Research Article
Volume 2 Issue 4 - 2018
Fungicidal Effect of Azadiracta Indica and Zingiber Officinale Extracts in the Control of Fusarium Oxysporum and Rhizoctonia Solani on Tomato (Solanum Lycopersicum) Fruits
Department of Crop Production and Protection, Faculty of Agriculture and Agricultural Technology, Federal University, Dutsin-Ma, PMB 5001, Katsina State, Nigeria
*Corresponding Author: Gwa V I, Department of Crop Production and Protection, Faculty of Agriculture and Agricultural Technology, Federal University, Dutsin-Ma, PMB 5001, Katsina State, Nigeria.
Received: November 23, 2017; Published: February 28, 2018
Abstract
Studies were carried out on the in-vitro evaluation of plant extracts in the control of some fungal pathogens from tomatoes (Solanum lycopersicum L) fruits collected from Darawa, Dutsin-Ma and Makera settlements in Dutsin-Ma Local Government Area of Katsina State, Nigeria using neem (Azadirachta indica) and ginger (Zingiber officinale) extracts. Rotted tomato fruits were collected from same locations and taken to Biological science laboratory for isolation, identification and subsequently pathogenicity test. Fusarum oxysporum, A. niger, Rhizoctonia solani, F. moniliforme and A. flavus were isolated from the rotten tomato fruits. Pathogenicity tests carried out confirmed that all the isolates were pathogenic on the tomato fruits. The most virulent pathogens (R. solani and F. oxysporum) were controlled, using the two plant extracts at 40g/l, 80g/l and 120g/l levels of concentrations respectively. The result obtained showed that A. indica was more effective in inhibiting the mycelial growth of R. solani at 40g/l (44.90%) and at 120g/l (97.22%) compare with Z. officnale which reduce the mycelial growth of pathogens to 43.83% and 63.31% at 40g/l and 120g/l respectively. The A. indica also proved to be more potent in controlling F. oxysporum at 40g/l, 80g/l and 120g/l with percentage growth inhibition of 37.51%, 40.56% and 45.48% respectively compared with 22.91%, 35.00% and 42.73% of Z. officinale. It is therefore, concluded that extracts of A. indica and Z. officinale can be used to manage fungal growth of tomato fruits by farmers since they have inhibitory effect on fungal pathogens and is easily available and cheap.
Keywords: Fungicidal effect; Extract; Azadiracta indica; Zingiber officinale; Fusarium oxysporum; Inhibition
Introduction
Tomato (Solanum lycopersicum) is grown all over the world (Agrios, 2005). According to Wachira., et al. (2014), tomatoes are widely grown and consumed. Tomatoes are the world second most important vegetable crop after potato (Dimphna, 2016). It is estimated that the crop is grown on more than 5 million hectares of land with a production of nearly 129 million tonnes worldwide (Bright, 2012) out of which Nigeria produces 1.5 million tonnes annually. Although the production of tomatoes is mostly concentrated in the northern part, it is consumed throughout the country (Kuntama., et al. 2007). The crop is grown for both fresh domestic uses as well as for export market but there is increasing demand for processed tomato products in Nigeria and beyond. The crop supplies a range of nutrients which makes it a widely accepted vegetable by consumers. It is characteristically rich in calcium, phosphorus, magnesium, copper, niacin, iron, foliate, vitamin A, B6, vitamin E, vitamin B2, vitamin C, iron and carbohydrates (Wamache, 2005).
The quality, quantity and to some reasonable extent, profitability of tomatoes are affected by insect pests and pathogens (Tijjani., et al. 2014). Tomatoes are also affected by disease-causing pathogens including bacteria, fungi, viruses and nematodes (Tijjani., et al. 2014). These pests and disease pathogens reduce the quality, quantity of the tomato fruits produced the shelf life of the tomato fruits as well as reduce its marketability income of famers (Goufo., et al. 2008). Synthetic chemicals have been used to manage pathogens in different crops. The major problems of these synthetic chemicals are that they cause environmental pollution and destabilize the ecosystem. Pesticides of plant origin are the best and most important because unlike the synthetic pesticides they are easily degradable, they are non-toxic to humans and the environment, they are target specific, are easily available and do not have residual effects on produce (Okigbo and Nmeka, 2005). In addition, pesticides of plant origin offer solutions to pest resistance, environmental and water body pollution, public concerns about food safety and improve agricultural productivity (Mishra., et al. 2015). It is against this backdrop that the study was carried out to isolate, identify and test the pathogenicity of fungal organisms associated with rot of tomato fruits as well as to inhibit the growth of these fungal pathogens with leaves of A. indica and rhizomes of Z. officinale extracts in vitro.
Materials and Methods
Description of Study Area
The experiment was conducted in the Biological Science Laboratory, Federal University, Dutsin-Ma, Local Government Area (LGA) in Katsina State, Nigeria (latitude 12° 27’ 18’’ N and longitude 07° 29’ 29’’ E) in 2016.
The experiment was conducted in the Biological Science Laboratory, Federal University, Dutsin-Ma, Local Government Area (LGA) in Katsina State, Nigeria (latitude 12° 27’ 18’’ N and longitude 07° 29’ 29’’ E) in 2016.
Collections of Tomato Fruits
Diseased samples of tomato fruits showing various rot symptoms were collected from three locations of Dutsin-ma Local Governments Area in Katsina State namely: Darawa, Dutsin-Ma and Makera at forth nightly interval and the samples were packaged in sterile polythene to prevent to prevent further attack by insects and pathogens.
