Mini Review
Volume 1 Issue 1 - 2017
Heat Shock Proteins in Brain Cancer: A mini Review
1Department of Bioscience, COMSATS Institute of Information Technology, Islamabad, Pakistan
2Department of Biomathematics, National Mathematical Centre, Abuja, Nigeria
3Department of Biochemistry, Sokoto State University, Nigeria
2Department of Biomathematics, National Mathematical Centre, Abuja, Nigeria
3Department of Biochemistry, Sokoto State University, Nigeria
*Corresponding Author: Sadeeq Muhammad Sheshe, Department of Bioscience, COMSATS Institute of Information Technology, Islamabad, Pakistan.
Received: October 12, 2017; Published: November 30, 2017
Abstract
This article provides an insight on different heat shock proteins and their roles as well as their expression in different brain cancers and tumors. Extensive review of various literatures associated with Brain cancers and heat shock proteins was carried out. All available data suggested abnormal high expression of different classes and families of the proteins in a wide range of brain cancers. Most of the results were also in correspondence with related results of expression of the proteins in other groups of cancers.
Keywords: Heat Shock proteins; Chaperones; Carcinogenesis; Brain; Oncogenesis; Cell lines
Introduction
Heat shock proteins (HSPs) are belong to a class of proteins that have significant roles in living organisms particularly humans [1]. HSPs are proteins that assist other proteins in folding after their synthesis [2]. Protein folding is a critical process after its synthesis. Unless a protein folds, then it cannot be able to carry out its function. Folding of a protein into three dimensional structures is akin to its function [3]. The 3D structure id the penultimate form for function and proteins that are unfolded or abnormally fold are incapable of their function [4].
HSPs are important component of a cell in most biological processes including cellular stresses, environmental factors, and pathological conditions [5]. These groups of proteins specialize in absorption of heat effects due to extreme temperature, facilitate protein folding, translocation and possibly degradation [6]. HSPs acting to facilitate protein folding are known as Molecular chaperones. HSPs expression has been found to be elevated in many types of Cancer including Breast cancer, colorectal cancer, Lung cancer and cervical cancer [7].
HSPs facilitate protein folding (Figure1) and are otherwise known as molecular chaperones [8]. These proteins are folded either directly after protein translation or after partial denaturation due to environmental factors such as elevated temperature [9]. The folding of many proteins, particularly large ones, is kinetically slow and is HSPs kinetically facilitate the process [10]. Most protein processes are thermodynamically not favorable as they require significant amount of energy.
In a similar mechanism with enzymes and their substrates, the HSPs tend to lower the required energy and make the process thermodynamically feasible. HSPs have therefore been implicated in many cancers and their expression has been studied. This review thus aims to summarize some key HSPs whose expression has been studied in brain cancers.
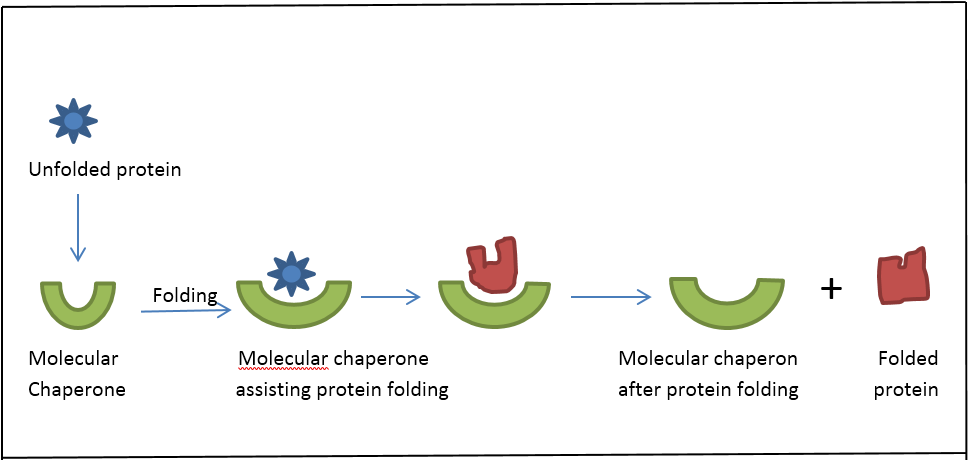
Figure 1: Folding of a protein assisted by a molecular chaperone (MC). Molecular
chaperones the thermodynamically highly unfavorable process of folding proceeds
and the proteins remaining folded after the process.
