MD, of the Massachusetts General Hospital, and Richard Cabot, MD, of Harvard, wrote: "One of the diseases most frequently mistaken for pulmonary tuberculosis is influenza, chronic or acute." According to Hawes and Cabot, the symptoms of both diseases were often identical [65].
Tufts pulmonologist Edward O. Otis also caught Park's attention. Otis not only mirrored Hawes and Cabot's view, he went a step further: "Often a patient gives the history of a previous attack of influenza which may have been an active outbreak of a latent tuberculous focus, which later again became inactive." [66]
Back in 1901, an editorial in the Journal of the American Medical Association specifically cited Liverpool physician R. Buchanan: "Dr. R.J.M. Buchanan makes the not improbable suggestion that many of the so-called sporadic cases of influenza are really symptomatic of the initial infection of tuberculosis, or possibly an exacerbation of a latent tuberculosis previously unsuspected or undetected." [67] In saying this, Buchanan had fired the shot heard around the world, and physician Walter Lindley, editor of the Southern California Practitioner, was quick to respond. Lindley on Buchanan: "The author, impressed by the large number of instances in which patients have referred the commencement of their ill-health to an attack of so-called influenza, conceives that many of the so-called sporadic cases of influenza are really symptomatic of the initial infection of tuberculosis or possibly an exacerbation of a latent tuberculosis." [68]
A complaint by Wakley and Wakley in the Lancet in 1899 regarding an epidemic of infectious fever raging in New York is also appropriate in that it portrayed how the public at large cherished and demanded their own right to self-diagnose their ‘flu’: "The name 'influenza' seems to have a strong attraction for some people. Every ache and pain, no matter where located and whether accompanied by fever or not, is at once put down as 'influenza'; every headache, every coryza [nasal congestion, common cold], every sore-throat, every attack of gastroenteritis, from whatever cause, is promptly self-diagnosed as 'influenza', and when the practitioner arrives upon the scene he will be expected to fall in with this view, and there is a great temptation to do so." [69] The authors go on to suggest that the rise in temperature and general malaise frequently met with in this New York epidemic wasn't influenza at all.
Hendrickson, who practiced during the Great Pandemic of 1918–19, spoke even more bluntly:
"No doubt there were many cases of tuberculosis whose death certificates were labeled influenza during the pandemic owing to lack of time to make a diagnosis by the overworked physician." [70]
And so, as the greatest influenza pandemic of all time continued to rage, other doctors were speaking out. In 1920, with the killing fields of 1918–19 laid out clearly before him, British physician Marcus Paterson took yet another jab at the "flu" epidemic. He wrote: "During the time I was Resident Medical Officer at Brompton, it was usual to classify a rise of temperature in an ordinary case of 'chronic' pulmonary tuberculosis as influenza, even when there was no epidemic." As for the well-known sick feeling (malaise) and preliminary symptoms of reactivated tuberculosis, Paterson asked: "Are these symptoms any different from those of the ordinary onset of influenza? They are not, simply because they denote not a particular disease, but a toxemia due to bacterial action." [71]
Paterson's reference to clinicians confusion of sorting out influenza from TB was pointed:
"Surely there is no more emphatic testimony to the clinical difficulties of a differentiation between the two diseases when one group of clinicians describes a rise of temperature in a proven case of tuberculosis to be influenza, and another section terms an arrested case of tuberculosis with pyrexia [fever] an active case of tuberculosis."
Writing in 1920, after the greatest pound-for-pound health catastrophe ever, Paterson said what every physician still learns as a resident: "Before the war, it was usual to classify a sudden onset of acute symptoms as influenza, and it is very easy to appreciate the reason. When a person is taken ill, the patient's friends demand to know at once what the ailment is: hence it must be given a name, and 'influenza' is a good enough term for the moment."
What Paterson left out, as a given, was the hysteria in the family and immediate community that would ensue were a diagnosis of tuberculosis rendered. Instead, he went on to say that he knew of no physician who could differentiate clinically between tuberculosis and influenza ̶ quickly adding that he "shudders to think" of the number of times that "tubercle bacillus have been classified as influenza without further investigation". To Paterson, this represented countless opportunities to cure the real cause, masked under the designation "influenza". His conclusion: "There is no research required here. It is a known fact that what is at present called influenza is often tuberculosis. But the knowledge is not practically applied. It is information of inappreciable value lying idle. It is no new discovery, or its import would ring throughout the world." In fact, for Paterson, much like it was for von Unruh, "influenza" was simply the first indication of tuberculosis.
Rockefeller Institute for Medical Research, NYC, 1931
American virologist Richard E. Shope, based in the Department of Animal Pathology at the Rockefeller Institute, was in direct communication with British investigators Smith, Andrewes and Laidlaw at Mill Hill, England, and sent samples of his flu virus and Pfeiffer's bacillus. But the British group, in return, wasn't being 100 per cent supportive.
American virologist Richard E. Shope, based in the Department of Animal Pathology at the Rockefeller Institute, was in direct communication with British investigators Smith, Andrewes and Laidlaw at Mill Hill, England, and sent samples of his flu virus and Pfeiffer's bacillus. But the British group, in return, wasn't being 100 per cent supportive.