Diseased samples of tomato fruits showing various rot symptoms were collected from three locations of Dutsin-ma Local Governments Area in Katsina State namely: Darawa, Dutsin-Ma and Makera at forth nightly interval and the samples were packaged in sterile polythene to prevent to prevent further attack by insects and pathogens.
Isolation and identification of pathogens
Diseased samples were washed under running tap water after which the samples were chopped into small pieces of about 2-3 mm in diameter and kept in a sterile Petri dish. The pieces were dipped into 5% hypochlorite solution for about 20 seconds. The pieces of the tomatoes were transferred into Petri dishes containing sterile distilled water and were washed thoroughly in three successive changes of sterile distilled water. About 15ml of the molten PDA was poured in Petri dishes of 9 cm in diameter and allowed to solidify. After solidification, four pieces of the diseased tomato tissues were aseptically placed at different distance in the Petri dishes. The Petri dishes were tightly covered with a masking tape to prevent contamination by air-borne pathogens. The dishes were incubated at ambient room temperature (33°C-37°C) for 7 days to allow for the growth of fungi.
Diseased samples were washed under running tap water after which the samples were chopped into small pieces of about 2-3 mm in diameter and kept in a sterile Petri dish. The pieces were dipped into 5% hypochlorite solution for about 20 seconds. The pieces of the tomatoes were transferred into Petri dishes containing sterile distilled water and were washed thoroughly in three successive changes of sterile distilled water. About 15ml of the molten PDA was poured in Petri dishes of 9 cm in diameter and allowed to solidify. After solidification, four pieces of the diseased tomato tissues were aseptically placed at different distance in the Petri dishes. The Petri dishes were tightly covered with a masking tape to prevent contamination by air-borne pathogens. The dishes were incubated at ambient room temperature (33°C-37°C) for 7 days to allow for the growth of fungi.
Preparation of sub-culture
The pathogens that grew on the Petri dishes were sub-cultured after incubation period of 7 days to have a pure culture of the isolates. This was done by transferring the fungi mycelial on agar plates containing the medium by using an inoculation needle to place the mycelial at the centre of the Petri dishes. The Petri dishes were tightly sealed with a masking tape and thereafter incubated for 7 days. When growth was established, growth patterns were determined based on microscopic and morphological characteristics and were compared with existing authorities (Ahmed and Ravinder, 1993; Burgess., et al. 2008). Test fungi in this study were Rhizoctonia solani and Fusarium oxysporum which were mostly isolated in the locations.
The pathogens that grew on the Petri dishes were sub-cultured after incubation period of 7 days to have a pure culture of the isolates. This was done by transferring the fungi mycelial on agar plates containing the medium by using an inoculation needle to place the mycelial at the centre of the Petri dishes. The Petri dishes were tightly sealed with a masking tape and thereafter incubated for 7 days. When growth was established, growth patterns were determined based on microscopic and morphological characteristics and were compared with existing authorities (Ahmed and Ravinder, 1993; Burgess., et al. 2008). Test fungi in this study were Rhizoctonia solani and Fusarium oxysporum which were mostly isolated in the locations.
Determination of frequency of occurrence of isolates
Records of the organisms isolated were kept on periodic basis to determine the frequency of occurrence of the isolates. Since isolation and characterization were carried out at fortnightly interval, the number of times each fungal pathogen was isolated at fortnightly interval was expressed as a percentage of the total of all the different fungal organisms isolated over the period (Okigbo and Ikediugwu, 2000), which was calculated as follows:
Records of the organisms isolated were kept on periodic basis to determine the frequency of occurrence of the isolates. Since isolation and characterization were carried out at fortnightly interval, the number of times each fungal pathogen was isolated at fortnightly interval was expressed as a percentage of the total of all the different fungal organisms isolated over the period (Okigbo and Ikediugwu, 2000), which was calculated as follows:
Where,
x = number of times an individual isolate has occurred over the period
n = total number of fungal organisms isolated in the study area over the period
x = number of times an individual isolate has occurred over the period
n = total number of fungal organisms isolated in the study area over the period
Pathogenicity test for isolated fungi
Fresh and healthy-looking tomato fruits were collected from the markets and were surface sterilized by dipping them in 5% sodium hypochlorite for 20 seconds and then rinsed with four successive changes of sterile distilled water. The healthy-looking fruits were then wounded with a sterile needle. Mycelial disc from R. solani and F. oxysporum were carefully lifted from the pure culture of the respective plates and introduced directly into the wounded tissues of healthy tomato fruits. In another experiment, three tomato fruits were each surface sterilized and wounded with a sterile needle and inoculated separately with sterile distilled water instead of the mycelial of R. solani and F. oxysporum. This served as the control experiment for both R. solani and F. oxysporum. The inoculated fruits and the control were placed separately lined with a moist filter paper and cover with aluminium foil and incubated at ambient room temperature (33°C-37°C). The fruits were observed for symptoms of rot development after five days of incubation.
Fresh and healthy-looking tomato fruits were collected from the markets and were surface sterilized by dipping them in 5% sodium hypochlorite for 20 seconds and then rinsed with four successive changes of sterile distilled water. The healthy-looking fruits were then wounded with a sterile needle. Mycelial disc from R. solani and F. oxysporum were carefully lifted from the pure culture of the respective plates and introduced directly into the wounded tissues of healthy tomato fruits. In another experiment, three tomato fruits were each surface sterilized and wounded with a sterile needle and inoculated separately with sterile distilled water instead of the mycelial of R. solani and F. oxysporum. This served as the control experiment for both R. solani and F. oxysporum. The inoculated fruits and the control were placed separately lined with a moist filter paper and cover with aluminium foil and incubated at ambient room temperature (33°C-37°C). The fruits were observed for symptoms of rot development after five days of incubation.