Heat Shock proteins
Heat shock proteins are also known as stress responsive proteins and are widely found in cells of all eukaryotes [11]. They are a class of proteins that are involved in a number of cellular activities including protein folding, apoptosis, cell proliferation and even protein translocation [12]. Heat shock proteins that assist in protein folding are referred to as molecular chaperones and include HSP10, HSP27, HSP40, HSP60, HSP70, HSP90, HSP110 etc.
Heat shock proteins are also known as stress responsive proteins and are widely found in cells of all eukaryotes [11]. They are a class of proteins that are involved in a number of cellular activities including protein folding, apoptosis, cell proliferation and even protein translocation [12]. Heat shock proteins that assist in protein folding are referred to as molecular chaperones and include HSP10, HSP27, HSP40, HSP60, HSP70, HSP90, HSP110 etc.
Heat shock proteins have been evolutionary conserved in eukaryotes and are name with acronym ‘’HSP’’ linked with their Molecular weight [13]. Some HSPs comprised several proteins forming a family of heat shock proteins. For example HSP70 represents a family of at least eight proteins some of which are tissue-specific and localized in several organelles in eukaryotes while the family includes two proteins in humans [14].
Hsp70 is the most prominent of the HSP70 family and is a 70kDa protein with two functional domains, a 44kDa ATP-Binding domain and a 28kDa C-terminal substrate binding domain [15]. The ATP-Binding domain is conserved in most of the members of the family while the substrate binding domain comprised of two-layered twisted β-sheets [16]. The member of the family is a constitutively expressed, 73kDa protein Hsc70 [14].
| HSP | Role/Function | Reference |
| HSP10 | Mitochondrial molecular chaperone, assist in protein folding. Plays role in cancer cell proliferation and Oncogenesis. | [17] |
| HSP27 | Interacts with a large number of proteins. Inhibits cellular apoptosis and promote cancer cell survival. | [18] |
| HSP40 | Co-chaperone with HSP70 proteins that’s assist to re-translocate misfolded proteins from one cellular compartment to another. Promote Oncogenesis and cancer cell survival. | [19] |
| HSP60 | Also known as chaperonin, assist in protein folding and translocation. Up-regulated in breast and colon cancers as well as brain tumors. | [20] |
| Hsp70 | Belongs to the HSP70 family. One of the most important chaperone, Promotes Oncogenesis and Cancer cell survival. Up-regulated in breast cancer, brain cancer; medulloblastomas and glioblastomas. Major therapeutic targets in cancers. | [20] |
| HSP90 | A major chaperone found abundantly in cells and plays role during cancers. Particularly it is highly expressed in colon, head and neck, breast as well as brain cancers. | [21] |
| HSP110 | a molecular chaperone with anti-aggregation properties, interacts with Hsp70 and assists with folding of newly synthesized and misfolded proteins. Highly expressed in Head and Neck Cancers, Colorectal cancer and meningiomas well as other brain tumors. | [22] |
Table 1: Brief classification of some Heat Shock Proteins (HSP) in humans.
Brain Cancers
Brain cancers or tumors refer to cancers of the brain in the central nervous system. The tumors can benign or malignant and ranges from lower to higher ages [23]. Brain tumors are one of the most life-threatening cancers and were reported as the 10th most common cause of death in both men and women around the world [24]. In 2015, about 700,000 people were reported to have been living with brain tumors out of which more than 70% were benign and less than 30 % were malignant [25]. In the first half of this year, about 80,000 new cases of brain cancers have been reported already which included over 20,000 malignant cases and over 50,000 non-malignant ones according to the American Brain tumor association.
Brain cancers or tumors refer to cancers of the brain in the central nervous system. The tumors can benign or malignant and ranges from lower to higher ages [23]. Brain tumors are one of the most life-threatening cancers and were reported as the 10th most common cause of death in both men and women around the world [24]. In 2015, about 700,000 people were reported to have been living with brain tumors out of which more than 70% were benign and less than 30 % were malignant [25]. In the first half of this year, about 80,000 new cases of brain cancers have been reported already which included over 20,000 malignant cases and over 50,000 non-malignant ones according to the American Brain tumor association.
