Research Article
Volume 3 Issue 1 - 2019
Efficacy and Safety of Anti-Vascular Endothelial Growth Factor Therapy for Diabetic Macular Edema in Mongolians
1Sondra Eye Clinic, Ulaanbaatar, Mongolia
2Department of Ophthalmology, School of Medicine, Mongolian National University of Medical Sciences, Ulaanbaatar, Mongolia
3Bolor melmii Eye Clinic, Ulaanbaatar, Mongolia
4Department of Epidemiology and Biostatistics, School of Public Health, Mongolian National University of Medical Sciences, Ulaanbaatar, Mongolia
5Mongolian National University of Medical Sciences, Ulaanbaatar, Mongolia
2Department of Ophthalmology, School of Medicine, Mongolian National University of Medical Sciences, Ulaanbaatar, Mongolia
3Bolor melmii Eye Clinic, Ulaanbaatar, Mongolia
4Department of Epidemiology and Biostatistics, School of Public Health, Mongolian National University of Medical Sciences, Ulaanbaatar, Mongolia
5Mongolian National University of Medical Sciences, Ulaanbaatar, Mongolia
*Corresponding Author: Anaraa Toishubai, Sondra Eye Clinic, Ulaanbaatar, Mongolia.
Received: May 15, 2019; Published: July 24, 2019
Objectives: To evaluate the efficacy and safety of bevacizumab monotherapy or combined with laser versus laser monotherapy in Mongolian patients with visual impairment due to diabetic macular edema.
Design: Prospective, randomized, single-center, a 12 month, laser-controlled, clinical trial.
Participants: One hundred twelve eligible patients, aged ≥18 years, with type 1 or 2 diabetes mellitus and best corrected visual acuity (BCVA) in the study eye of 35 to 69 Early Treatment Diabetic Retinopathy Study (ETDRS) letters at 4 m (Snellen equivalent: ≥6/60 or ≤6/12), with visual impairment due to center-involved diabetic macular edema (DME).
Methods: Patients were randomized into three treatment groups: (I) intravitreal bevacizumab monotherapy (n = 42), (II) intravitreal bevacizumab combined with laser (n = 35), (III) laser monotherapy (n = 35). Bevacizumab injections were given for 3 initial monthly doses and then pro re nata (PRN) thereafter based on BCVA stability and DME progression. The primary efficacy endpoints were the mean change in BCVA and central retinal subfield thickness (CRST) from baseline to month 12.
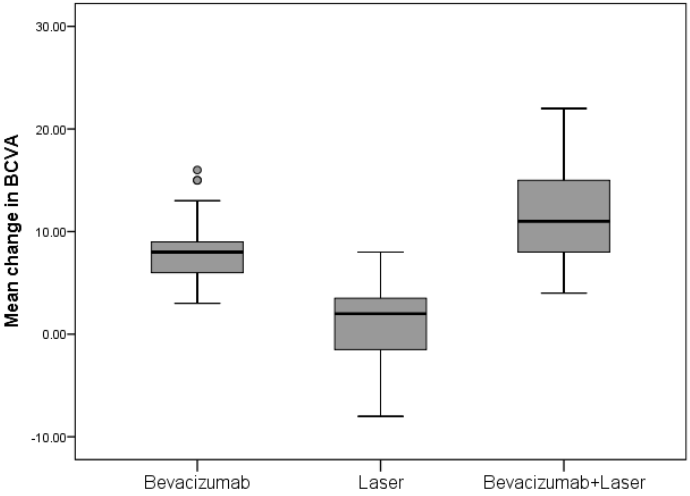
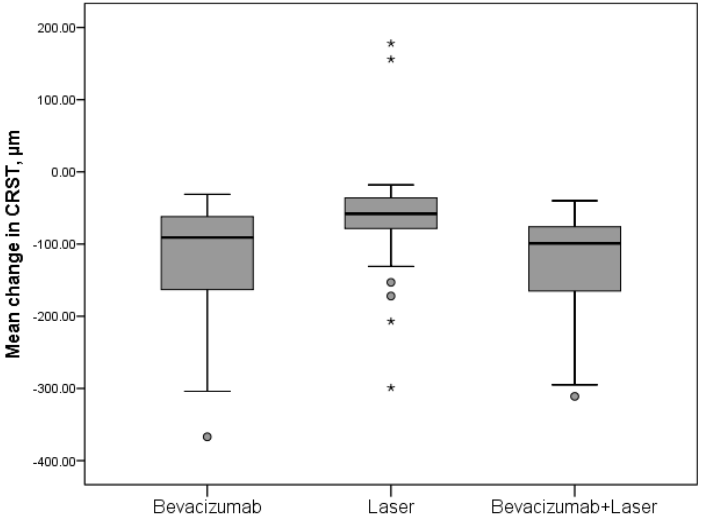
Results: Bevacizumab monotherapy or combined with laser were superior to laser monotherapy in improving mean change in BCVA letter score from baseline to month 12 (+8.3 and +11.3 vs +1.1 letters; both p < 0.0001). There were significant difference detected between the bevacizumab and bevacizumab combined with laser treatment groups (p = 0.004). At month 12, greater proportion of patients gained ≥10 and ≥15 letters and with BCVA letter score >73 (Snellen equivalent: >6/12) with bevacizumab monotherapy (23.8% and 7.1% and 4.8%, respectively) and bevacizumab + laser (57.1% and 28.6% and 14.3%, respectively) versus laser monotherapy (0% and 0% and 0%). The mean central retinal subfield thickness was significantly reduced from baseline to month 12 with bevacizumab (−124.4 μm) and bevacizumab + laser (−129.0 μm) versus laser (−62.0 μm; both p<0.0001). Conjunctival hemorrhage was the most common ocular events. No endophthalmitis cases occurred.