Virologists like Shope and Laidlaw saw a great opportunity for virology provided by the 1918–19 pandemic. Shope began the first salvo on swine "influenza", again falling back on the stale conception that the mild disease and flu-like symptoms were created in pigs by what he believed to be a "filterable virus"[73]. Shope had the singular advantage of realizing that since 1918, pigs had been coming down with the same "influenza" each year. Having lived in Iowa, he had grown up with the knowledge. But beginning his investigations in earnest, Shope became perplexed. Not a virus but a bacterium kept cropping up in swine mucous secretions, and it resembled Pfeiffer's bacillus or Haemophilus influenzae (H. flu) more than anything else. The problem was that he couldn't infect most of his subjects with the bacterium alone. So he took the mucous secretions of sick pigs and put them through a filter which he thought would only yield a virus. However, incredibly, even the filtrate from the discharge just gave low-grade symptoms. So if it wasn't a "virus" that had caused the deadly strains of flu, and if it wasn't a bacterium present in most malignant "flu", what could it be? To Shope’s mind, possibly both, working in conjunction with one another. In 1931, he introduced both the "virus" and Pfeiffer's bacillus into animals [74], which subsequently came down with just the deadly "flu" complicated with pneumonia that killed between 20 and 100 million people in 1918–19.

Figure 28: Dr. Richard Shope. Originally Shope was looking for simply a bacterial cause such as Pfeiffer’s bacillus for “swine influenza”, as the medical orthodoxy of his day dictated. There is no evidence in the literature that Shope even knew about the filterable forms of H. influenza (Pfeiffer’s bacillus) which also appeared “viral”
As late as 1944, Shope still was insisting that the pandemic influenza was this meld of "virus" plus Pfeiffer's bacillus [75]. But as opposed to what's written in today's revisionist history, the idea wasn't really his. When Shope was a teenager, the announcement that hog cholera (swine flu) was due to the combined action of a bacterium and a virus stimulated French-Canadian microbiologist Félix d'Hérelle in 1917 to publish his discovery of bacteriophages –viruses which live in and can destroy or alter the shape of bacteria –followed by d'Hérelle’s 1921 classic book on such bacterial viruses [76]. Not only could such viral bacteriophages alter the shape of a bacteria [or mycobacteria in the case of mycobacteriophages], causing virus-like, cell-wall-deficient forms, but such phages could also change a microbe’s staining characteristics and moreover, its virulence. Therefore an individual with a latent case of TB could potentially be at risk for the reactivation of previously quiescent infection when another mycobacterial infection [M. avium, M. Influenzae] occurred –through the injection of phage DNA into previously latent TB.
Actually, it was only sometime after joining Rockefeller in 1928, that Richard Shope switched his interest from tuberculosis to virology. There is no evidence in the literature that Shope even knew about the filterable forms of H. influenza (Pfeiffer's bacillus) which also appeared "viral". To accept Shope's conclusion, an exception had to be made. There wasn't a single cause behind the 1918–19 pandemic, but two: a virus and a bacterium.
UK Medical Research Council, Mill Hill Farm, 1932

Figure 29: Mill Hill ‘Farm’ Laboratories, at the UK MRC’s National Institute for Medical Research facilities in north London. As far back as 1921, the MRC purchased this 40 acre agricultural site, then part of Rhodes Farm, to breed experimental animals at and do studies in
British virologists Smith, Andrewes and Laidlaw, as was the case for head of the UK’s MRC (Medical Research Council) Walter Fletcher, all saw the study and cure of canine distemper in dogs as the soft underbelly to promote finding the virus that caused the Influenza Pandemic of 1918. The only problem was, that the vast weight of evidence was pointed in an entirely different direction, towards the fact that canine distemper was caused by a filterable bacterial bacillus, and not a virus. In fact Laidlaw’s entire set of criteria for calling a pathogen a virus: such as that it could pass through a filter, and that it could not grow on artificial media was on shaky grounds, and he knew it.
Nor was the fact lost on many that Patrick Playfair Laidlaw's team seemed to fly directly in the face of Shope's multifactorial conclusion, which said that both a virus and Pfeiffer's bacillus from swine were necessary to acquire the flu. Just the "virus" itself was necessary, claimed the British trio [77].
Christopher Howard Andrewes, who would subsequently receive the lion’s share of credit for discovering the human influenza virus, thought that he had discovered the viral cause of rheumatic fever at the Rockefeller Institute as far back as 1923 [78]. When that didn't pan out, and back in England, he, similar to Shope in America, put his efforts into the futile attempt to find a virus behind all cancers.
But when virus-admirer Walter Fletcher, first secretary of Britain’s MRC, started his new scheme to once and for all viralize Influenza, Andrewes soon found himself at the Mill Hill farm under virologist Patrick P. Laidlaw and his colleague Wilson Smith. The MRC was originally founded as a consequence of the recommendations of the Royal Commission on Tuberculosis, but if Walter Fletcher had anything to say about it, it would now go viral.

Figure 32: Sir Walter Fletcher of England’s MRC. Fletcher seemed to have a great affection for viral research: “There are many reasons for suspecting that cancer may prove to be a virus disease, in which case all the elaborate work done in studying viruses will in due time find its use here.”
