Research Article
Volume 1 Issue 1 - 2017
Proximate Composition and Functional Properties of Rice (Oryza Sativa L.) Germinated-Cowpea (Vigna Unguiculata L.) Flour Blends
1Department of Home Sciences, Federal University, PMB1005 Gashua Yobe state
2Department of Food Science and Technology, Federal university of Agriculture, PMB 2240, Abeokuta Ogun state
2Department of Food Science and Technology, Federal university of Agriculture, PMB 2240, Abeokuta Ogun state
*Corresponding Author: Ajayi FF, Department of Home Sciences, Federal University, PMB1005 Gashua Yobe state.
Received: June 02, 2017; Published: June 15, 2017
Abstract
The study was conducted to investigate the proximate composition and functional properties of various blends of rice-cowpea flour that will provide convenient material base for food product development and processing. Rice Flour (RF) was blended with Germinated Cowpea Flour (GCF) at ratios of 100:0, 90:10, 80:20, 70:30, 50:50 and 0:100 to obtain six composite blends. Moisture Content (MC), ash, crude fibre, protein, carbohydrate (CHO), fat, methionine and lysine were determined for each blend. Functional properties namely; Water Absorption Capacity (WAC), Oil Absorption Capacity (OAC), bulk density, Least Gelation Capacity (LGC), dispersibility was determined. Results obtained showed that there were no significant (p < 0.05) differences in the MC, ash content, crude fibre and fat content. The protein content increased steadily with increase in cowpea flour addition from 7.58% in 100% RF to 19.57% in 50:50 (RF: GCF) while the carbohydrate content decreased. Lysine, the limiting amino-acid in rice increased significantly (p < 0.05) in the blends from 3.52% in 100% RF to 6.45% in 50:50 (RF: GCF) while methionine, the limiting amino-acid in cowpea, also increased from 1.21% in 100% CF to 2.38% in 90:10 (RF: GCF). The OAC increased significantly from 120% in 100% RF to 220.06% in 50:50 (RF: GCF). The blend of 50:50 (RF: GCF) gave the best improvement in terms of limiting amino-acid lysine and protein content. This study showed that significant stable probiotics, nutrient pockets and security as well as functional properties of rice were attained through the use of germinated cowpea flour in blend formulation.
Keywords: Chemical Composition; Functional property; Germinate cowpea-Rice Blend
Introduction
Rice (Oryza sativa L.) is a starchy food and a major staple food especially in Nigeria. It has many unique attributes such as ease of digestion; bland taste and hypoallergenic properties. However, rice has relatively low amounts of proteins and most of them are very hydrophobic and therefore resist swelling in water at neutral pH (Lumduwong and Seib, 2000). The low protein contents and absence of gliadin moiety in rice however make it an ideal food material to feed patients suffering from celiac sprue (gluten-sensitivity disease), other chronic diarrhea diseases, and conditions needing low protein diets (Hartsook, 1984). It is cholesterol, fat, sodium and gluten free (Hui, 1991). Rice varieties which include Rough, Brown, White, Parboiled and Rice Polish in the United states are classified for trading purposes, by grain size and shape into three types; long, medium and short grain, which have distinct cooking, eating and processing qualities (Hui, 1991).
Rice flour has a growing market with many diverse food applications (Bean and Nishita, 1985). Most rice flour is made from white rice, and major component is starch which finds uses in infant foods, extruded breakfast cereals and snack products. Rice flour (also rice powder) is a form of flour made from finely milled rice. It is distinct from rice starch, which is usually produced by steeping rice in lye. Rice flour may be made from either white rice or brown rice.
The cowpea (Vigna Unguiculata L.) is the most popular grain legume in tropical Africa. When grown for its dry seeds. It is also known as black-eyed bean, the black-eyed pea, the southern bean, the china pea, the kaffir pea and the marvel pea. Other varieties grown for their long immature pods, are the yard-long bean, the asparagus bean, the bodi bean and the snake bean. Apart from its high protein value, it is a good source of phosphorus, magnesium, potassium, calcium and iron. It contains a fair amount of the B vitamins and some trace elements (Kordylas, 1990). The development of flatulence upon consumption of cowpea is one of the constraints limiting the consumption of this legume seeds by human.