Conclusion: Bevacizumab monotherapy or combined with laser showed superior BCVA improvements over macular laser treatment alone in Mongolian patients with visual impairment due to diabetic macular edema.
Keywords: Diabetic macular edema; Diabetic retinopathy; Vascular endothelial growth factor; Bevacizumab
Introduction
Diabetic retinopathy is the most common microvascular complication of diabetes mellitus and the leading cause of blindness in working-age adults in the United States, Europe, and increasingly worldwide. [1] Diabetic macular edema is a major cause of the vision loss (DME visual impairment) associated with diabetic retinopathy. [2] In 2010, of an estimated 92.6 million adults with diabetic retinopathy worldwide, 20.6 million were estimated to have DME. [3] The increasing prevalence of diabetes worldwide highlights the importance of diabetic macular edema as a global health issue. [4]
The Early Treatment Diabetic Retinopathy Study (ETDRS) established the role of laser in preventing up to 15 letters (ETDRS scale) loss of best-corrected visual acuity (BCVA) with prompt therapy. [5] Although laser photocoagulation has been the standard treatment for DME for nearly 3 decades, there is increasing evidence that superior outcomes can be achieved with anti-vascular endothelial growth factor (anti-VEGF) therapy. [6-11] Vascular endothelial growth factor (VEGF) plays a pivotal role in the development of DME. [12] A decade of clinical trials demonstrated anti-VEGF drugs that bind soluble VEGF restore the integrity of the blood-retinal barrier, resolve macular edema, and improve vision in most patients with DME. [13-18] In 2007, the DRCR.net reported results from a phase two randomized clinical trial that suggested intravitreal bevacizumab treatment had an effect on the reduction of DME in some eyes (Protocol H). [19] The Pan-American Collaborative Retina Group (PACORES) also reported an apparent benefit of bevacizumab treatment for DME. [20] A Prospective Randomized Trial of Intravitreal Bevacizumab or Laser Therapy in the Management of Diabetic Macular Edema (BOLT) study randomized 80 participants to intravitreal bevacizumab or macular laser treatment and found that whereas the bevacizumab group gained a median of eight letters in visual acuity over 12 months, the laser group lost a median of 0.5 letters over the same time period.[21,22] Three commonly used intravitreous VEGF inhibitors: Ranibizumab (Lucentis, Genentech), Bevacizumab (Avastin, Genentech), and Aflibercept (Eylea, Regeneron Pharmaceuticals) have been shown to be beneficial and relatively safe for the treatment of diabetic macular edema. Bevacizumab is a full-length recombinant humanized monoclonal antibody that, in contrast to pegaptanib’s isoform-specific actions, blocks all isoforms of VEGF-A. It shares a similar molecular structure with ranibizumab, which was designed as a monoclonal antibody fragment from the same parent murine antibody.[23] In 2015, the Diabetic Retinopathy Clinical Research Network published the results of Protocol T study.[24] In this comparative-effectiveness, randomized clinical trial of center-involved DME causing decreased visual acuity, treatment with intravitreous aflibercept, bevacizumab, or ranibizumab was associated with a substantial improvement in mean visual acuity by 1 month, with the improvement sustained through 1 year.
Diabetes is becoming major public health concern in Mongolia. Most recent report from the Mongolian STEPS Survey on the Prevalence of Non-communicable Disease and Injury Risk Factors 2009 estimated the prevalence of diabetes was 6.5% (95% CI 4.5-8.4) in the study population.[25] It has been reported that in 2010, in Mongolia prevalence of any grade DR was 30.2%, DME 17.7% and sight threatening retinopathy was 6.4%, but 96.3% of these patients could not been treated due to the shortage of trained personnel especially vitreo-retinal specialists, lack of diagnostics and therapeutic instruments at that time.[26] Last few years, however, as a result of the improved training personal and the introduction of the latest technology in our practice, we were able to diagnose and treat patients with diabetic retinopathy at a qualitatively new level. The purpose of this study was to treat and evaluate the clinical efficacy and safety of bevacizumab alone or combined with laser versus laser monotherapy in Mongolian patients with DME visual impairment.
Materials and Methods
Study Design
The study was a prospective, randomized, laser-controlled, 12 month, single-center, clinical trial, and was undertaken at Infinity Eye Clinic, Ulaanbaatar, Mongolia. Patients were randomized into three treatment groups: (I) intravitreal bevacizumab monotherapy, (II) intravitreal bevacizumab combined with laser, (III) laser monotherapy for 12 months. One eye was selected and treated as the study eye. If both eyes were eligible, the eye with the worse visual acuity (VA; assessed at visit 1) was selected for treatment, unless, based on medical reasons, the investigator deemed the other eye more appropriate to receive study treatment. The study was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Ethical Committee of the School of Medicine, Mongolian National University of Medical Sciences. All study participants provided written informed consent before entering the study.