So when influenza re-appeared in epidemic proportions during the autumn of 1932, Andrewes and Smith executed an experimental outline planned long in advance by Laidlaw and designed to reveal their hypothetical virus. In the midst of this, Andrewes began to feel unwell with flu-like symptoms. Immediately, Smith, having passed Andrewes' respiratory secretions through a filter, began to inject his colleague’s mucus intra-cerebrally and intra-testicularly into mice, rabbits and guinea pigs. Nothing happened immediately, but just afterwards Laidlaw was informed by the director of Wellcome laboratory, their reference lab, that some of their ferrets appeared to be suffering from influenza at the same time as the epidemic of influenza was raging among Wellcome's staff. Ferrets, traditionally used for rabbit hunting and in rat control, had never been used in medical research until Laidlaw and Dunkin used them in their dog distemper studies. And it turned out that these Wellcome ferrets didn't have influenza but were suffering from distemper. In addition ferrets are extremely sensitive to tubercular mycobacteria as well, and Mycobacterium avium, M. bovis and human M. tuberculosis have all been isolated from ferrets [79]. In fact the very same techniques used by Andrews Smith and Laidlaw could easily have transmitted tubercular infection to their ferrets [80].
But never mind, Smith had another idea and then inoculated several other ferrets through their nose with filtrates from Andrewes nasal secretions. Shortly thereafter “influenza” appeared. Subsequently, Wilson Smith himself came down with a flu that the rest of the group suspected was from a ferret. From the filtrate of this strain, the infectious agent WS (Wilson Smith) was isolated.

Figure 33: Nasal injection of the ferret. Andrewes is the one holding the pipette, and his assistant holds the ferret, demonstrating the standard technique of instilling ‘virus’ material into the nose of a ferret. The ferret was anaesthetized with ether, to ease injection of the virus material. Source: Picture Post, ‘Can We Beat Influenza?’ 2nd of February 1946, p. 10.
But as head of a government lab at that time, Dr. William Crofton actually examined the famed WS strain that had afflicted Wilson Smith, and that today is still being used in research; bearing the alphanumeric notation A/WS/1933, for Influenza A/Wilson Smith/1933. Crofton, of all people, knew that there was nothing "viral” about strain WS, which he had personally investigated. Nor did it have anything to do with "influenza". Smith, Andrewes and Laidlaw where in pathologist William Crofton’s mind clearly perpetrating a deceptive fraud, and Crofton fully intended to stop them.
During the summer and winter of 1918, Crofton was isolating Pfeiffer’s bacillus from 100 per cent of influenza cases. But to do so, he was using improved special-growth agents and a sufficiently high-powered microscope. Crofton's "moist-chamber method" kept his culture medium warm and moist. If the microbe wasn't kept warm, Crofton found, then it couldn't be isolated in every case. Pfeiffer's bacillus was clearly pleomorphic (many forms) with viral-like forms that could easily pass through a filter.
So, in 1938, when Crofton was final able to corner Andrewes, who was presenting a paper before the Epidemiology Section of the Royal Society of Medicine, Crofton said this:
"I asked him how then he knew that his colleague Wilson Smith, from whom was first isolated the virus, had, in fact influenza, and how he and his fellows dared to advertise to the four corners of the earth that at long last the [viral] cause of influenza had been discovered. I told him that I had ascertained that Wilson Smith had, in fact, influenza, because he was swarming with [bacterial or mycobacterial] influenza bacilli. I asked him why no cultures on proper medium were made from the infected ferret to ascertain if the Pfeiffer bacillus could be grown, as it would have been inevitably if, in fact, influenza had been transmitted. Andrewes replied not one word, and the authorities of the section would not publish my criticism." [25]
Apparently the fix was already in through powerful monied UK governmental forces at Mill Hill’s MRC led by Sir Walter Fletcher. Crofton didn’t stand a chance. Neither did the rest of the world, which was forced to accept influenza as a virus.
Christopher Andrewes, now flushed with victory, suggested with the help of Burnet and Bang that the term "myxovirus", meaning "mucous virus", be incorporated into a family name for the influenzas [81]. This, one imagines, was because the organism came from mucous secretions. By May 1935, another from this group, Patrick Playfair Laidlaw, who in his deceptive viral studies did anything but play fair, wrote "Epidemic Influenza: A Virus Disease"–an article seemingly titled to assure himself that this was the case. In this document, Laidlaw would give the only credence he saw fit to Crofton and the many scientists like Crofton who did not believe him, saying that although he thought that it was "gradually" being proven that a "filterable virus" was primarily responsible for epidemic influenza, "Pfeiffer's bacillus or Haemophilus influenzae is still regarded by many observers as the prime cause of the disease and many of its complications" [82]. Actually, it was the MRC and its influence not only in the UK but abroad that would make sure that this “gradually” became much more accelerated –much, much more accelerated.
Crofton was convinced by the confirmation of scientists like Calmette at Pasteur regarding how certain forms of tuberculosis, appearing both minuscule and viral, could pass through the smallest of filters. Crofton himself then established that tuberculosis could disappear into tissues as viruses did, and then go through filters which stopped cold even most of those "now invariably called viral disease". "Surely, then", Crofton concluded, "Tuberculosis has more right to be considered a true virus than these (25)." So, at a time when viral forms of TB where scarcely being documented, Crofton struggled to link H. Flu with the TB it so often infected in coordination with, as historical and political momentum carried Laidlaw's study through for posterity. Pfeiffer's strongly resembled TB; it was just smaller. A great opportunity was missed to correct the record.
The Name Game
After the pandemic of 1918–19, the name of Pfeiffer's bacillus, Mycobacterium influenzae, was officially changed to Haemophilus influenzae. American bacteriologist Margaret Pittman [83], pinpointed the influenza bacillus's name change to a 1920 report by Charles-Edward A. Winslow and colleagues for the Society of American Bacteriologists' nomenclature committee.