Heat Shock protein in Brain cancer and tumors
Heat shock proteins assist many proteins to fold, thus their high expression during cancers, which involves expression of a large number of oncoproteins, is expected [26]. Many studies have indicated high expression of many heat shock protein in breast cancer, head and neck carcinomas and brain tumors [27]. In medulloblastoma, one of the most common malignant brain tumors in children, heat shock protein levels have been found to be elevated and have been implicated in tumor cell proliferation [28].
Heat shock proteins assist many proteins to fold, thus their high expression during cancers, which involves expression of a large number of oncoproteins, is expected [26]. Many studies have indicated high expression of many heat shock protein in breast cancer, head and neck carcinomas and brain tumors [27]. In medulloblastoma, one of the most common malignant brain tumors in children, heat shock protein levels have been found to be elevated and have been implicated in tumor cell proliferation [28].
Similarly, Graner., et al. [29] reported atypical expression pattern of HSP70 in brain tumor cell lines. Their results showed substantial over expression of the heat shock proteins even on the surface of the cells both constitutive and in response to shock conditions. A novel small heat shock protein, Hsp16.2 was remarkably reported to be highly expressed in necroectodermal tumors [30]. Similar result was obtained when the expression of this small heat shock protein was studied in different types of brain tumors including benign and malignant meningeoma, oligodendroglioma, glioblastoma multiforme, ependymoma and medulloblastoma [31].
Mirzaei et al., [32] reported a down regulation of Hsp40 in U-87MG (brain tumor) cell lines when the variant protein Oct4B1 was silenced, suggesting a correlative interaction between expression of the protein and Hsp40. Increased Membrane surface expression of some heat shock proteins at the onset of carcinogenesis suggest a possible role of the proteins in signaling pathways [33]. These and so many studies have described the role of Heat shock protein carcinogenesis of many brain cancers and tumors.
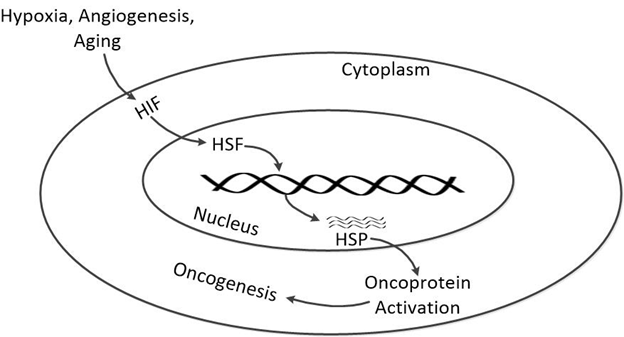
Figure 2: Hypoxia in cancer as well as angiogenesis is sensed by many factors such as Hypoxia
inducing factors (HIF). The HIF activates a transcription factor, Heat shock factor (HSF)
which promotes transcription of genes coding for variety of HSPs. These HSPs promotes oncoproteins
activation by assisting in folding of these proteins thus stimulating Oncogenesis.
Significance and Potentials of Heat shock proteins in brain cancer therapy
Brain cancers are a major threat in various part of the world [34]. Thus development of various therapeutic approaches is imperative to provide cure to brain carcinomas [35]. Multiple studies have described roles of HSPs in carcinogenesis and tumor progression [36]. Here in our studies, we have the shown roles played by HSPs in various processes of carcinogenesis of brain cancer including anti-apoptosis, angiogenesis and tumor cell proliferation (Figure 2).
Brain cancers are a major threat in various part of the world [34]. Thus development of various therapeutic approaches is imperative to provide cure to brain carcinomas [35]. Multiple studies have described roles of HSPs in carcinogenesis and tumor progression [36]. Here in our studies, we have the shown roles played by HSPs in various processes of carcinogenesis of brain cancer including anti-apoptosis, angiogenesis and tumor cell proliferation (Figure 2).