The study was a prospective, randomized, laser-controlled, 12 month, single-center, clinical trial, and was undertaken at Infinity Eye Clinic, Ulaanbaatar, Mongolia. Patients were randomized into three treatment groups: (I) intravitreal bevacizumab monotherapy, (II) intravitreal bevacizumab combined with laser, (III) laser monotherapy for 12 months. One eye was selected and treated as the study eye. If both eyes were eligible, the eye with the worse visual acuity (VA; assessed at visit 1) was selected for treatment, unless, based on medical reasons, the investigator deemed the other eye more appropriate to receive study treatment. The study was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Ethical Committee of the School of Medicine, Mongolian National University of Medical Sciences. All study participants provided written informed consent before entering the study.
Patients
The study population consisted of 112 male and female patients ≥ 18 years of age with either type 1 or 2 diabetes mellitus, and visual impairment due to DME.
The study population consisted of 112 male and female patients ≥ 18 years of age with either type 1 or 2 diabetes mellitus, and visual impairment due to DME.
The key inclusion criteria were: (1) patients of either gender aged ≥ 18 years; (2) diabetes mellitus (type 1 or 2); (3) best-corrected visual acuity (BCVA) in the study eye between 35 and 69 Early Treatment Diabetic Retinopathy Study (ETDRS) letters at 4 m (Snellen equivalent ≥6/60 or ≤6/12); (4) center-involving diabetic macular edema (DME) with central macular thickness (CMT) on optical coherence tomography (OCT) of ≥270 µm; (5) media clarity, pupillary dilation, and subject cooperation sufficient for adequate fundus imaging; (6) intraocular pressure (IOP) <30 mmHg; (7) ability to return for regular study visits. The key exclusion criteria were: (1) macular ischemia (foveal avascular zone [FAZ] ≥1000 µm greatest linear dimension (GLD) or severe perifoveal intercapillary loss on FFA; (2) macular edema due to a cause other than DME; (3) coexistent ocular disease; (4) history of an anti-VEGF treatment for DME in the past 12 months in the study eye; (5) any other treatment for DME in the past four months (such as focal/grid macular photocoagulation, intravitreal or peribulbar corticosteroids); (6) medical history of chronic renal failure; (7) pregnancy (8) uncontrolled glaucoma.
Baseline Evaluation
After informed consent, medical and ophthalmic history was recorded and ophthalmologic examination was performed, including BCVA, applanation tonometry, and anterior segment and dilated slit-lamp biomicroscopic examination. All subjects had standard ETDRS 7 field fundus photographs, FFA and OCT imaging.
After informed consent, medical and ophthalmic history was recorded and ophthalmologic examination was performed, including BCVA, applanation tonometry, and anterior segment and dilated slit-lamp biomicroscopic examination. All subjects had standard ETDRS 7 field fundus photographs, FFA and OCT imaging.
Efficacy and Safety Assessments
Best Corrected Visual Acuity. At baseline and each follow-up visit, investigators assessed the BCVA using the ETDRS-like VA testing chart at a starting distance of 4 m. The primary efficacy end point was the mean change in BCVA letter score from baseline to month 12. Secondary efficacy end points included the proportion of patients with a BCVA letter score >73 (Snellen equivalent: >6/12); the proportion of patients who gained ≥10 and ≥15 ETDRS letters (improvement); the proportion of patients who lost <15 ETDRS letters (stabilization) at month 12.
Best Corrected Visual Acuity. At baseline and each follow-up visit, investigators assessed the BCVA using the ETDRS-like VA testing chart at a starting distance of 4 m. The primary efficacy end point was the mean change in BCVA letter score from baseline to month 12. Secondary efficacy end points included the proportion of patients with a BCVA letter score >73 (Snellen equivalent: >6/12); the proportion of patients who gained ≥10 and ≥15 ETDRS letters (improvement); the proportion of patients who lost <15 ETDRS letters (stabilization) at month 12.
Optical Coherence Tomography. Optical coherence tomography was performed at every study visit using spectral-domain OCT (Cirrus™, Carl Zeiss Meditec, Germany). Retinal thickness was determined using individual A-scans along with each of 6 B-scans. Baseline and 1 year OCT scans were graded at the Infinity Eye Clinic by the investigators. The end points included the mean change in CRST and the proportion of patients with <250 µm (“dry macula”) from baseline over time.
Stereoscopic Color Fundus Photography and Fluorescein Angiography
Stereoscopic color fundus photography and fluorescein angiography (VX-10, Kowa Company, Ltd, Nagoya, Japan) were performed at baseline, month 4, month 8 and month 12. After pupil dilation and before fluorescein dye injection, red-free and ETDRS 7-field color photographic images of the retina of the study eye were taken.
Stereoscopic color fundus photography and fluorescein angiography (VX-10, Kowa Company, Ltd, Nagoya, Japan) were performed at baseline, month 4, month 8 and month 12. After pupil dilation and before fluorescein dye injection, red-free and ETDRS 7-field color photographic images of the retina of the study eye were taken.
Safety Assessments. Safety was assessed by analysis of the incidence of adverse events (AEs) and serious adverse events (SAEs), by ophthalmic examinations, intraocular pressure measurements, and by changes in vital signs over the 12-month assessment period. All ocular and non ocular AEs and SAEs were recorded.
Treatment
Bevacizumab monotherapy
Bevacizumab injections were given for 3 initial monthly (every 4 weeks) doses and then pro re nata (PRN) thereafter based on BCVA stability and DME progression. Subjects were subsequently reviewed every 4 weeks. At each visit, a full history was taken, ETDRS BCVA was recorded by an investigator, and a complete ocular examination (including anterior chamber reaction, IOP and dilated fundoscopy) and OCT were performed.