After the pandemic of 1918–19, the name of Pfeiffer's bacillus, Mycobacterium influenzae, was officially changed to Haemophilus influenzae. American bacteriologist Margaret Pittman [83], pinpointed the influenza bacillus's name change to a 1920 report by Charles-Edward A. Winslow and colleagues for the Society of American Bacteriologists' nomenclature committee.
By any stretch, C.-E. A. Winslow was far from the ideal choice to head a panel to rename Mycobacterium influenzae to Haemophilus influenzae. Not only was he an avid one-form-only bacteriologist [84], leaving very little room for a microbe which could appear in both fungal and bacterial forms, but his unique thought processes became obvious when, as Professor of Public Health at Yale, he said he believed that posture was a neglected cause of tuberculosis, a disease known since antiquity for its spine-bending changes.
Yet Winslow and his colleagues decided to ignore Pfeiffer's bacillus's previously documented fungal forms, concluding that, unlike the mycobacteria, it stained Gram-negative and liked hemoglobin. But neither was conclusive enough to warrant the name change that Winslow had in mind. Tuberculosis researcher Stephen J Maher of the Connecticut State Tuberculosis Commission wrote in 1913: "All non-acid coccal and bacillary derivatives of the tubercle bacillus are, strange to say, Gram-negative." [85] Krylow confirmed Maher's observation that TB could stain Gram-negative, [86] –just like Pfeiffer’s bacillus.
The Gram stain, developed by Hans Christian Gram, separates bacteria based on their cell walls. The thick layers in "Gram-positive” cell walls stain purple, while the thin "Gram-negative" cell walls appear pink. Like other bacilli, cultures of TB, on the other hand, could be Gram-positive when young but might become Gram-negative as they aged. Hans Much saw this in 1907 [87], and Chandrasekhar reported it again in 1983 [88]. Therefore, the fact that Pfeiffer's influenza bacillus was Gram-negative still didn’t rule it out as a bacillary derivative of the tubercle bacillus. Furthermore, the organism was arbitrarily named Haemophilus influenzae (from the Greek haemophilus, meaning blood-loving), but it grew on the same blood-based cultures on which Mycobacterium tuberculosis had long been known to thrive [89].
To many in the lay and scientific communities, bacterial names and classifications are hallowed ground. But Sneath and Brenner's 1992 paper [90] for the American Society for Microbiology clarified that there was no such thing as an official classification of bacteria or "approved lists" and that even Bergey’s Manual was not "official", but merely the best consensus at the time. Therefore, Sneath and Brenner said, bacterial lists and classifications (called "taxonomy") are partly a matter of judgment and opinion, as is all science, and, until new information is available, different bacteriologists may legitimately hold different views. In the same vein, George Fox and colleagues remind us that even with regard to today's sacred 16S rRNA sequence identity as a criterion for species identification, 16S rRNA may not be sufficient alone to guarantee species identity[91]. Today, we are carefully taught that Pfeiffer’s bacillus, historically Mycobacterium influenzae, was erroneously thought to have caused and been behind the Great Pandemic of 1918–19. We are not taught that Pfeiffer's bacillus itself has a virus-like cell-wall-deficient phase which goes right through a filter. Nor are we taught that its original difficulty in cultivation on normal media alone, without blood's hemoglobin, almost classified Pfeiffer’s bacillus, a priori, as a "virus" in the minds of those who relentlessly track a virus as being behind influenza.
Virology on the Ropes
It was in 1952 that Cornelius P. Rhoads, Director of the Sloan Kettering Institute for Cancer Research in New York City, remarked in the introduction to a conference on viruses and cancer that the term "virus" had achieved "a high professional status with doubtful credentials" [92]. Rhoads wasn’t alone.
It was in 1952 that Cornelius P. Rhoads, Director of the Sloan Kettering Institute for Cancer Research in New York City, remarked in the introduction to a conference on viruses and cancer that the term "virus" had achieved "a high professional status with doubtful credentials" [92]. Rhoads wasn’t alone.
Papers such as that of Peter Palese [93], of the Mount Sinai School of Medicine in Manhattan, remind us that, even in 1992, millions in China already had antibodies to H5N1, meaning that they had contracted it and that their immune system had little trouble fending it off.
And as Dutch science historian Ton van Helvoort accurately pointed out, by the 1950s the word "virus" had become so moldable a concept that one could speak of viruses without the existence of any consensus whatsoever of what viruses were [94]. Extremely supportive of this moldability among virologists was biophysicist Max Delbrück's subtitle for "Viruses 1950", a conference held at the California Institute of Technology: "Proceedings of a conference on the similarities and dissimilarities between viruses attacking animals, plants, and bacteria, respectively" [95]. Indeed, in the 1930s and 1940s, the concept of "filterable virus" was subjected to such criticism that its very foundations were threatened.
But in truth, until the late 1940s influenza viruses were studied as infections, which, although filterable, were conceived of as analogous to bacteria, a kind of ultra-bacteria.
Not to be deterred, and still seeing Influenza as a great opportunity for virology, in 1941 virologist Hirst [96] claimed that influenza ‘‘virus’’ could agglutinate red blood cells of fowl and other animal species. Hirst showed that ‘‘virus’’ particles first adsorbed to the red cells and, after a certain time, eluted again as a result of what could be interpreted as an enzymatic reaction. But six years later, Middlebrook and Dubos (1948) made this seem nothing more than a cheap hat trick by showing that similarly red blood cell agglutination could be produced by sera from patients with tuberculosis [97]. Takahashi and Ono then reviewed similar red cell agglutination occurring in the presence of tubercular serums [98,99].