Nnanna and Philips (1990) reported that germination of seed for 24h at 30°C reduced the oligosaccharide responsible for flatulence to almost nil and permitted minimal protease and amylose activities as was desired. Altschull (1974) reported that cereals have low protein content and are in general lysine deficient but are adequate in sulphur containing amino acids. Legume protein rich in lysine but deficient in sulphur containing amino acids should complements the protein in cereal grains since the chemical and nutritional characteristics of legumes make them natural complements to cereal-based diets so as to achieve optimal nutritional level.
Cowpea flour is a convenient food ingredient with the potential to promote industrial utilization of cowpea. It could replace wet paste as the starting ingredient for some traditional West African cowpea foods (Henshaw, 2000). It could also become and ingredient in other food applications such as baked foods (bread, cake) extruded products (noodles, spaghetti) comminuted met products (sausage, ham) and weaning foods (Falcone and Phillips, 1988). According to Mc Watters (1986), the successful performance of legume flours as food ingredients depends on the functional characteristics and sensory quality they impart to end products and functionality of food ingredients is the sum total effect of the properties which affect utilizations such as foaming and emulsification properties (Poul-El, 1981).
In Nigeria, whole rice and cowpea grains mixture is prepared by boiling and this requires long time to cook and produce a softened product that can be eaten. This constraint tends to limit the regularity of combining rice and cowpea in meals. The availability of a ready-to-use flour blends from rice-cowpea would improve the nutritional profile and provide convenient material base for food product development and processing. Therefore, the objective of this study is to evaluate the chemical composition and functional properties of rice-cowpea flour blends.
Materials and Methods
Rice and cowpea grains were purchased from a popular market in Abeokuta, Ogun state, Nigeria.
Methods
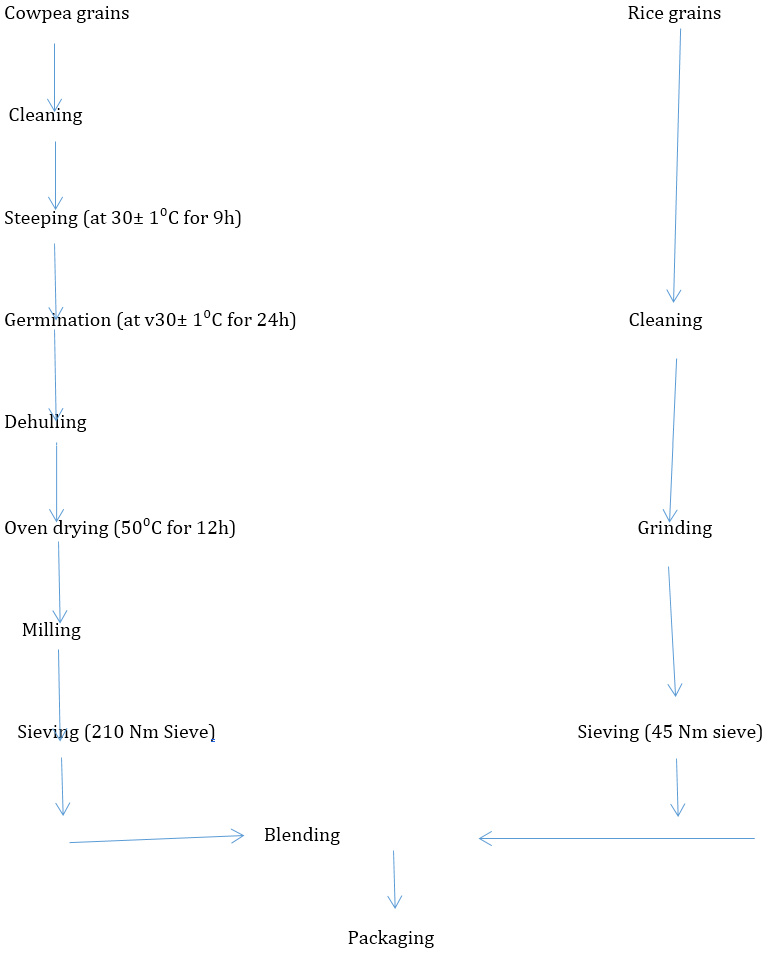
The cowpea was processed according to the method of Nnanna and Phillips (1990). The cowpea grains were soaked in water at 30 ± 1°C for 9h after cleaning. The hydrated grain was germinated in the dark cupboard for 24 h at 30 ± 1°C. the germinated seeds was collected and dried at a low temperature of 50°C in air oven for 12h, milled and passed through 210 Nm sieve to obtain germinated cowpea flour.