Bevacizumab monotherapy
Bevacizumab injections were given for 3 initial monthly (every 4 weeks) doses and then pro re nata (PRN) thereafter based on BCVA stability and DME progression. Subjects were subsequently reviewed every 4 weeks. At each visit, a full history was taken, ETDRS BCVA was recorded by an investigator, and a complete ocular examination (including anterior chamber reaction, IOP and dilated fundoscopy) and OCT were performed.
Retreatment Criteria
As of month 3, one injection per month was to be continued if stable VA was not reached. Treatment was suspended if either of the following criteria were met: (1) if the investigator's opinion was that no (further) BCVA improvement was attributable to treatment with intravitreal injection at the last 2 consecutive visits, or (2) BCVA letter score ≥ 84 was observed at the last 2 consecutive visits. After suspension, injections were resumed pro re nata (PRN]) if there was a decrease in BCVA due to DME progression, confirmed by clinical evaluation and/or OCT or other anatomic and clinical assessments, in the opinion of the investigator. Patients were treated at monthly intervals until stable VA was reached again.
As of month 3, one injection per month was to be continued if stable VA was not reached. Treatment was suspended if either of the following criteria were met: (1) if the investigator's opinion was that no (further) BCVA improvement was attributable to treatment with intravitreal injection at the last 2 consecutive visits, or (2) BCVA letter score ≥ 84 was observed at the last 2 consecutive visits. After suspension, injections were resumed pro re nata (PRN]) if there was a decrease in BCVA due to DME progression, confirmed by clinical evaluation and/or OCT or other anatomic and clinical assessments, in the opinion of the investigator. Patients were treated at monthly intervals until stable VA was reached again.
Intravitreal Bevacizumab Injection Technique
Intravitreal bevacizumab (Avastin, Genentech) injections (1.25 mg in 0.05 ml) were performed in the operating theatre of the Infinity Eye Clinic by the investigators. Bevacizumab injections were done under sterile conditions, using topical anesthesia and povidone-iodine 5% into the conjunctival sac and onto the lid margins, and following application of a drape and insertion of a lid speculum. The injections were undertaken with a 30-gauge needle through the supra- or infratemporal quadrant, with a drop of gatifloxacin placed in the fornix at the end of the procedure. Patency of the central retinal artery was determined by indirect ophthalmoscopy and VA of hand movements. The IOP was checked 30 minutes after the injection. After the injection, topical gatifloxacin was instilled 4 times per day for 5 days.
Intravitreal bevacizumab (Avastin, Genentech) injections (1.25 mg in 0.05 ml) were performed in the operating theatre of the Infinity Eye Clinic by the investigators. Bevacizumab injections were done under sterile conditions, using topical anesthesia and povidone-iodine 5% into the conjunctival sac and onto the lid margins, and following application of a drape and insertion of a lid speculum. The injections were undertaken with a 30-gauge needle through the supra- or infratemporal quadrant, with a drop of gatifloxacin placed in the fornix at the end of the procedure. Patency of the central retinal artery was determined by indirect ophthalmoscopy and VA of hand movements. The IOP was checked 30 minutes after the injection. After the injection, topical gatifloxacin was instilled 4 times per day for 5 days.
Laser Treatment
All patients in the intravitreal bevacizumab combined with laser and laser monotherapy groups underwent modified ETDRS macular laser therapy (MLT) at their baseline visit or within 7 days of randomization. ETDRS MLT was performed using the VISULAS® 532s (Carl Zeiss, Germany). Subjects were subsequently reviewed every 4 months. Re-treatments were performed if clinically indicated by ETDRS guidelines. [27] Modified ETDRS MLT comprised 50 µm argon laser spot size, laser applied only greater than 500 µm from the edge of the foveal avascular zone (FAZ), with focal treatment aiming to cause mild blanching of the retinal pigment epithelium and not darkening/whitening of microaneurysms. Areas of diffuse leakage or non-perfusion were similarly treated in a grid pattern. At each visit, a full history was taken and a complete ocular examination was performed (including IOP and dilated fundoscopy); ETDRS BCVA was recorded by the investigators; and 7-field color fundus photography, FFA, and OCT were undertaken.
All patients in the intravitreal bevacizumab combined with laser and laser monotherapy groups underwent modified ETDRS macular laser therapy (MLT) at their baseline visit or within 7 days of randomization. ETDRS MLT was performed using the VISULAS® 532s (Carl Zeiss, Germany). Subjects were subsequently reviewed every 4 months. Re-treatments were performed if clinically indicated by ETDRS guidelines. [27] Modified ETDRS MLT comprised 50 µm argon laser spot size, laser applied only greater than 500 µm from the edge of the foveal avascular zone (FAZ), with focal treatment aiming to cause mild blanching of the retinal pigment epithelium and not darkening/whitening of microaneurysms. Areas of diffuse leakage or non-perfusion were similarly treated in a grid pattern. At each visit, a full history was taken and a complete ocular examination was performed (including IOP and dilated fundoscopy); ETDRS BCVA was recorded by the investigators; and 7-field color fundus photography, FFA, and OCT were undertaken.
Statistical Analysis
All statistical analyses were carried out using IBM SPSS version 20.0 (IBM Corp., Armonk, NY, USA) software for Windows. P-values of less than 0.05 were taken as significant. One-way ANOVA was used to compare baseline BCVA, Brown-Forsythe test was used for BCVA at 12 month and mean change in ETDRS BCVA, and Kruskal-Wallis test was used for CRST. Differences between treatment groups were calculated using multiple comparisons Games-Howel test and non-parametric post-hoc test.