Preparation of plant extracts using leaves of A. indica and rhizomes of Z. officinale
The methods of Gwa and Akombo, (2016) and Gwa and Nwankiti, (2017) were adopted for this experiment. Accurately, 40g, 80g and 120 g of powdered leaves of neem (A. indica) and rhizomes of ginger (Z. officinale)were measured respectively using an electric weighing machine. Sterile water was heated using hot plate to a temperature of 100oC. 1-litre of the sterile distilled water was each measured using a measuring cylinder and was poured into conical flasks containing the neem leaves powder and ginger rhizome powder at different level of concentrations respectively. The mixtures were vigorously stirred and left to settle for 24 hours, after which they were filtered through three layers of muslin cloth. Concentrations of 40 g/L, 80 g/L and 120 g/L of the neem leaves and ginger rhizomes were prepared accordingly. 5 ml each of the prepared plant extracts at the different level of concentrations were used to amend in 15 ml of potato dextrose agar.
The methods of Gwa and Akombo, (2016) and Gwa and Nwankiti, (2017) were adopted for this experiment. Accurately, 40g, 80g and 120 g of powdered leaves of neem (A. indica) and rhizomes of ginger (Z. officinale)were measured respectively using an electric weighing machine. Sterile water was heated using hot plate to a temperature of 100oC. 1-litre of the sterile distilled water was each measured using a measuring cylinder and was poured into conical flasks containing the neem leaves powder and ginger rhizome powder at different level of concentrations respectively. The mixtures were vigorously stirred and left to settle for 24 hours, after which they were filtered through three layers of muslin cloth. Concentrations of 40 g/L, 80 g/L and 120 g/L of the neem leaves and ginger rhizomes were prepared accordingly. 5 ml each of the prepared plant extracts at the different level of concentrations were used to amend in 15 ml of potato dextrose agar.
Effect of A. indicaand Z. officinale in inhibiting the mycelial growth of R. solani and F. oxysporum in vitro
The method of Amadioha and Obi (1999) was used to measure the fungitoxicity of the extracts. This method involved direct treatment of potato dextrose agar (PDA) medium with plant extracts before inoculation of fungus. This involved creating four equal sections on each plate by drawing two perpendicular lines at the bottom of the plate. The point of intersection indicates the centre of the plates. These were done before dispensing PDA into each of the plates. The prepared medium was poured into sterilized Petri dishes and 5 ml of each plant extracts at different levels of concentration were poured into Petri dishes containing the media separately, mixed well and allowed to solidify, the solidified medium was inoculated centrally at the point of intersection of the two perpendicular lines drawn at the bottom of the plate with discs (5 mm diameter) which was obtained from one-week old cultures of R. solani and F. oxysporum. Three dishes were plated with extract of each plant at different concentrations. The control experiment had 5 ml of sterile distilled water added to the PDA plates in place of plant extracts respectively, the treatment and control plates were replicated three times and were incubated for 4 days at ambient room temperature (33°C-37°C) and measurement of mycelial radial growth as radius of growing fungal colony were undertaken at intervals of 1 day for 4 days using a transparent ruler. The absence of growth in any of the plates was indication of the potency of the extract against the test fungal. Fungitoxicity was determined as percentage growth inhibition (PGI) according to the method described by Gwa and Akombo, (2016).
The method of Amadioha and Obi (1999) was used to measure the fungitoxicity of the extracts. This method involved direct treatment of potato dextrose agar (PDA) medium with plant extracts before inoculation of fungus. This involved creating four equal sections on each plate by drawing two perpendicular lines at the bottom of the plate. The point of intersection indicates the centre of the plates. These were done before dispensing PDA into each of the plates. The prepared medium was poured into sterilized Petri dishes and 5 ml of each plant extracts at different levels of concentration were poured into Petri dishes containing the media separately, mixed well and allowed to solidify, the solidified medium was inoculated centrally at the point of intersection of the two perpendicular lines drawn at the bottom of the plate with discs (5 mm diameter) which was obtained from one-week old cultures of R. solani and F. oxysporum. Three dishes were plated with extract of each plant at different concentrations. The control experiment had 5 ml of sterile distilled water added to the PDA plates in place of plant extracts respectively, the treatment and control plates were replicated three times and were incubated for 4 days at ambient room temperature (33°C-37°C) and measurement of mycelial radial growth as radius of growing fungal colony were undertaken at intervals of 1 day for 4 days using a transparent ruler. The absence of growth in any of the plates was indication of the potency of the extract against the test fungal. Fungitoxicity was determined as percentage growth inhibition (PGI) according to the method described by Gwa and Akombo, (2016).
Where PGI = Percentage growth inhibition
R = Distance of fungal growth from the point of inoculation to the colony margin in the control plate
R1 = Distance of fungal growth from the point of inoculation to the colony margin in treated plate.
R = Distance of fungal growth from the point of inoculation to the colony margin in the control plate
R1 = Distance of fungal growth from the point of inoculation to the colony margin in treated plate.
Experimental design and data analysis
Data collected were subjected to Analysis of variance (ANOVA) using GenStat Discovery Edition 12 for ANOVA and means separation, Minitab Release 17 for descriptive statistics and Graph Pad Prism 6 for trend graphs. Statistical F-tests were evaluated at P ≤ 0.05. Differences among treatment means for each measured parameter were separated using Fisher’s least significant difference (FLSD) (Cochran and Cox, 1992).