The cowpea was processed according to the method of Nnanna and Phillips (1990). The cowpea grains were soaked in water at 30 ± 1°C for 9h after cleaning. The hydrated grain was germinated in the dark cupboard for 24 h at 30 ± 1°C. the germinated seeds was collected and dried at a low temperature of 50°C in air oven for 12h, milled and passed through 210 Nm sieve to obtain germinated cowpea flour.
Processing method for rice flour
The rice grain was processed according to the method of Abulude (2004). The rice grains were cleaned by handpicking to remove impurities, ground to pass through a 45 Nm sieve, packaged and stored for further analyses.
The rice grain was processed according to the method of Abulude (2004). The rice grains were cleaned by handpicking to remove impurities, ground to pass through a 45 Nm sieve, packaged and stored for further analyses.
Flour Blending
The blends were prepared as put in Table 1
The blends were prepared as put in Table 1
| Samples | Rice Flour (%) | Germinated Cowpea Flour (%) |
| A | 100 | - |
| B | 90 | 10 |
| C | 80 | 20 |
| D | 70 | 30 |
| E | 50 | 50 |
| F | - | 100 |
Table 1: Ratios of Rice-Germinated Cowpea flour blends.
Sample Analysis
Moisture was determined by hot-air oven drying at 105°C to constant weight (AOAC, 2000). Ash, protein (microKjeldahl, N × 6.25), crude fiber and fat (Solvent extraction) were determined by the AOAC (2010) methods. Calorie was calculated using Atwater factors of 4 × % Protein, 4 × % carbohydrate and 9 × % fat and then taking the sum. Total energy value was determined by multiplying the percentage value obtained from protein, carbohydrate and fat by the value of 4, 4, 9, respectively. The calorie content was calculated as follows: Cal/100g = (Protein × 4) + (CHO × 4) + (Fat × 9).
Moisture was determined by hot-air oven drying at 105°C to constant weight (AOAC, 2000). Ash, protein (microKjeldahl, N × 6.25), crude fiber and fat (Solvent extraction) were determined by the AOAC (2010) methods. Calorie was calculated using Atwater factors of 4 × % Protein, 4 × % carbohydrate and 9 × % fat and then taking the sum. Total energy value was determined by multiplying the percentage value obtained from protein, carbohydrate and fat by the value of 4, 4, 9, respectively. The calorie content was calculated as follows: Cal/100g = (Protein × 4) + (CHO × 4) + (Fat × 9).
Determination of pH and Titratable Acidity
The pH and Titratable acidity were determined using method described by Egan., et al. (1981). Ten gram of the sample were weighed and homogenized in 50 ml of distilled water for 8 min using a shaker. The pH was measured using a jenway pH meter. The homogenized samples were filtered; 25 ml of the filtrate was titrated with 0.1M NaOH using phenolphthalein as an indicator and the percentage total acidity expressed as lactic acid equivalent.
The pH and Titratable acidity were determined using method described by Egan., et al. (1981). Ten gram of the sample were weighed and homogenized in 50 ml of distilled water for 8 min using a shaker. The pH was measured using a jenway pH meter. The homogenized samples were filtered; 25 ml of the filtrate was titrated with 0.1M NaOH using phenolphthalein as an indicator and the percentage total acidity expressed as lactic acid equivalent.