All statistical analyses were carried out using IBM SPSS version 20.0 (IBM Corp., Armonk, NY, USA) software for Windows. P-values of less than 0.05 were taken as significant. One-way ANOVA was used to compare baseline BCVA, Brown-Forsythe test was used for BCVA at 12 month and mean change in ETDRS BCVA, and Kruskal-Wallis test was used for CRST. Differences between treatment groups were calculated using multiple comparisons Games-Howel test and non-parametric post-hoc test.
Results
A total of 112 participants were randomized to receive bevacizumab (42 participants), bevacizumab combined with laser (35 participants), or laser (35). The mean age of the participants was 54.5±10 years; 55.4% were women. A total of 94.6% of the participants had type 2 diabetes, and the mean duration of diabetes was 8.5±4.6 years. The mean visual acuity letter score at baseline was 55.7±8.9, and the mean central retinal subfield thickness was 399.4±114.36 µm. The baseline characteristics of each treatment group are summarized in Table 1. Overall, baseline demographics and diabetes or ocular characteristics were comparable across the 3 treatment groups.
| Variable | Bevacizumab | Bevacizumab + Laser |
Laser | P value |
| No. of patients (eyes) | 42 | 35 | 35 | |
| Mean age ± SD (years) | 54.4±9.76 | 54.03±12.24 | 55.3±8.38 | 0.872† |
| Gender, n (%) | 0.139‡ | |||
| Male | 20 (47.6) | 11 (31.4) | 19 (54.3) | |
| Female | 22 (52.4) | 24 (68.6) | 16 (45.7) | |
| Diabetes type, n (%) | 0.703‡ | |||
| Type 1 | 3 (7.1) | 2 (5.7) | 1 (2.9) | |
| Type 2 | 39 (92.9) | 33 (94.3) | 34 (97.1) | |
| Mean HbA1c ± SD (%) | 9.8±3.08 | 10.8±3.26 | 9.9±3.07 | 0.357† |
| Mean duration of DM (years) | 8.1±3.61 | 8.6±4.73 | 8.8±5.52 | 0.812† |
| Mean BCVA ± SD (letters) | 56.6±8.9 | 54.9±8.6 | 55.5±9.5 | 0.710† |
| Mean CRST ± SD (µm) | 397.3±114.8 | 410.1±116.9 | 391.2±113.8 | |
| Previous vitrectomy, n | 0 | 0 | 0 | |
| Previous laser treatment, n | 0 | 0 | 0 | |
| Pseudophakic, n | 1 | 0 | 1 | |
| Phakic, n | 41 | 35 | 34 | |
| BCVA = best-corrected visual acuity; CRST = central retinal subfield thickness; DM = diabetes mellitus; HbA1c = glycosylated hemoglobin A1c; SD = standart deviation; † One-Way ANOVA;‡ Pearson Chi-Square test. | ||||
Table 1: Key Baseline Demographics, Diabetes, and Ocular Characteristics.
Efficacy
Best-Corrected Visual Acuity
The mean change ± SD in the BCVA letter score from baseline to month 12 improved significantly with bevacizumab and bevacizumab + laser treatment versus laser monotherapy (8.3 ± 3.2 letters and 11.3 ± 4.5 letters vs 1.1 ± 3.7 letters), hence the primary end point was achieved (Table 2). There was a significant difference between the mean ETDRS BCVA at 12 months in the bevacizumab and laser monotherapy group (mean difference 7.2; p < 0.0001); bevacizumab + laser and laser monotherapy group (mean difference 10.2; p < 0.0001) (Table 3). There was also significant difference detected between the two bevacizumab treatment groups (mean difference -3.0; p=0.004) (Table 3). In the laser group, mean BCVA stabilized around baseline level at month 12. At month 12, 4.8% of patients in the bevacizumab group and 14.3% of patients in the bevacizumab + laser group had a BCVA letter score >73 (Snellen equivalent: >6/12).
Best-Corrected Visual Acuity
The mean change ± SD in the BCVA letter score from baseline to month 12 improved significantly with bevacizumab and bevacizumab + laser treatment versus laser monotherapy (8.3 ± 3.2 letters and 11.3 ± 4.5 letters vs 1.1 ± 3.7 letters), hence the primary end point was achieved (Table 2). There was a significant difference between the mean ETDRS BCVA at 12 months in the bevacizumab and laser monotherapy group (mean difference 7.2; p < 0.0001); bevacizumab + laser and laser monotherapy group (mean difference 10.2; p < 0.0001) (Table 3). There was also significant difference detected between the two bevacizumab treatment groups (mean difference -3.0; p=0.004) (Table 3). In the laser group, mean BCVA stabilized around baseline level at month 12. At month 12, 4.8% of patients in the bevacizumab group and 14.3% of patients in the bevacizumab + laser group had a BCVA letter score >73 (Snellen equivalent: >6/12).