The "H" and "N" of influenza sub-typing, revolves around two glycoproteins called hemagglutinin (HA) and neuraminidase (NA), both of which can be, and are, associated with infectious diseases such as the minuscule, viral forms of tuberculosis, a disease which ought to be high on the differential diagnosis for 'flu-like illness' . Since August, 2008, a Medline study in the Journal of Clinical Biochemistry showed that sputum neuraminidase levels over 1.0 mU per mL were proven associated with having tuberculosis in 92% of cases, previous to which bacteria closely related to TB were shown, through crystallization, to produce the same protein neuraminidase used to subtype 'Influenza' [100]. Furthermore, as of 2006, it has become obvious in Menozzi's study that similar to Influenza, tuberculosis not only uses hemagglutinin to attach to the lung's epithelial cells it invades, but requires hemagglutinin for dissemination of the disease to the rest of the body [101].
There was much the same “Influenza” talk when in 1990, a new multi-drug-resistant (MDR) tuberculosis outbreak took place in a large Miami municipal hospital. Soon thereafter, similar outbreaks in three New York City hospitals left many sufferers dying within weeks. By 1992, approximately two years later, drug-resistant tuberculosis had spread to seventeen US states, with mini-epidemics in Florida, Michigan, New York, California, Texas, Massachusetts, and Pennsylvania and was reported, not by the American, but the international media, as out of control. By 1993 the World Health Organization (WHO), proclaimed tuberculosis a global health emergency, an emergency which since then it has never lifted.
To say that the history of the theoretical underpinning of virology has been a tortuous one is probably an understatement, and, incredibly, many virologists even to this day persist in using the flawed reasoning that that which passes through a microfilter is a virus. Certainly, one exception to these is virologist/molecular biologist Stefan Lanka, who questions the very existence of H1N1 and its allied forms as a swine flu virus altogether. Lanka, after reviewing the data, came to the conclusion that studies, micropictographs and even the data have been manipulated. Through personal communication, he has assured this author that his repeated investigative letters to the National Institutes of Health (NIH) regarding supportive material for the "influenza virus" have gone unaddressed and unanswered [102]. Virologist Lanka therefore concludes that the pictures the CDC is showing are liposomes from dead cell cultures that have been reduced, and then centrifuged with a solvent. In addition he feels that US attempts that supposedly wrested a virus from cadaver specimens were contrived and deceptive.
Shannon Brownlee and Jeanne Lenzer are not new to writing sharp warnings regarding public health. Among their writings listed on Medline is a British Medical Journal treatment entitled "Doctor takes 'march of shame' to atone for drug company payments" [103]. That influenza vaccination and oral antiviral studies have been consistently pushed and paid for by pharmaceutical companies themselves is no secret. But once in a while, unsponsored papers such as Brownlee and Lenzer's 2009 article in The Atlantic clear the misinformation:
"Whether this season's swine flu turns out to be deadly or mild, most experts agree that it's only a matter of time before we're hit by a truly devastating flu [or coronavirus] pandemic— one that might kill more people worldwide than have died of the plague and AIDS combined. In the US, the main lines of defense are pharmaceutical—vaccines and antiviral drugs to limit the spread of flu and prevent people from dying from it. Yet now some flu experts are challenging the medical orthodoxy and arguing that for those most in need of protection, flu shots and antiviral drugs may provide little to none. So where does that leave us if a bad pandemic strikes?…" [104]
The authors added: "But what if everything we think we know about fighting influenza [or coronavirus] is wrong? What if flu vaccines do not protect people from dying –particularly the elderly, who account for 90 percent of deaths from seasonal flu? And what if the expensive antiviral drugs that the government has stockpiled over the past few years also have little, if any power to reduce the number of people who die or are hospitalized? The US government—with the support of leaders in the public-health and medical communities—has put its faith in the power of vaccines and antiviral drugs to limit the spread and lethality of swine flu. Other plans to contain the pandemic seem anemic by comparison. Yet some top flu researchers are deeply skeptical of both flu vaccines and antivirals. Like the engineers who warned for years about the levees of New Orleans, these experts caution that our defenses may be flawed, and quite possibly useless against a truly lethal flu. And that unless we are willing to ask fundamental questions about the science behind flu vaccines and antiviral drugs, we could find ourselves, in a bad epidemic, as helpless as the citizens of New Orleans during Hurricane Katrina."
This paper has intentionally questioned perhaps the most fundamental question about the science behind influenza and its vaccine and antiviral cures: is it really a virus at all? For if it is indeed a form of viral, cell-wall-deficient mycobacteria such as Mycobacterium influenzae or Mycobacterium tuberculosis, or Mycobacterium avium, as many physicians and scientists in the past have suggested, we could indeed find ourselves in the midst of another devastating, infectious Hurricane Katrina, worldwide.
References
- Muller DG., et al. “A Virus Infection in the Marine Brown Alga Ectocarpus siliculosus (Phaeophyceae)”. Botanica Acta 103.1 (1990): 72-82.
- Lanka STJ., et al. “Genome Structure of a Virus Infecting the Marine Brown Alga Ectocarpus siliculosus”. Virology 193.2 (1993): 802-811.
- Klein M., et al. “Coat Protein of the Ectocarpus siliculosus Virus”. Virology 206.1 (1995): 520-526.