Determination of Methionine
Methionine was determined using method described by Lunder (1973). About 0.2g of samples were weighed into a 30 ml test tube. Trisbuffer reagent (10 ml, pH 7.2) was added with 0.1 mg papain and incubated at 55°C for 18h overnight. It was removed from incubator and 4 drops of orthophosphoric acid (88 %) to stop enzymatic hydrolysis. The mixture was transferred into a 25 ml volumetric flask and made up to volume with distilled water. The solution obtained was shaken thoroughly and filtered through a Whatman No 4 Filter paper into a test tube. About 5 ml alcquot was pipetted out of this into another test tube to which 1 ml of 5N NaOH, 1 ml 1% aqueous glycine and 1ml 1% of sodium nitroprusside were added. The tubes were kept in the water bath at 40°C for 10 min after thorough shaking. The contents were allowed to cool and 6ml of 88% orthophophoric acid added with thorough shaking to obtain a red colour. Methionine standard of range 0.5 to 2.5 mg/ml were prepared and treated as above. Absorbance of samples and standard were read on the spectronic 20 spectrophotometer at wavelength 520 Nm.
Methionine was determined using method described by Lunder (1973). About 0.2g of samples were weighed into a 30 ml test tube. Trisbuffer reagent (10 ml, pH 7.2) was added with 0.1 mg papain and incubated at 55°C for 18h overnight. It was removed from incubator and 4 drops of orthophosphoric acid (88 %) to stop enzymatic hydrolysis. The mixture was transferred into a 25 ml volumetric flask and made up to volume with distilled water. The solution obtained was shaken thoroughly and filtered through a Whatman No 4 Filter paper into a test tube. About 5 ml alcquot was pipetted out of this into another test tube to which 1 ml of 5N NaOH, 1 ml 1% aqueous glycine and 1ml 1% of sodium nitroprusside were added. The tubes were kept in the water bath at 40°C for 10 min after thorough shaking. The contents were allowed to cool and 6ml of 88% orthophophoric acid added with thorough shaking to obtain a red colour. Methionine standard of range 0.5 to 2.5 mg/ml were prepared and treated as above. Absorbance of samples and standard were read on the spectronic 20 spectrophotometer at wavelength 520 Nm.
% Methionine or Methionine in g/100g protein is calculated using the formula:
Determination of lysine
Lysine was determined using the method described by Jambunathan., et al. (1983). About 1g of samples were weighed into 50 ml centrifuge tube. Forty miles (40 ml) of acid orange dye solution was added and shaked for 1h. The mixture was filtered through a Whatman No 4 filter paper into a 50 ml volumetric flask and made up to mark with distilled water and incubated at 60°C for 18h overnight. Standard lysine was also prepared from range of 0.35 to 0.85 mg/ml and treated with acid orange dye to develop colour. The absorbance for standard as well as sample was read on the spectronic 20 Spectrophotometer at wavelength 415 Nm.
Lysine was determined using the method described by Jambunathan., et al. (1983). About 1g of samples were weighed into 50 ml centrifuge tube. Forty miles (40 ml) of acid orange dye solution was added and shaked for 1h. The mixture was filtered through a Whatman No 4 filter paper into a 50 ml volumetric flask and made up to mark with distilled water and incubated at 60°C for 18h overnight. Standard lysine was also prepared from range of 0.35 to 0.85 mg/ml and treated with acid orange dye to develop colour. The absorbance for standard as well as sample was read on the spectronic 20 Spectrophotometer at wavelength 415 Nm.
% Lysine or lysine in g/100g protein is calculated using the formula:
Functional properties
Water and oil absorption capacity were determined as described by Hayta., et al. (2002). Bulk density was determined using the method of Akapapunam and Markakis (1981).Least gelation capacity was determined as described by Abulude (2001). Dispersibility was determined according to the procedure of Chukwu., et al. (1995). All determination was done in triplicates.
Water and oil absorption capacity were determined as described by Hayta., et al. (2002). Bulk density was determined using the method of Akapapunam and Markakis (1981).Least gelation capacity was determined as described by Abulude (2001). Dispersibility was determined according to the procedure of Chukwu., et al. (1995). All determination was done in triplicates.
Statistical Analysis
All data were subjected to analysis of variance (ANOVA) to determine any significant difference at 5% level (Steel and Torrie, 1980) and was reported as means of three replicates. Means were separated by Duncan’s multiple range tests to establish if there were significant differences between the samples (SAS, 1999).
All data were subjected to analysis of variance (ANOVA) to determine any significant difference at 5% level (Steel and Torrie, 1980) and was reported as means of three replicates. Means were separated by Duncan’s multiple range tests to establish if there were significant differences between the samples (SAS, 1999).