| Efficacy Outcome Measure | Treatment groups | P value | ||
| Bevacizumab (n=42) | Laser (n=35) | Bevacizumab+ Laser (n=35) | ||
| Baseline ETDRS BCVA | ||||
| Mean (SD) | 56.6 (8.9) | 55.5 (9.5) | 54.9 (8.6) | 0.710† |
| Median | 58 | 58 | 57 | |
| (P25, P75) | (50.5, 63.3) | (51.0, 63.0) | (49.0, 59.0) | |
| ETDRS BCVA at 12 month | ||||
| Mean (SD) | 64.9 (8.6) | 56.0 (10.1) | 66.3 (6.6) | 0.0001‡ |
| Median | 67 | 58 | 67 | |
| (P25, P75) | (59.8, 70.3) | (49.0, 63.0) | (63.0, 71.0) | |
| Change in ETDRS BCVA | ||||
| Mean (SD) | +8.3 (3.2) | +1.1 (3.7) | +11.3 (4.5) | 0.0001‡ |
| Median | 8 | 2 | 11 | |
| (P25, P75) | (+6.0, +9.0) | (-1.5, +3.5) | (+8.0, +15.0) | |
| Baseline CRST, µm | ||||
| Mean (SD) | 397.3 (114.8) | 391.2 (113.8) | 410.1 (116.9) | 0.448§ |
| Median | 353.5 | 345 | 375 | |
| (P25, P75) | (312.0, 479.0) | (309.0, 494.0) | (318.0, 479.0) | |
| CRST at 12 month, µm | ||||
| Mean (SD) | 272.9 (51.6) | 329.2 (106.7) | 281.1 (52.9) | 0.059§ |
| Median | 269.5 | 279 | 271 | |
| (P25, P75) | (230.0, 305.0) | (254.0, 381.0) | (238.0, 310.0) | |
| Change in CRST, µm | ||||
| Mean (SD) | -124.4 (82.4) | -62.0 (80.8) | -129.0 (74.6) | 0.0001§ |
| Median | -91.0 | -58 | -99.0 | |
| (P25, P75) | (-163.0, -62.0) | (-85.0, -35.0) | (-167.0, -75.0) | |
| ETDRS = Early Treatment Diabetic Retinopathy Study; BCVA = best-corrected visual acuity; CRST = central retinal subfield thickness;† One-Way ANOVA test; ‡ Brown-Forsythe test; § Kruskal-Wallis test | ||||
Table 2: Efficacy Outcome Measures in the Three Treatment groups.
| Outcomes | Bevacizumab vs Laser |
Bevacizumab+Laser vs Laser | Bevacizumab vs Bevacizumab+Laser |
| Change in BCVA | |||
| Mean Difference | 7.2 | 10.2 | -3.0 |
| 95% Confidence Interval | (+5.3, +9.2) | (+7.9, +12.6) | (-5.2, -0.9) |
| P-value† | <0.0001 | <0.0001 | 0.004 |
| Change in CRST | |||
| Mean Difference | -62.5 | -67.1 | 4.6 |
| 95% Confidence Interval | (-107.1, -17.8) | (-111.6, -22.5) | (-38.2, +47.4) |
| P-value‡ | <0.0001 | <0.0001 | 0.529 |
| BCVA = best-corrected visual acuity; CRST = central retinal subfield thickness; BOLD = significant p<0.05 † -Multiple comparisons Games-Howel test, ‡- NPar Kruskal-Wallis test | |||
Table 3: Treatment Group Comparisons: Differences in Mean change BCVA and CRST.
The mean number of bevacizumab injections administered was 8.07 in the bevacizumab group, 7.51 in the bevacizumab + laser treatment group. The percentages of eyes with a change in the letter score of 10 or more and 15 or more are provided in Table 4.
| Efficacy Outcome Measure | Bevacizumab (n=42) | Bevacizumab + Laser (n=35) | Laser (n=35) | P value |
| Proportion with final VA >73 | 2 (4.8) | 5 (14.3) | 0 (0) | 0.044† |
| Proportion with <10 letter gain | 32 (76.2) | 15 (42.9) | 23 (100.0) | 0.0001‡ |
| Proportion with ≥10 letter gain | 10 (23.8) | 20 (57.1) | 0 (0) | |
| Proportion with <15 letter gain | 39 (92.9) | 25 (71.4) | 23 (100.0) | 0.002† |
| Proportion with ≥15 letter gain | 3 (7.1) | 10 (28.6) | 0 (0) | |
| Proportion with <30 letter gain | 42 (100.0) | 35 (100.0) | 23 (100.0) | |
| Proportion with ≥30 letter gain | 0 (0) | 0 (0) | 0 (0) | |
| Proportion with <10 letter loss | 0 (0) | 0 (0) | 10 (83.3) | |
| Proportion with ≥10 letter loss | 0 (0) | 0 (0) | 2 (16.7) | |
| Proportion with <15 letter loss | 0 (0) | 0 (0) | 12 (100.0) | |
| Proportion with ≥15 letter loss | 0 (0) | 0 (0) | 0 (0) | |
| Proportion with <30 letter loss | 0 (0) | 0 (0) | 12 (100.0) | |
| Proportion with ≥30 letter loss | 0 (0) | 0 (0) | 0 (0) | |
| Proportion with final CRST <250 | 17 (40.5) | 10 (28.6) | 5 (14.3) | 0.040‡ |
| BCVA = best-corrected visual acuity; CRST = central retinal subfield thickness; VA= visual acuity; BOLD = significant p<0.05; † -Fisher's Exact test; ‡ -Pearson Chi-Square test; | ||||
Table 4: Categorized BCVA letter score and CRST outcomes at month 12.
Central Retinal Subfield Thickness
The mean change CRST from baseline to month 12 decreased significantly for bevacizumab (-124.4 μm; p<0.0001) and bevacizumab + laser (129.0 μm; p<0.0001) compared with laser (62.0 μm). There was no difference detected between the two bevacizumab treatment groups (p=0.529). At month 12, the proportion of patients with CRST < 250 μm was greater in the bevacizumab monotherapy group (40.5%) and the bevacizumab + laser group (28.6%) compared with the laser group (14.3%).