Data collected were subjected to Analysis of variance (ANOVA) using GenStat Discovery Edition 12 for ANOVA and means separation, Minitab Release 17 for descriptive statistics and Graph Pad Prism 6 for trend graphs. Statistical F-tests were evaluated at P ≤ 0.05. Differences among treatment means for each measured parameter were separated using Fisher’s least significant difference (FLSD) (Cochran and Cox, 1992).
Results
Isolation, identification and pathogenicity tests
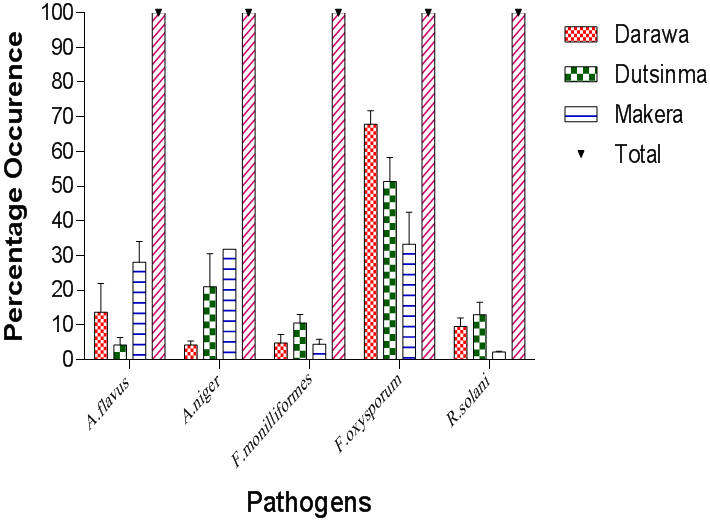
Results presented in Plates I shows some cultures and photomicrographs of the fungi pathogens isolated from tomato fruits. Results presented in Figure 1 show the frequency of the fungi organisms that were isolated and identified in the different locations. F. oxysporium, F. monilliforme, A. niger, A. flavus and R. solaniwere identified. Among the pathogenic fungi, F. oxysporum was the most frequently occurring fungus constituting 67% in Darawa, 51.36% in Dutsin-ma and 33.30% in Makera. A. niger was the second most encountered pathogen constituting 4.17% in Darawa, 21.01% in Dutsin-ma, and 31.85% in Makera. The percentage frequency of occurrence of A. flavus was 13.69%, 4.17%, and 28.10% in Darawa, Dutsin-ma and Makera regions, respectively. R. solani recorded percentage frequency of 9.52% in Darawa, 12.94% in Dutsin-ma, and 2.22% in Makera. The least occurred isolate was F. monilliforme with percentage frequency of occurrence of 4.17% in Darawa, 10.50% in Dutsin-ma and 4.44 % in Makera. Fungi pathogenic organisms were identified based on their morphological growth patterns as well as microscopic characteristics and were compared with existing authorities
Results presented in Plates I shows some cultures and photomicrographs of the fungi pathogens isolated from tomato fruits. Results presented in Figure 1 show the frequency of the fungi organisms that were isolated and identified in the different locations. F. oxysporium, F. monilliforme, A. niger, A. flavus and R. solaniwere identified. Among the pathogenic fungi, F. oxysporum was the most frequently occurring fungus constituting 67% in Darawa, 51.36% in Dutsin-ma and 33.30% in Makera. A. niger was the second most encountered pathogen constituting 4.17% in Darawa, 21.01% in Dutsin-ma, and 31.85% in Makera. The percentage frequency of occurrence of A. flavus was 13.69%, 4.17%, and 28.10% in Darawa, Dutsin-ma and Makera regions, respectively. R. solani recorded percentage frequency of 9.52% in Darawa, 12.94% in Dutsin-ma, and 2.22% in Makera. The least occurred isolate was F. monilliforme with percentage frequency of occurrence of 4.17% in Darawa, 10.50% in Dutsin-ma and 4.44 % in Makera. Fungi pathogenic organisms were identified based on their morphological growth patterns as well as microscopic characteristics and were compared with existing authorities
Table 1 show that there was no significant difference in the occurrence of the isolated pathogens in all the locations. However, there was a significant difference in the total number of fungi pathogens isolated in all the study locations with the highest number of fungi pathogens in Dutsin-ma (12.33) followed by Makera (9.67) and least number of fungi organisms occurred in Darawa (5.67).
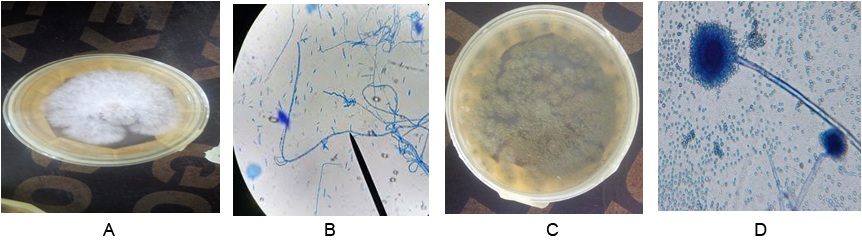
Plate 1: Culture of F. oxysporum (A), Photomicrogragh of F. oxysporum (B), culture of A. flavus (C) and photomicrograph of F. oxysporum (D) showing conidia on conidiophores (x10).
| Pathogens | Locations | P-Value | ||
| Darawa | Dutsinma | Makera | ||
| A.flavus | 1.00 ± 0.57 | 0.33 ± 0.03 | 2.00 ± 0.57 | 0.14ns |
| A.niger | 0.33 ± 0.03 | 2.33 ± 0.88 | 3.00 ± 1.00 | 0.12ns |
| F.monilliformes | 0.33 ± 0.03 | 2.33 ± 0.20 | 0.66 ± 0.06 | 0.62ns |
| F.oxysporum | 3.33 ± 1.33 | 5.66 ± 0.33 | 3.67 ± 1.86 | 0.45ns |
| R.solani | 0.66 ± 0.06 | 2.00 ± 1.53 | 0.33 ± 0.03 | 0.48ns |
| Total | 5.67 ± 1.86a | 12.33 ± 3.38b | 9.67 ± 2.91ab | 0.04 |
Means on the same row with different superscript are statistically significant (P ≤ 0.05); ns = not significant
Table 1: Number of fungi pathogens isolated from different locations.