Results and Discussion
Table 2 shows the percentage moisture of Rice-Germinated Cowpea flour blends samples. There was significant difference (p < 0.05) among the samples. Moisture content ranged between 8.30%-12.70%. The observed values for the blends are within the value (12%-15%) designated as sample grade under U.S. standard whereas 12% is recommended for long-term storage (Spadaro., et al. 1980). This implies that the blends may have long shelf life. From this study, high ash content was recorded in [Table 2] by 100% Germinated-cowpea flour (2.83 %), suggesting that germination process increases the mineral content. The values reported are consistent with the values reported by Agu and Aluyah (2004) for maize, soybean and fluted pumpkin seed flour. The fibre content of the blends increased with increasing level of germinated-cowpea flour substitution. The fibre content of 100% germinated-cowpea flour (2.16%) was higher than 100% rice flour with the least crude fiber content (1.56%). The amount of crude fibre in the flour samples may influence the digestibility of menu or diets prepared from the products, and may also help to maintain the normal internal distention of the intestinal tract and thus, aid peristaltic movements (Edem., et al. 1984).
The protein content of the blends increased as germinated-cowpea flour substitution increased. The highest value was observed in sample F (Germinated-cowpea flour) and lowest in sample A (100% Rice flour). Since legumes, including cowpeas are low in sulphur amino acids but high in lysine (Okoh., et al. 1981) the proteins in cowpeas flour complement that of rice and thus improve nutritional quality of the blends. In a similar study, Achi (1999) observed a steady increase in the protein content of the yam flour supplemented by soy flour in the blends. In this study, such significant increases were observed both at 0% and 50% rise in cowpea flour content.
The highest value was observed in sample A (100% Rice flour) with and lowest in sample E (50:50 blends). The value (2.00%) obtained by sample A is in accordance to that reported by King and Puwastein (1987), but low when compared with the value reported for selected seafood (2.4-4.7 %) by Ogunlade., et al. (2005) and 2.29 - 5.1% for maize, soybean and fluted pumpkin seed flour (Agu and Aluyah, 2004).
The Carbohydrate level in 100% Rice flour (75.18%) was significantly (p < 0.05) higher than 100% Germinated-Cowpea flour (60.70%). This also might be an indication of increase in the level of carbohydrate in Rice flour. The value found in germinated cowpea flour was similar to the findings of Akpapum and Achienewhu (1985) for germinated cowpeas.
| Samples | Moisture Content (%) | Ash Content (%) | Crude Fibre (%) | Protein (%) | Fat (%) | Carbohydrate (%) |
| A | 12.70d | 2.13c | 1.56a | 7.58a | 2.00d | 75.18d |
| B | 12.40d | 1.60a | 1.60b | 12.53b | 1.15b | 70.10c |
| C | 12.20d | 1.70b | 1.64b | 16.70c | 1.07a | 66.99b |
| D | 11.40c | 1.73b | 1.76c | 18.50d | 1.06a | 66.34b |
| E | 9.37b | 2.33c | 1.82d | 19.57e | 1.03a | 66.04b |
| F | 7.84a | 2.83d | 2.16e | 22.30f | 1.42c | 60.70a |
A = 100% Rice Flour, B = 90% Rice Flour; 10% Germinated-Cowpea Flour, C = 80% Rice Flour; 20% Germinated-
Cowpea Flour, D = 70 % Rice Flour; 30% Germinated-Cowpea Flour, E = 50 % Rice Flour; 50% Germinated-Cowpea
Flour, F = 100 % Germinated-Cowpea Flour.
Table 2: Proximate composition of Rice-Germinated Cowpea Flour Blends.