- Holcombe J and Jacobson D. The Great 1918 Flu Pandemic Was Not Due to Flu ... or A Virus. April 14, 2011. Salem-News. http://www.salem news.com/articles/april142011/1918-flu.php.
- Lehman KB, Neumann R. Atlasund Grundriss der Bakeriologie und Lehrbuch der speziellen bakteriologischen Diagnositk. 1st Ed., (1896) J.F. Lehmann, Munchen
- Chester FD. A manual of determinative bacteriology. The Macmillan Co New York, (1901): 1-401.
- Wolbach SB. “The Filterable Viruses, a Summary”. The Journal of Med Research 27.1 (1912): 1-25.
- Torrey JC and Rank AH. “Studies in Canine Distemper”. The Journal of Medical Research 27.3 (1913): 291-364.
- Ferry NS. “Etiology of Canine Distemper”. The Journal of Infectious Diseases 8.4 (1911): 399-420.
- Van Helvoort T. “History of Virus Research in the Twentieth Century: The Problem of Conceptual Continuity”. History of Science 32.2 (1994): 185-235.
- Laidlaw PP and Dunkin GW. “Studies in dog-distemper: III.―The nature of the virus”. Journal of Comparative Pathology and Therapeutics 39 (1926): 222-230.
- Kendall AI. “Observations Upon the Filterability of Bacteria, Including a Filterable Organism Obtained From Cases of Influenza”. Science 74 (1931) 74: 129-139.
- Bird C. “What has become of the Rife Microscope?” New Age Journal (1976): 41-47.
- Smith W., et al. “A virus obtained from influenza patients”. Lancet 222.5732(1933): 66-68.
- Lwoff A. "The Concept of Virus: The Third Marjory Stephenson Memorial Lecture". Journal of General Microbiology 17.1 (1957): 239-253.
- Noymer A and M Garenne, "The 1918 influenza epidemic’s effects on sex differentials in mortality in the United States". Population Development Review 26.3(2000): 565-581.
- Pearl R. Influenza studies. Public Health Reports. The United States Public Health Service, Washington Government Printing Office 34.32 (1919): 1743-1792.
- Oei W and Nishiura H. “The Relationship between Tuberculosis and Influenza Death during the Influenza (H1N1) Pandemic from 1918-19”. Computational and Mathematical Methods in Medicine (2012): 9
- Tekaia F., et al. "The Genomic Tree as Revealed from Whole Proteome Comparisons". Genome Research 9.6 (1999): 550-557.
- Stobie W., "Prognosis in Pulmonary Tuberculosis". British Medical Journal 1 (1933): 507-509.
- Koch R. "The Aetiology of Tuberculosis". Mitteilungen aus dem Kaiserlichen Gesundheitsamte 2 (1884): 1-88.
- Cornet G., Nothnagel's Encyclopedia of Practical Medicine. Tuberculosis and Acute General Miliary Tuberculosis, W.B. Saunders Co Philadelphia (1907): 483.
- Avery OT and Lyall HW. “Concerning Secondary Infection in Pulmonary Tuberculosis”. The Journal of Medical Research 28.1 (1913): 111-139.
- Rosenow EC. “Studies in Influenza and Pneumonia” Journal of the Mississippi State Medical Association (1919): 423-424.
- Crofton WM. “The True Nature of Viruses”. John Bale Sons & Curnow (1939): 165-166.
- Wiltschur AJ. "Ueber den Einfluss der Gippe auf den Verlauf der Phthise und deren Krankheitsbild bei Complicationen mit Gippe". Petersburger med Wochenschrift (1890): 5.
- Anderson M. “Clinical Lectures on the Curability of Attacks of Tubercular Peritonitis and Acute Tuberculosis (Galloping Consumption)”. James Maclehose Publisher Glascow (1877): 56.
- Abrams A. “A Popular Catechism of Consumption”. WM Doxey San Francisco 40 1897: 8-9.
- Dubos R and Dubos J. “The White Plague: Tuberculosis, Man, and Society”. Rutgers University Press (1987): 205.
- Mays TJ. “Types of Pulmonary Consumption in Pulmonary Consumption, Pneumonia and Allied Diseases of the Lungs. EB Treat & Company”. New York 539 (1901): 309-319.
- Wade HW and Manalang C. “Fungous Developmental Growth Forms of Bacillus Influenzae”. Journal of Experimental Medicine 31.1 (1920): 95-103.
- Pfeiffer RFJ. "Die Aetiologie der Influenza" ("The Aetiology of Influenza"), Zeitschrift für Hygiene und Infektionskrankheiten 13 (1893): 357-386.
- Wainwright M. "Extreme Bacterial Pleomorphism and the Bacterial Life Cycle: A Forgotten Controversy". Perspectives in Biology and Medicine 40.3 (1997): 407-414.
- Birt C Leishman WB. “A new Acid-Fast Streptothrix, Pathogenic to Man and Animals”. Journal of Hygiene 2.2 (1902): 120-128.
- Macpherson WG and Leishman WB. History of the Great War –Medical Services Pathology. His Majesty’s Stationary Office London (1923): 600.
- Flexner S. "Pseudo-tuberculosis Hominis Streptothricha". Journal of Experimental Medicine 3.4.5 (1898): 435-450.
- Von Unruh V. "A Comparative Study of the Acid Fast Bacilli". The Eclectic Medical Journal (ed. Harvey Wickes Felter) 76.6 (1916): 289-300.