Table 1: Number of fungi pathogens isolated from different locations.
Pathogenicity Test
The study revealed that the fungi organisms isolated from the infected tomato fruits were pathogenic on the healthy tomato fruits. A. niger was more virulent where the inoculated fruits were completely rotten at the end of the fifth day of incubation. The fruits were completely disintegrated with extensive mycelial growth forming a dark colour covering the fruit skin. Fruits inoculated with F. oxysporum had water-soaked lesions with some white to pink mycelial while fruits inoculated with A. flavus had whitish cheesy like lesions. Samples inoculated with F. monilliforme had small water soaked lesion with slightly brownish appearance on the inoculated areas while tomato fruits inoculated with R. solani had small hard dark lesion around the inoculated area. The fruits that were not inoculated with any fungi pathogen however, showed no signs and symptoms of rot.
The study revealed that the fungi organisms isolated from the infected tomato fruits were pathogenic on the healthy tomato fruits. A. niger was more virulent where the inoculated fruits were completely rotten at the end of the fifth day of incubation. The fruits were completely disintegrated with extensive mycelial growth forming a dark colour covering the fruit skin. Fruits inoculated with F. oxysporum had water-soaked lesions with some white to pink mycelial while fruits inoculated with A. flavus had whitish cheesy like lesions. Samples inoculated with F. monilliforme had small water soaked lesion with slightly brownish appearance on the inoculated areas while tomato fruits inoculated with R. solani had small hard dark lesion around the inoculated area. The fruits that were not inoculated with any fungi pathogen however, showed no signs and symptoms of rot.

Plate 2: Tomato fruit inoculated with A. niger (A), A. flavus (B), R. solani (C), F. oxysporum (D) and Control without fungus mycelial (E).
Efficacy of A. indica and Z. officinale on Radial Growth inhibition of R. solani
The result presented in Table 2 revealed that the plant extracts inhibited the growth of test fungi, although the rate of inhibition varied with different extracts and concentrations used. However, growth inhibition in all the test pathogens took a similar trend in all extracts as the concentration of the tested plant extracts were found to increase with increase in the concentration as incubation period increased. The highest concentration of 120 g/L was the most potent and had the highest inhibition on the pathogens. At concentration of 120 g/L, Z. officinale produced lower effect on R. solani compared with A. indica. The effectiveness of the extracts differed significantly (P ≤ 0.05) comparing them at 120g/L on mycelial growth inhibition of R. solani. The effectiveness of neem and ginger extracts did not differ significantly (P ≤ 0.05) at 40g/L and 80g/L. A. indica inhibited the mycelial growth of R. solani recording percentage growth inhibition of 44.90% compared with Z. officinale which inhibited the growth of R. solani by 43.38%. Neem crude plant extract was the most effective on R. solan but less effective on F. oxysporum. There was however, no significant difference (P < 0.05) between the extracts at each level of comparison when they were tested on F. oxysporum (Table 3).
The result presented in Table 2 revealed that the plant extracts inhibited the growth of test fungi, although the rate of inhibition varied with different extracts and concentrations used. However, growth inhibition in all the test pathogens took a similar trend in all extracts as the concentration of the tested plant extracts were found to increase with increase in the concentration as incubation period increased. The highest concentration of 120 g/L was the most potent and had the highest inhibition on the pathogens. At concentration of 120 g/L, Z. officinale produced lower effect on R. solani compared with A. indica. The effectiveness of the extracts differed significantly (P ≤ 0.05) comparing them at 120g/L on mycelial growth inhibition of R. solani. The effectiveness of neem and ginger extracts did not differ significantly (P ≤ 0.05) at 40g/L and 80g/L. A. indica inhibited the mycelial growth of R. solani recording percentage growth inhibition of 44.90% compared with Z. officinale which inhibited the growth of R. solani by 43.38%. Neem crude plant extract was the most effective on R. solan but less effective on F. oxysporum. There was however, no significant difference (P < 0.05) between the extracts at each level of comparison when they were tested on F. oxysporum (Table 3).
| Concentration (g/L) | Plant Extract | df | T-Value | P-Value | |
| A. indica | Z. officianale | ||||
| 40 | 44.90 ± 11.80 | 43.38 ± 6.88 | 17 | 0.11 | 0.91 |
| 80 | 41.07 ± 9.27 | 61.84 ± 6.49 | 19 | 1.84 | 0.08 |
| 120 | 97.22 ± 2.78 | 63.31 ± 6.79 | 14 | 4.62 | < 0.01* |
*indicates statistical significance at 95% CL
Table 2: Percentage Growth Inhibition of R. solani at different levels of Concentrations of A. indica and Z. officinale.
Table 2: Percentage Growth Inhibition of R. solani at different levels of Concentrations of A. indica and Z. officinale.
| Concentration (g/L) | Plant Extracts and Growth Inhibition (%) | df | T-Value | P-Value | |
| A. indica | Z. officianale | ||||
| 40 | 37.51 ± 6.97 | 22.91 ± 4.92 | 19 | 1.71 | 0.10 |
| 80 | 40.56 ± 6.30 | 35.00 ± 7.24 | 21 | 0.58 | 0.56 |
| 120 | 45.48 ± 6.79 | 42.73 ± 6.67 | 21 | 0.29 | 0.77 |
Table 3: Percentage Growth Inhibition of F. oxysporum at different levels of Concentrations of A. indica and Z. officinale.