Table 2: Proximate composition of Rice-Germinated Cowpea Flour Blends.
| Samples | Energy (kcal/100g) | pH | TTA (%) | Methionine(g/100g protein) | Lysine (g/100g Protein) |
| A | 337.28b | 6.17b | 0.03b | 3.29d | 3.52a |
| B | 342.55c | 6.01b | 0.02a | 2.38c | 6.04b |
| C | 343.83c | 6.21c | 0.03b | 2.33c | 6.25c |
| D | 346.13c | 6.27c | 0.04b | 2.13b | 6.33c |
| E | 355.60d | 6.60d | 0.06c | 2.09b | 6.45cd |
| F | 329.25a | 5.45a | 0.11d | 1.27a | 6.58d |
A = 100% Rice Flour, B = 90% Rice Flour; 10% Germinated-Cowpea Flour, C = 80% Rice Flour; 20% Germinated-Cowpea Flour, D = 70% Rice Flour; 30% Germinated-Cowpea Flour, E = 50% Rice Flour; 50% Germinated-Cowpea Flour, F = 100% Germinated-Cowpea Flour.
Table 3: Chemical composition of Rice Germinated-Cowpea Flour Blends.
Table 3: Chemical composition of Rice Germinated-Cowpea Flour Blends.
From table 3, it can be deduced that the high energy observed in germinated cowpea flour samples may be due to the breakdown of starch, which is broken down into simple sugar, which are used to generate energy for germinating seeds. The pH of the flour blends ranged between 5.45-6.60 in table 3 agrees with the findings of pearson (1976) who reported pH range of flours of 6.0-6.8. The lysine contents of the blends increase with increasing level of cowpea flour substitution while reversal effect was observed for methionine value. Both the lysine and methionine value of the samples was in the range (4.32 and 2.30g/100g protein) reported by Ene-Obong and Carbovale (1992) for cowpea and 3.9 and 3.0g/100g protein by Oyenuga (1968) for rice respectively, though germinated cowpea flour samples were observed to be high.
| Samples | WAC (%) | OAC (%) | Bulk Density (g/ml) | LGC (%) | Dispersibilty (%) |
| A | 225.03d | 120.80b | 0.79a | 22.00d | 64.67a |
| B | 189.43a | 103.67a | 0.81ab | 16.00b | 85.70b |
| C | 191.70ab | 158.20c | 0.82ab | 18.00c | 86.63b |
| D | 206.70bc | 160.00c | 0.83ab | 18.00c | 86.80b |
| E | 211.40c | 220.60d | 0.83ab | 18.00c | 87.93bc |
| F | 199.60b | 210.20d | 0.85b | 15.00a | 89.27c |
A = 100% Rice Flour, B = 90% Rice Flour; 10% Germinated-Cowpea Flour, C = 80% Rice Flour; 20% Germinated-Cowpea Flour, D = 70% Rice Flour; 30% Germinated-Cowpea Flour, E = 50 % Rice Flour; 50% Germinated-Cowpea Flour, F = 100% Germinated-Cowpea Flour.
Table 4: Functional Properties of Rice-Cowpea Flour Blends.
Table 4: Functional Properties of Rice-Cowpea Flour Blends.
Table 4 shows the water absorption capacity (WAC) of the flour blends. The WAC was observed highest in sample A (225.03%) and lowest in sample B (189.43%). Water absorption capacity or characteristics represent the ability of a product to associate with water under conditions where water is limited (Singh, 2001). The highest WAC of potato flour could be attributed to the presence of higher amount of carbohydrates (starch). Aletor., et al. (2002) reported that the WAC ranging from 149.1% to 491.5% is considered critical in viscous foods such as soups and gravies. Thus, the flours might find use as functional ingredients in soups, sausages, dough and baked product.
Oil absorption capacity (OAC) has been attributed to the physical entrapment of oil. This is important since fat acts as flavour retainer and increases the mouth feel of foods (Eke and Akobundu, 1993). The highest value was observed in sample E (50:50 blends) and lowest in sample B (90:10 blends). Germination increased the OAC of the cowpea flour. The values reported for 100% Rice flour (120.80%) were close to the report of rice flour (89.75%) by Chandra and Samsher (2013) and quinoa flour (84.6%) by Ogungbenle (2003).
The highest bulk density was observed for 100 % germinated-cowpea flour (0.85 g/ml) and lowest for 100% rice flour (0.79 g/ml). The high bulk density of flour suggests their suitability for use in food preparations. On contrast, low bulk density would be an advantage in the formulation of complementary foods (Akpata and Akubor, 1999).