- Stengel A and Fox H. “A Text-book of Pathology”. W.B. Saunders & Co Philadelphia 6th ed (1915): 298.
- Youmans GP., Tuberculosis, W.B. Saunders Co., Philadelphia, (1979).
- Schlossberg D. Tuberculosis (Praeger Mongographs in Infectious Disease)”. 2 (1983).
- Gernez-Rieux C., et al. “Experimental study of interactions between pneumoconiosis and mycobacterial infections”. Annals of the New York Academy of Sciences(1972): 106-26.
- Rosenzweig DY. “Pulmonary mycobacteria infections due to Mycobacterium avium complex. Clinical features and course in 100 consecutive cases”. Chest 75.2 (1979): 115-119.
- Rosenzweig DY. “Atypical mycobacteriosis”. Clinics in Chest Medicine 1 (1980): 273–284.
- Evans AS., et al. “Pulmonary mycobacterium kansasii infection: comparison of the clinical features, treatment and outcome with pulmonary tuberculosis”. Thorax 51. 12 (1996): 1248-1252.
- Fox H. Comparative Lessons in Infection, Detection and Control of Tuberculosis. Report of the Proceedings of the Twenty-Eighth Annual Meeting of the US Livestock Sanitary Association. Chicago, Illinois. December (1924): 106-111.
- Miner L. Public Health Reports US Gov Printing Office 33. 14 (1918).
- Vaughan WT. Influenza, an Epidemiologic Study, American Journal of Hygiene Monograph Series No. 1 Baltimore (1921).
- Butler WJ. Tuberculosis. Proceedings of the Twenty-Eighth Annual meeting of the United States Live Stock Sanitary Association. Chicago, December 3.5, (1924): 97.
- Hollenbeck JE. “An avian connection as a catalyst to the 1918-9 influenza pandemic”. International Journal of Medical Sciences 2.2 (2005): 7-90.
- Hall MW. Communicable and other Diseases in the Medical Dept of the United States Army in the World War, Volume IX. Washington. U.S. Government Printing Office (1928).
- Linder FE and Grove RD. “Vital Statistics Rates in the United States 1900-1940”. Washington, D.C.: U.S. Government Printing Office. (1947).
- Grove RD and Hetzel AM. “Vital Statistics Rates in the United States 1940-1960”. Washington, D.C.: U.S. Government Printing Office. (1968).
- Flick KF. Third Annual Report (1905-1906), Henry Phipps Institute, Philadelphia. (1906).
- Baum OS and Amberson JB JR. “Non-tuberculous pulmonary infections complicating pulmonary tuberculosis”. American Review of Tuberculosis and Pulmonary Diseases 45.2 (1942): 243-279.
- Rist, E. “An Address on the Sudden Onset of Lung Tuberculosis in the Adult and Its Lobar Localization”. Canadian Medical Association Journal 21.2 (1929):143-152.
- Farber JE and Clarke WT. “Unrecognized tuberculosis in a general hospital”. American Review of Tuberculosis (1943):129-134.
- M.W. Ireland and Siler JF. “The Medical Department of the United States Army in the World War: Volume IX, Communicable and other Diseases”. Washington: U.S. Government Printing Office (1928): 628.
- Knopf SA. “A History of the National Tuberculosis Association”. National Tuberculosis Association, New York. (1922): 470.
- Fishberg. “M Pulmonary Tuberculosis 3rd Edition”. Lea & Febiger Philadelphia and New York. (1922): 891.
- War Department (US). “Order of battle of the United States land forces in the World War” Washington: U.S. 3 (1931):
- McDougall JB. “Tuberculosis: a Global Study in Social Pathology”. E&S Livingstone Ltd., Edinburgh (1950):
- Coffman EM. “The War to End All Wars: The American Military Experience in World War I”. University Press of Kentucky Press (1986): 440.
- Park WH and Williams AW. “Pathogenic micro-organisms, including bacteria and Protozoa; a practical manual for students, physicians and health officers”. 4th Edition New York Lea & Febiger (1910):
- Flick L. "The Hygiene of Phthisis". The Medical and Surgical Reporter (ed. Charles W. Dulles). Philadelphia (1888) 58:136.
- Hawes JB, Cabot RC. “Early Pulmonary Tuberculosis –Diagnosis, Prognosis and Treatment”. William Wood and Co. New York (1918): 50.
- Otis EO. “Pulmonary Tuberculosis: A Handbook for Medical Students”. W.M. Leonard, Boston, (1917): 95.
- JAMA, "Influenza or Pulmonary Tuberculosis?" Journal of the American Medical Association 36.23(1901):1628.
- Lindley W. “Influenza and Tuberculosis”. Southern California Practitioner. Los Angeles. (1901): XVI: 338-339.
- Wakley TH and Wakley T. “Influenza”. The Lancet 1 (1899): 103.
- Hendrickson G. “Influenza and Tuberculosis”. Transactions of the Minneapolis, St Paul & Sault Ste Marie Railway Surgical Association, Thirteenth Annual Meeting, Minneapolis, Minnesota (1920): 149-161.
- Paterson M. “The Shibboleths of Tuberculosis”. (1920): 239, 85-88, 224.
- Rich AR. “The Pathogenesis of Tuberculosis”. Thomas Publisher Ltd, Springfield, Illinois (1946): 627.
- Shope RE and Francis T. “The Susceptibility of Swine to the Virus of Human Influenza”. Journal of Experimental Medicine 64.5 (1936): 791-801.