Measurement of radial mycelial growth of F. oxysporum in potato dextrose agar amended with Z. officinale
Growth of F. oxysporum on potato dextrose agar amended with Z. officinaleis presented in figure 2. The results indicated that mycelial extension was higher in the control (0 g/L of Z. officinale). This study reveals that the radial growth decreases with increase in the concentration of ginger. At 120 g/L the radial mycelial growth was lowest compared to 80 g/L and 40 g/L respectively. Results presented in figure 3 revealed the growth inhibition of F. oxysporumthroughout the period of incubation. It showed that radial mycelial growth increased with increase in incubation period but decreased with increase in concentration. At 120 g/L the radial mycelial growth was lowest compared to 80 g/L and 40 g/L.
Growth of F. oxysporum on potato dextrose agar amended with Z. officinaleis presented in figure 2. The results indicated that mycelial extension was higher in the control (0 g/L of Z. officinale). This study reveals that the radial growth decreases with increase in the concentration of ginger. At 120 g/L the radial mycelial growth was lowest compared to 80 g/L and 40 g/L respectively. Results presented in figure 3 revealed the growth inhibition of F. oxysporumthroughout the period of incubation. It showed that radial mycelial growth increased with increase in incubation period but decreased with increase in concentration. At 120 g/L the radial mycelial growth was lowest compared to 80 g/L and 40 g/L.
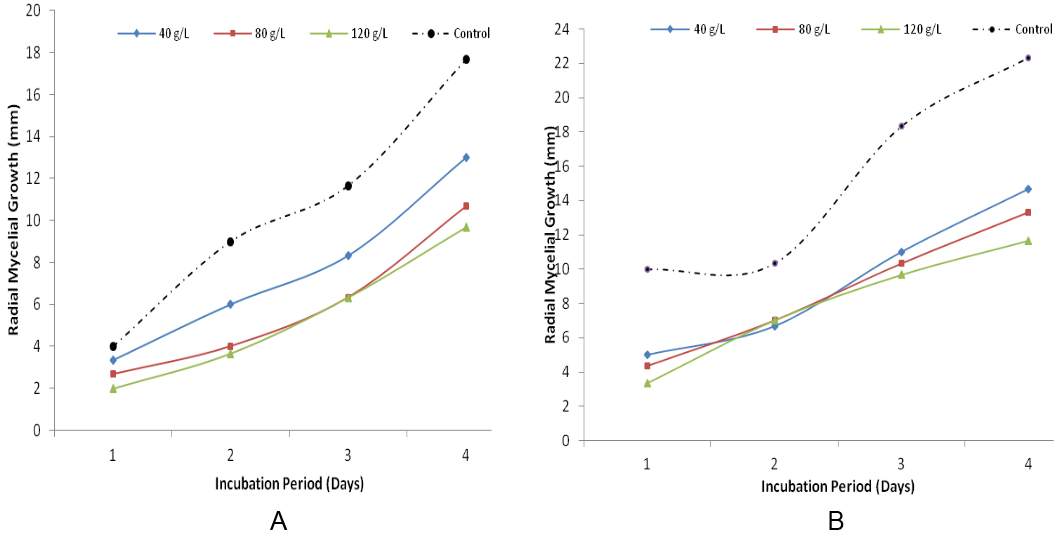
Figure 2: Radial Mycelia Growth of F. oxysporum on Potato Dextrose Agar amended with Z. officinale (A) and A. indica (B) after 4 days of incubation.
The results presented in Figure 3 show the growth inhibition of R. solanion potato dextrose agar amended with Z. officinale. The results revealed that mycelial growth inhibition was lowest in the control plates beginning from first day of incubation at 12 mm and rose to 19 mm on the fourth day. R. solaniwas most inhibited at concentration of 80 g/l and 120 g/l with mycelial growth reduction of 4 mm and 3 mm at first day of incubation and 12 mm and 10 mm at fourth day of incubation respectively. Figure 5 shows a similar trend of growth inhibition of R. solani on potato dextrose agar amended with A. indicaextracts at different concentrations. The result indicated that the pathogen grew more in the control plates that were not inoculated with A. indica. Growth was only observed in plates that were inoculated with the pathogen at first day of incubation in 120 g/L of A. indica.
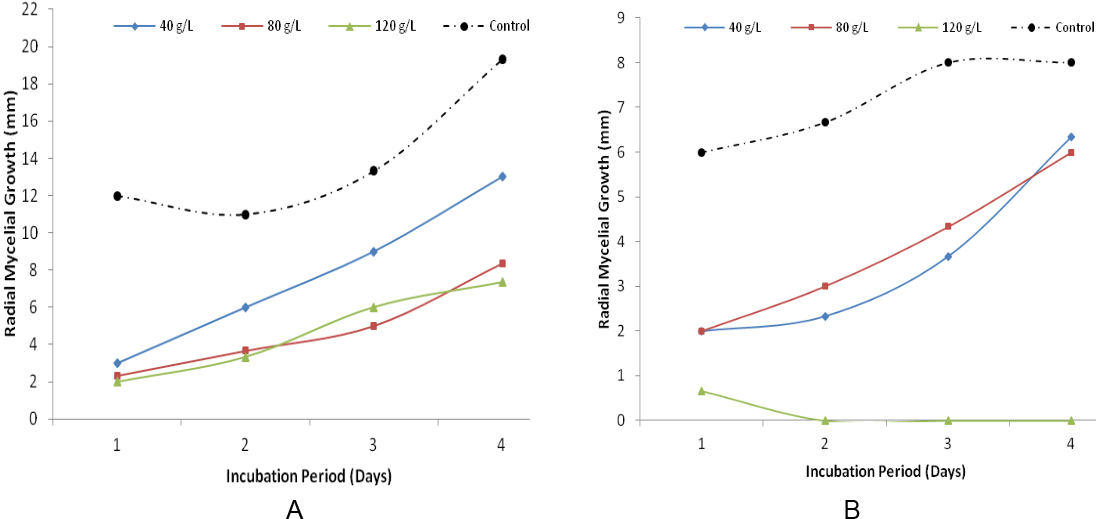
Figure 3: Radial Mycelial Growth of R. solani on Potato Dextrose Agar amended with Z. officinale (A) and A. indica (B) after four days of incubation.