Least gelation capacity is a measure of the minimum amount of flour or blends of flours that is needed to form a gel when mixed with water and heated. The higher the least gelation capacity, the higher the amount of flour needed to form a gel (Adebowale, 2002). The values obtained were similar and lower than the fermented locust bean (30%) reported by Ikpeme., et al. (2003) but higher than the value (10%) observed by Sathe., et al. (1982) for great northern bean and (14%) for lupin seed flour.
The higher the dispersibility, the better the flour reconstitutes in water (Kulkarni., et al. 1991). An increased in values was observed for the blends but higher values was observed in germinated cowpea flour. However, the values are relatively high and the flours will reconstitute easily to give fine consistent pastes (Kulkarni., et al. 1991).
Conclusion
This work has shown that significant improvement in the chemical composition and functional properties can be attained through the use of germinated cowpea in a blend formulation. Substitution up to 50% (50% Rice Flour and 50% Germinated-Cowpea Flour) is recommended, since the blend gave the best improvement in terms of limiting amino acid lysine and protein content.
References
- Abulude FO. “Composition and Properties of Cola acuminate flour in Nigeria”. Global Journal of Pure and Applied Science10.1 (2004): 11-16.
- Abulude FO. “Effect of processing on Nutritional composition Phytate and functional properties of Rice (Oryza sativa) flour”. Nigerian Food Journal 22.1 (2004): 97-104.
- Achi OK and Ibrahim R. “Chemical composition and selected functional properties of Daniella olveri kernel flour”. Journal of Management and Technology 2 (2001): 78-82.
- Adebowale AA., et al. “Effect of texture modifiers on the physicochemical and sensory properties of dried Fufu”. Food Science and Technology International 11.5 (2005): 28-49.
- Agu HO and Aluyah E. “Production and chemical analysis of weaning food from maize, soybean and fluted pumpkin seed flour”. Nigeria Food Journal 22 (2004): 171-177.
- Akapapunam MA and Markakis P. “Physicochemical and Nutritional aspects of cowpea flour”. Journal of Food Science 46.3 (1981): 972-973.
- Akpapunam MA and Achinewhu SC. “Effect of cooking, germination and fermentation on chemical composition of Nigeria cowpea (Vigna unguculata). Quality Plant”. Plant Foods Human Nutrition 35.4 (1985): 353-358.
- Aletor O., et al. “Chemical composition of common leafy vegetables and functional properties of their leaf protein concentrations”. Food Chemistry 78 (2002): 63-68.
- Altschull AM. “New Protein Foods”. Academic Press 1 (1974): 20-23.
- Alok C. Samal., et al. “Heavy Metal Accumulation Potential of Some Wetland Plants Growing Naturally in the City of Kolkata, India”. American Journal of Plant Sciences 7.15 (2016): 148-150.
- Bean MM and Nishita KD. “Rice flours for baking. In Rice Chemistry and Technoloogy”. St. Paul MN: AACC (1985): 539 -556.
- Chandra S and Samsher. “Assessment of functional properties of different flours”. African Journal of Agriculture Research 8.38 (2013): 4849-4852.
- Chukwu U., et al. “Development, production, properties and acceptability of snacks and weaning food made from extruded cooking banana (ABB)”. Proceedings, conference on post harvests Technology and Commodity marketing in West Africa, Accra, Ghana (1995): 120-131.
- Egan JK., et al. “Pearson Chemical Analysis of Foods”. 8th edition, Edinburgh: Churchill Livingstone(1981): 511.
- Eke OS and Akobundu EN. “Functional properties of African yam bean (Spherus tenocarpa) seed flour as affected by processing”. Food Chemistry 48 (1993): 337-340.
- Ene-Obong HN. “A comparison of the proximate, mineral and amino acid composition of some known and lesser-known legumes in Nigeria”. Food Chemistry 43.3 (1992): 169-175.
- Falcone RG and Phillips RD. “Effect of feed composition, feed moisture. And barrel temperature on the physical and Rheological properties of snack-like products prepared from cowpea and sorghum flours by extrusion”. Journal of food science 53.5 (1988): 1464-1469.
- Hartsook ET. “Celiac Spress: Sensitivity to gliandin Cereal Foods World”. Advances in Food and Nutrition Research 29.2 (1984): 157-158.