- Shope RE. “Swine Influenza: III. Filtration Experiments and Etiology”. Journal of Experimental Medicine 54.3 (1931): 373-385.
- Shope RE. “Old, Intermediate, and Contemporary Contributions to Our Knowledge of Pandemic Influenza”. Medicine 23.4 (1944): 415-455.
- Adams MH. “Bacteriophages”. Interscience Publishers (1959): 592-593.
- Smith W., et al. “A Virus Obtained from Influenza Patients”. Lancet 222.5732 (1933): 66-68.
- Anonymous. “Profile. Dr C. H. Andrewes: A life's work on virus research”. New Scientist 7.182 (1960): 1200-1201.
- Quesenberry K and Carpenter JW. “Ferrets, Rabbits and Rodents”. Elsevier Health Sciences: Clinical Medicine and Surgery (2011): 558-608.
- McCallan L., et al. “A New Experimental Infection Model in Ferrets Based on Aerosolised Mycobacterium bovis”. Veterinary Medicine International2011.981410 (2011): 9.
- Andrewes CH., et al. “A short description of the Myxovirus group (influenza and related viruses)”. Virology 1.2 (1955): 176-184.
- Laidlaw PP. “Epidemic Influenza: A Virus Disease”. The Lancet 228 (1935): 1119-1120.
- Pittman M. "Variation and Type Specificity in the Bacterial Species Hemophilus influenzae". Journal of Experimental Medicine 53.4 (1931): 471-492.
- Winslow C –EA and Winslow AR. “The Systematic Relationships of the Coccaceae with a Discussion of the Principles of Bacterial Classification”. John Wiley & Sons New York (1908): 130.
- Maher SJ. "The Progeny of the Tubercle Bacillus". Medical Record 84.26 (1913): 1162.
- Krylow O. "Uber die Bedeutung und das Vorkommen der Much'schen Granula" ("On the importance of the presence of Much's granules)”. Zeitschrift für Hygiene 70.1 (1911): 135-148.
- Much H. "Uber die granulare, nach Zieh nicht farbbare. Form des Tuberkulosevirus". Beit z Klin d Tuberk 8.85 (1907): 357.
- Chandrasekhar S. "Studies on Non Acid Fast Variants of Mycobacterium tuberculosis". Indian Journal of Tuberculosis 30.3 (1983): 42-44.
- Mathur ML., et al. "Rapid culture of Mycobacterium tuberculosis on blood agar in resource limited setting". Danish Medical Bulletin 56.4 (2009): 208-210.
- Sneath PHA and Brenner DJ. "'Official' Nomenclature Lists" (Letter). ASM News 58 (1992): 175.
- Fox GE., et al. "How Close Is Close:16S rRNA Sequence Identity May Not Be Sufficient To Guarantee Species Identity". International Journal of Systematic Bacteriology 42.1 (1992): 166-170.
- Rhoads CR. "Introduction: "Viruses as Causative Agents in Cancer". Annals of the New York Academy of Sciences 74.5 (1952): 872-873.
- Palese P. "Influenza: old and new threats". Nature Medicine 10.12 (2004): 82-87.
- Van Helvoort T. "History of virus research in the 20th century: the problem of conceptual continuity." History of Science 32.2 (1994): 185-235.
- Delbrück M. “Viruses 1950. Proceedings of a conference on the similarities and dissimilarities between viruses attacking animals, plants, and bacteria, respectively". California Institute of Technology Pasadena (1950): 1950.
- Hirst GK. “The agglutination of red cells by allantoic fluid of chick embryo infected with influenza virus”. Science 94 (1941): 22-23.
- Pound A. “Observation on the agglutination and haemolysis of red cells treated with extracts of Mycobacterium tuberculosis: an evaluation of methods”. The Journal of Pathology 64.1(1952):131-43.
- Takahashi Y, Ono K. “Hemagglutination reaction by the phosphatides of the tubercle bacillus”. Kekkaku no Kenkyu (Tuberculosis Res) 7.1 (1957).
- Takahashi Y and Ono K. “Study on the passive hem agglutination reaction by the phosphate of M. tuberculosis. 1. The reaction and its specificity”. American Review of Respiratory Disease 83.2 (1961): 381.
- Miller K., et al. “Quantitative sputum analysis: Neuraminidase and tuberculosis.” Clinical Biochemistry 41.12 (2008): 950-954.
- Menozzi FD., et al. “Mycobacterium tuberculosis heparin-binding haemagglutinin adhesin (HBHA) triggers receptor-mediated transcytosis without altering the integrity of tight junctions”. Microbes & Infect 8.1 (2006): 1-9.
- Lanka S. communications with the author.
- Lenzer J and S Brownlee. "Doctor takes 'march of shame' to atone for drug company payments". British Medical Journal 336.7634 (2008): 20-21.
- Brownlee S and J Lenzer. "Does the Vaccine Matter?" The Atlantic (2009).
Citation: Dr. Lawrence Broxmeyer MD. “The Great Influenza Pandemic: What Really Happened in 1918?” Pulmonology Research and Respiratory
Care 1.2 (2017): 53-89.
Copyright: © 2017 Dr. Lawrence Broxmeyer MD. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





































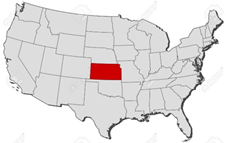








 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.