The mean change CRST from baseline to month 12 decreased significantly for bevacizumab (-124.4 μm; p<0.0001) and bevacizumab + laser (129.0 μm; p<0.0001) compared with laser (62.0 μm). There was no difference detected between the two bevacizumab treatment groups (p=0.529). At month 12, the proportion of patients with CRST < 250 μm was greater in the bevacizumab monotherapy group (40.5%) and the bevacizumab + laser group (28.6%) compared with the laser group (14.3%).
Safety
Serious Adverse Events. There were no ocular and nonocular SAEs reported in any of the treatment arms. No endophthalmitis cases occurred.
Serious Adverse Events. There were no ocular and nonocular SAEs reported in any of the treatment arms. No endophthalmitis cases occurred.
Adverse Events. Conjunctival hemorrhage was the most common ocular events.
Bevacizumab monotherapy or combined with laser was not associated with an increased risk of cardiovascular or cerebrovascular events in this study.
Discussion
This prospective, randomized clinical trial has demonstrated that treatment with bevacizumab as monotherapy or combined with laser treatment is superior to laser treatment alone in improving and sustaining visual acuity in Mongolian patients with DME visual impairment. A greater proportion of patients treated with bevacizumab gained ≥ 10, ≥ 15 BCVA letter scores and with BCVA letter score >73 from baseline compared with the laser-treated patients. Bevacizumab treatment consistently improved BCVA across all subgroups regardless of baseline characteristics as compared with the laser treatment alone (Figure 1).
The functional improvement in BCVA was accompanied by a significant improvement in anatomic end points, CRST on OCT, and resolution of leakage on fluorescein angiography. The mean change CRST from baseline to month 12 decreased significantly for bevacizumab and bevacizumab + laser (both; p < 0.0001) compared with laser (Figure 2). At month 12, 40.5% (bevacizumab), 28.6% (bevacizumab + laser), and 14.3% (laser) patients had CRST < 250 μm. This study showed that the bevacizumab monotherapy or combined with the laser therapy was safe and well tolerated in DME visual impairment patients. There were no ocular and nonocular SAEs reported in any of the treatment groups. This study was not sufficiently large enough to make a definitive safety statement. However, there were no cases of endophthalmitis, no unusual or previously unrecognized complications related to the intravitreal injection. The most commonly reported ocular AE was conjunctival hemorrhage across all treatment groups. There were no cases of glaucoma reported in the bevacizumab treated patients.
The treatment efficacy reported in the bevacizumab studies to date is comparable to that described in this report (Table 5). Although the DRCR.net Protocol H study was uncontrolled with only two intravitreal injection protocol. [19] The Pan-American Collaborative Retina Study (PACORES) was a nonrandomized, uncontrolled and the study duration was for 6 months with only a 3 injection protocol. [20] The BOLT study was based on 6-weeks injections, and 2-arm, randomized, controlled, masked clinical trial. The results of this study on bevacizumab replicate the observations made with ranibizumab in DME in larger randomized controlled trials, suggesting that pan-VEGF-A inhibitors appear to have similar effects on DME. [21]
| DRCR.net Protocol H 2007 | PACORES 2008 | BOLT Study 2010 | DRCR.net Protocol T 2015 | Present study | |
| Age (years) | 65 (57, 73) | 59.7±9.3 | 64.9±9.4 | 62±10 | 54.4±9.76 |
| Duration of DM (years) | 17 (11, 23) | 14 | 17 [11, 24] | 8.1±3.61 | |
| HbA1c (%) | 6.9 (6.3, 8.1) | 7.6±1.4 | 7.7 [6.8, 8.8] | 9.8±3.08 | |
| Change in BCVA | 5 (1,12) | 2.9 ± 3.6 lines of BCVA | 8 [1-10] | 9.7±10.1 | 8.3±3.2 |
| Change in CRST, µm | -56 (-120, -6) | -150.9 | -130±122 | -101±121 | -124.4±82.4 |
| Number of injections | 2 | 3 | 9 | 10 | 8 |
Table 5: Comparison of present study results to other studies of bevacizumab monotherapy groups.
A recent prospective randomized 3-arm trial (1.25 mg intravitreal bevacizumab alone, intravitreal bevacizumab in combination with intravitreal triamcinolone acetonide, and MLT) in patients with clinically significant macular edema (CSME) has reported positive visual outcomes similar to those of our trial.[28] However, the findings were at the 36-week time point, ETDRS VA charts were not used, re-treatments were performed at 12-week intervals, and a significant reduction of CRST from baseline was observed for a shorter 6 week period. Additionally, all those studies were used Stratus OCT, a time-domain OCT which is subject to frequent artifacts and lower repeatability compared to the spectral-domain Cirrus OCT which we used.
Our study used initiation of treatment with 3 initial monthly (every 4 weeks) doses and then pro re nata (PRN) dosing regimen addressing individual patient needs with reduced treatment burden. Currently, monthly injections (RISE and RIDE [9,10]), pro re nata (PRN) approach (RESOLVE [8]), and treat-and-extend (RETAIN [29]) are the main strategies of treating DME with anti-VEGF agents. The PRN and treat-and-extend strategies are considered a more favorable compared with the monthly approach because of the reduced cost burden.