- Hayta M., et al. “Effect of drying method on functional properties of Tarhana. Wheat flour-yoghurt mixture”. Journal of Food Science 67.2 (2002): 740-744.
- Henshaw FO. “Functionality of flour in relation to physical and chemical properties of seeds of selected cowpea (Vigna unguiculata) varieties”. Encyclopedia of Food Science and Technology 4 (200): 227.
- Ikpeme CA., et al. “Functional properties of soybean and locustbean dawadawa; a bacterial fermented product”. Global Journal of Pure and applied Sciences 9.1 (2002): 47-52.
- Jambunathan R., et al. “Rapid method for estimating lysine and protein in sorghum”. Journal of Cereal Chemistry 60 (1982): 192-194
- King RD and Puwastein P. “Effect of germination on the proximate composition and nutritional quality of winged bean (Psophocarpus tetragonolobus) seeds”. Journal of Food Science 52.1 (1987): 106-108.
- Kordylas JM. “Description of beans or pulses. Processing and Preservation of tropical and subtropical foods”.
- Kulkarni KD., et al. “Sorghum malt-based weaning formulations preparation, Functional properties and Nutritive value”. Food and nutrition Bulletin 13.4 (1987): 322-327.
- Lumduwong N and Seib PA. “Rice starch isolation by alkaline protease digestion of Wet-milled rice flour”. Journal of Cereal Science 31.1 (2000): 63-74.
- Lunder TJ. “An improved method for the colometric determination of methionine in acid hydrolysates of biological products industry”. Aliment 12 (1973): 94-98.
- McWatters KH. “Use of peanut and cowpea flours in selected fried and baked foods. In plant proteins: applications, biological effects and functionality”. ACS Symposium series2 (1986): 8-18.
- Nnanna IA and Phillips RD. “Protein digestibility and flatulence potential of Germinated Cowpea (Vigna unguiculata)”. Journal of Food Science 55.1 (1990): 151-153.
- Ogungbele HN. “Nutritional evaluation and functional properties of quinoa flour”. International Journal of Food Science 54.2 (2003): 153-158.
- Ogunlade I., et al. “Chemical composition, amino acids and functional properties of selected seafoods”. Journal of Food Agriculture and Environment3.2 (2005): 130-133.
- Okoh PN., et al. “Proximate analysis, amino acid composition and tannin content of improved Nigeria sorghum varieties and their potential in poultry feeds”. Animal Feeds Science and Technology 7.4 (982): 359-364.
- Oyenuga VA. “Nigerian’s food and feeding stuffs”. Journal of Food Agriculture and Environment (1968): 99.
- Pearson D. “Chemical analysis of foods. 6th edition”. Churchill Livingstone (1976):
- Poul-El A. “Protein functionality, classification, definition and methodology”. Journal of American Chemical Society 147 (1981): 1-9.
- Sathe SK.,et al. “Functional properties of winged bean protein”. Journal of Food Science 47.2 (1982): 503-509.
- Spadero JJ., et al. “Milling in Rice: Production and Utilization”. Westport. C.T: AVI (1980): 360-402.
- Steel RG and Torrie JH. “Principles and procedures of Statistics- A biometrical approach”. McGraw-Hill(1980):
- Edem DO., et al. “Chemical evaluation of the value of the fruit of African Star apple (chrysophyllum albidum)”. Food Chemistry14.4 (1984): 303-311.
Citation:
Ajayi FF., et al. “Proximate Composition and Functional properties of Rice (Oryza Sativa L.) Germinated-Cowpea (Vigna Unguiculata
L.) flour blends”. Innovative Techniques in Agriculture 1.1 (2017): 32-40.
Copyright: © 2017 Ajayi FF., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























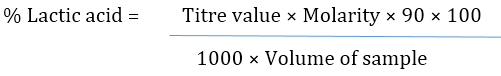
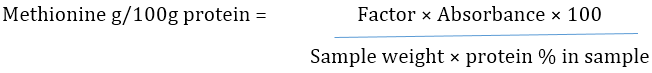

 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.