Discussion
The isolated fungi pathogens were F. oxysporum, F. moniliforme, R. solani, A. niger, A. flavus. The most frequently occurring fungi were F. oxysporum followed by A. niger while the least was F. moniliforme in all the locations. The result of the pathogenicity test from this study revealed that all tomato fruits showed symptoms of rot on the inoculated healthy tomato fruits while the uninoculated (control) fruits showed no symptoms of rot. However, the rate of rot varied from one tomato fruit to another depending on the pathogen inoculated. These fungi organisms have been previously found to be associated with rotted tomato fruits. Similar results were obtained by Tijjani., et al (2013) who used some plant extracts to inhibit the growth of Rhizopus stolonifer onmechanically injured tomato fruits. Results obtained showed that both A. indica and Z. officinale extracts inhibited the mycelial growth of F. oxysporum and R. solani. Neem leaf extract was found to be the most effective on R. solani. This is like the findings of Mugao, (2015) who reported that neem leaf extracts were more effective in the inhibition of Fusarium spp growth in tomato fruits. This also agreed with the report of Hycenth, (2008) who evaluated the effect of different plant extracts on R. stolonifer and found out that all the extracts effectively suppressed mycelial growth of R. stolonifer. According to Meena and Mariappan, (1993), neem leaf extracts inhibited the growth and spore germination of seed microflora including A. tenuis, A. flavus, C. lunata, F. moniliformeand R. stolonifer. Sharma and Jandaik (1994) reported that different extracts from neem leaves have inhibitory effect on R. solani. Cassava anthracnose caused by C. gloeosponiodes was controlled using neem extracts (Fokunang., et al. 2000). Hoque., et al. (2007) reported that neem contains a compound known as mahmoodin which is active against gram-positive and gram-negative bacteria. According to Suleiman (2010), neem extracts controlled the growth of fungi Alternaria solani causal organism of yam rot.
Stangarlin., et al. (2011) revealed that aqueous extract of ginger at different concentrations had effect on the mycelial growth and sclerotia production of Sclerotina sclerotium in vitro. The anti-microbial property of ginger in reducing the mycelial growth of fungal pathogens agreed with the results of this study. The inhibitive effect was proportional to the concentration of the crude extract used: the higher the concentration the higher the inhibitory effect. According to Ijato, (2011) extracts of Z. officinaleand Ocimum gratissimum were mycotoxic to F. oxysporum, A. flavus and A. nigerthat caused post-harvest rot of yam tubers and that the effectiveness of the extracts increased with increase in concentration as was observed in this study. Gwa and Akombo, (2016) used extracts of P. nigrum, Z. officinale, A. indica, C. papaya and N. tabacum and inhibited the mecylial growth of A. flavus in vitro. The authors found out that the growth of the pathogen decreased as the concentrations of the extracts were increased. Similar results were obtained by Gwa and Nwankiti, (2017) who reduced the growth of Colletotrichumsp isolated from yam tubers with extracts of P. nigrum, Z. offcinale, A. indica, C. papaya and N. tabacum in vitro.
Conclusion
F. oxysporum, F. moniliforme, R. solani, A. niger, and A. flavus are responsible for rot of tomato fruits in Darawa, Dutsin-ma, and Makera. Extracts of A. indica and Z. officinale origin showed inhibitory activities on both R. solani and F. oxysporum. However, the concentration of 120 g/L was found to be effective compared to 80 g/L and 40 g/L for both R. solani and F. oxysporum. It is therefore concluded that extracts of plant origin especially A. indica and Z. officinale should be considered in managing fungi pathogens of tomato fruits by farmers.
Conflict of Interest Disclosure
The authors declare that there is no conflict of interest regarding the publication of this paper.
The authors declare that there is no conflict of interest regarding the publication of this paper.
Funding Acknowledgement
This research received no specific grant from any funding agency in the public, commercial or not-for- profit sectors
This research received no specific grant from any funding agency in the public, commercial or not-for- profit sectors
References
- Agrios GN. “Plant Pathology 5th Edition. Academic press. London”. (2005): 410-430.
- Ahmed KM and Ravinder Reddy Ch. “A pictorial Guide to the Identification of Seed Borne Fungi of Sorghum, Pear Millet, Finger Millet, Chickpea, Pigeonpea and Groundnut. Information Bulletin no. 34: (In En summaries in Fr, Es and Ar.) Patancheru, A. P. 502324, India”. International Crops Research Institute for the Semi Arid Tropics (1993): 200.
- Amadioha AC and Obi V. “Control of Anthracnose Disease of Cowpea Cymbopogon citrates and Ocimum gratissimum”. Acta of phytopathology and Entomology Hungerica 34.92 (1999): 85-89.