The limitations of our clinical trial were small number of patients and relatively short follow-up time course. Further large multicenter studies are required with longer follow-up (at least 3 years). Because of the chronic nature of the underlying disease process and the mechanism of action of anti-VEGF agents, monotherapy with anti-VEGF drugs is likely to be impractical, although the development of slow delivery systems may yet address this issue. Nevertheless, one would anticipate that treating patients with the clinically significant macular edema with the repeated intravitreal bevacizumab at an earlier time point, before irreversible structural damage has been sustained, will result in even better visual outcomes. Furthermore, more rapid reduction in macular edema compared with MLT may lead to a superior longer-term visual acuity. In conclusion, this study demonstrated the superiority of bevacizumab therapy with or without laser therapy over laser monotherapy in improving BCVA and reducing CRST in Mongolian patients with DME visual impairment.
References
- Bourne RR., et al. “Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990-2010”. British Journal of Ophthalmology 98.5 (2014): 629-638.
- Boyer DS., et al. “Anti-vascular endothelial growth factor therapy for diabetic macular edema”. Therapeutic Advances in Endocrinology and Metabolism 4.6 (2013): 151-169.
- Yau JW., et al. “Global prevalence and major risk factors of diabetic retinopathy”. Diabetes Care35.3 (2012): 556-564.
- International Diabetes Foundation. “IDF Diabetes Atlas”. (2015):
- “Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy: ETDRS report number 9”. Ophthalmology 98 (1991): 766-785.
- Diabetic Retinopathy Clinical Research Network. “Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema”. Ophthalmology 117.6 (2010): 1064-1077 .
- Nguyen QD., et al. “Two-year outcomes of the Ranibizumab for Edema of the Macula in Diabetes (READ-2) study”. Ophthalmology 117.11 (2010): 2146-2151 .
- Massin P., et al. “Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study”. Diabetes Care 33.11 (2010): 2399-2405.
- Nguyen QD., et al. “Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE”. Ophthalmology119 (2012): 789-801 .
- Brown DM., et al. “Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE”. Ophthalmology 120.10 (2013): 2013-2022 .
- Do DV., et al. “Ranibizumab for edema of the macula in diabetes study: 3-year outcomes and the need for prolonged frequent treatment”. JAMA Ophthalmology 131.2 (2013): 139-145.
- Simo R., et al. “Ocular Anti-VEGF therapy for diabetic retinopathy: the role of VEGF in the pathogenesis of diabetic retinopathy”. Diabetes Care 37.4 (2014): 893-899.
- Mitchell P., et al. “The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema”. Ophthalmology 118.4 (2011): 615-625 .
- Do DV., et al. “One-year outcomes of the DaVinci Study of VEGF Trap-Eye in eyes with diabetic macular edema”. Ophthalmology 119.8 (2012): 1658-1665.
- Schmidt-Erfurth U., et al. “Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study”. Ophthalmology 121.5 (2014): 1045-1053.
- Elman MJ., et al. “Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results”. Ophthalmology 122.2 (2015): 375-381.
- Ishibashi T., et al. “The REVEAL Study: Ranibizumab Monotherapy or Combined with Laser versus Laser Monotherapy in Asian Patients with Diabetic Macular Edema; REVEAL Study Group”. Ophthalmology 122.7 (2015): 1402-1415.
- Korobelnik JF., et al. “Intravitreal aflibercept for diabetic macular edema”. Ophthalmology 121.11 (2014): 2247-2254 .
- Scott IU., et al. “A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema”. Ophthalmology114.10 (2007): 1860-1867.
- Arevalo JF., et al. “Primary Intravitreal Bevacizumab (Avastin) for Diabetic Macular Edema. Results from the Pan-American Collaborative Retina Study Group (PACORES) at 6-Month Follow-up”. Ophthalmology 114.4 (2007): 743-750.
- Michaelides M., et al. “A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2”. Ophthalmology 117.6 (2010): 1078-1086 .
- Rajendram R., et al. “A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema; 24-month data: report 3”. Archives of Ophthalmology 130.8 (2012): 972-979.
- Genentech I. “AVASTIN (bevacizumab) injection”. (2010):
- Diabetic Retinopathy Clinical Research Network. “Comparative effectiveness study of intravitreal aflibercept, bevacizumab, and ranibizumab for DME (Protocol T)”. (2014):
- “Ministry of Health Mongolia, World Health Organization Western Pacific Region. Mongolian STEPS Survey on the Prevalence of Noncommunicable Disease and Injury Risk Factors 2009”. Geneva: World Health Organizatio (2010):
- Chimgee Ch. “Evaluating diabetic retinopathy amongst registered diabetic patients in urban and rural Mongolia”. (2010):
- “Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. ETDRS research group”. Archives of Ophthalmology 103.12 (1985): 1796-1806.
- Soheilian M., et al. “Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema”. Ophthalmology 116.6 (2009): 1142–1150.
- Pruente C and RETAIN Study Group. “Efficacy and safety of ranibizumab in two treat-and-extend versus pro-re-nata regimes in patients with visual impairment due to diabetic macular edema: 24-month results of RETAIN study”. ARVO Meeting Abstracts 55.13 (2014): 1700.
Citation: Anaraa Toishubai., et al. “Efficacy and Safety of Anti-Vascular Endothelial Growth Factor Therapy for Diabetic Macular Edema in Mongolians”. Ophthalmology and Vision Science 3.1 (2019): 6-16.
Copyright: © 2019 Anaraa Toishubai., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.