- Bright AO. “Incidence and severity of major fungal diseases of tomato in three districts within forest and forest savannah agro-ecological zones of Ghana”. Msc thesis Kwame Nkrumah University of science and technology (2012):
- Burgess LW., et al. “Diagnostic Manual for Plant Diseases in Vietnam”. ACIAR Monograph 129 (2008): 210.
- Cochran GW and Cox GM. “Experimental Designs. 2nd Edn”. John willey and Sons Inc (1992): 611.
- Dimphna NE. “Isolation and Identification of Fungi Associated with PostharvestDecay of Lycopersicum esculentum M. sold in Abakaliki, Nigeria”. Journal of Agriculture and Veterinary Science 9.7 (2016): 87-89.
- Fokunang CN., et al. “Efficacy of anti-microbial plant crude exracts on the growth of Colletotrichum gloesporoides f.sp manihotis Pakistan”. Journal of Biological Sciences 3.6 (2000): 928-932.
- Goufo P., et al. “High Efficacy of Extracts of Cameroon Plants against Tomato Late Blight Disease”. Agronomy for Sustainable Development 28.4 (2008): 567-573.
- Gwa VI and Akombo RA. “Studies on the antimicrobial potency of five crude plant extracts and chemical fungicide in the in-vitro of Aspergillus flavus causal agent of white yam (Dioscorea rotundata) tuber rot”. Journal of plant science and Agriculture research 1.1 (2016): 1-8.
- Gwa VI and Nwankiti AO. “Efficacy of Some Plant extracts in vitro control of collectotrichum Species, Causal agent of yam (Doscorea rotundata Poir) tuber rot”. Asia Journal of Plant Science and Research 7.2 (2017): 8-16.
- Hoque MD., et al. “Antibacterial activity of guava (Psidium guajava L.) and neem (Azadirachta indica, A. juss.) extracts against foodborne pathogens and spoilage bacteria”. Foodborne Pathogens and Diseases 4.4 (2007): 481-488.
- Hycenth N. “Effects of different plant extracts in the control of yam rot induced by Rhizopus stolonifer on stored yam (Dioscorea sp). In Yola, Adamawa state, Nigeria”. Agricultural Journal 3.5 (2008): 382-387.
- Ijato JY. “Inhibitory effect of two indigenous plant extracts of Zingiber officinale and Ocimum gratissimum on post-harvest yam (Dioscorea rotundata) rot in vitro”. Journal of American Science 7.1 (2011): 43-47.
- Kutama AS., et al. “Fungal Pathogens Associated with Tomato Wicker Storage Baskets”. Science World Journal 2 (2007): 345-378.
- Meena SS and Mariappan V. “Effect of plant products on seed borne mycoflora of sorghum”. Madras Journal of Agriculture 80.7 (1993): 383-387.
- Mishra J., et al. “Biopesticides; where we stand”. Springer (2015): 37-75.
- Mugao GL. “Tomato post-harvest spoilage, causes and uses of selected botanical extracts in their management in Mwea,kirinyaga county”. Msc thesis of the school of pure and applied sciences Kenyatta University (2015):
- Okigbo RN and Ikediugwu FEO. “Studies on Biological Control of Post-Harvest Rot in Yams (Dioscorea rotundata) using Trichoderrma viride”. Journal of Phytopathology 148 (2000): 351-355.
- Okigbo RN and Nmeka IA. “Control of Yam Tuber with Leaf Extracts of Xylopia aethiopica and Zingiber officinale”. African Journal Biotechnology 4.8 (2005): 804-807.
- Sharma VN and Jandaik CL. “Effect of some plant materials in controlling different moulds in Agaricus bisporus (Lang)”. Indian Journal of Mycology and plant pathology 24.30 (1994): 183-185.
- Stangarlin JA., et al. “Control of plant diseases using extracts from medicinal plants and fungi”. Communicating Current Research and Technological Advances 25 (2011): 1033-1042.
- Suleiman MN. “Fungitoxic Activity of Neem and Pawpaw Leaves Extracts on Alternaria Solani, Causal Organism of Yam Rots”. Advances in Environmental Biology 4.2 (2010): 159-161.
- Tijjani A., et al. “Efficacy of Some Botanicals for the Control of Wet Rot Disease on Mechanically Injured Sweet Potato Caused by Rhizopus Stolonifer in Bauchi State”. International Journal of Science Research and Publication 3.6 (2013): 1-10.
- Tijjani A., et al. “In vitro and In vivo Efficacy of Some Plant Extracts for the Control of Tomato Fruit Rot Caused by Aspergillus Aflavus”. International Journal of Scientific and Research Publications 4.4 (2014): 1-5.
- Wachira MJ., et al. “Comparison of Profitability of Small Scale Green House and Open-Field Tomato Production Systems in Nakuru North District, Kenya”. Asian Journal of Agricultural Sciences 6.2 (2014): 54-61.
- Wamache A. “Vegetable Seeds Handbook. Regina Seeds Seminis”. Printed by Bizone ltd. Nairobi Kenya (2005): 23-25.
Citation:
Gwa V I and Sani S. “Fungicidal Effect of Azadiracta Indica and Zingiber Officinale Extracts in the Control of Fusarium
Oxysporum and Rhizoctonia Solani on Tomato (Solanum Lycopersicum) Fruits”. Innovative Techniques in Agriculture 2.4 (2018): 439-448.
Copyright: © 2018 Gwa V I and Sani S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























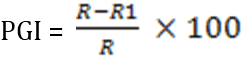
 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.