Thus these roles can easily be exploited to develop therapeutic agents in brain carcinomas. For example as Alexiou., et al. [37] reported anti-apoptotic roles of HSPs 27, 60, 70 and 90 in medullocarcinomas, a therapeutic agent targeting each of the HSPs could be a promising approach (Figure 3). Similarly certain HSPs have complementary function with one other during protein folding, for instance Hsp70 complements Hsp90 chaperonic activity [38]. As such targeting one of the proteins could have a corresponding effect to the other, therefore achieving higher folds of chaperone inhibition.
As Tutar [14] showed, inhibition of Hsp90 results into overexpression of Hsp27 and Hsp70 in brain cancers such as glioblastomas. However it has been shown that continuous to exposure to inhibitors of Hsp90 resulted into resistance to chemotherapy in gliomas and as well as oligodendroglioma [39]. This could be attributed to overexpression of Hsp70 as a result of Hsp90 inhibition. Therefore exploiting the complementary roles of these HSPs particularly in brain cancers and tumors could be significant in developing efficient therapeutic approach with less chances of resistance.
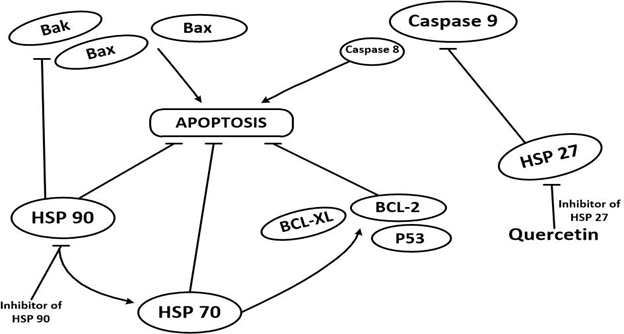
Figure 3: Roles played by some HSPs in apoptosis. Hsp70 inhibits apoptosis through anti-apoptotic proteins such
as Bcl-2 and Bcl-XL. Similarly oncoproteins such p53 are client proteins of Hsp70. Hsp90 also inhibits apoptosis by
inhibiting pro-apoptotic proteins such as Bax and Bad. However inhibition of Hsp90 promotes activity of Hsp70
which complements Hsp90 function and promotes inhibition of apoptosis. Hsp27 inhibits Caspases 8 and 9 thus inhibiting
apoptosis. Inhibitors of Hsp27 such as Quercetin targets the protein thus has been used in cancer therapy
Conclusion
It is evident from a large number of studies carried out and bulk data available that many Heat shock proteins play crucial roles in carcinogenesis of many malignant and benign brain tumors. Such proteins, giving their roles in folding of proteins (oncoproteins), apoptosis and increased cell survival and also, signaling roles could present a target for therapeutic agents. Inhibitors of some HSPs e.g 17-AAG have already been approved for use in patients with different level of brain tumors [40]. However direct inhibition of an individual HSP might not be as efficient as seen in Hsp90 [41]. Thus use of more diverse approaches in developing inhibitors could be valuable.
Schultz., et al. [41] reported a combine approach of inhibition of HSP27 and pAKT in SPARC-induced glioma cell. Although these approaches have been tested in many forms of brain cancers and tumors particularly gliomas and medulloblastoma, a number of brain cancers have yet to be tried upon by utilizing HSPs as potential therapeutic targets [42]. From our studies, we can conclude that HSPs massive role in promoting brain cancers could spell a potential target for a holistic approach in this complicated group of cancers.
Recommendations
We recommend that more studies on expression of heat shock proteins in different brain tumors. This we enable studies to determine specific classes and families of the protein specifically involved in the development of brain cancers. Similarly, we recommend that such proteins should be targeted as a potential therapeutic measure in management of brain cancers.
We recommend that more studies on expression of heat shock proteins in different brain tumors. This we enable studies to determine specific classes and families of the protein specifically involved in the development of brain cancers. Similarly, we recommend that such proteins should be targeted as a potential therapeutic measure in management of brain cancers.
References
- S Hariharan., et al. “HEAT SHOCK PROTEINS – A MINI REVIEW”. International journal Of Pharmacy and Pharmaceutical Analysis 1.1 (2014):
- FU Hartl., et al. “Molecular chaperones in protein folding and proteostasis”. Nature 475.7356 (2011): 324–332.
- M Haslbeck. “sHsps and their role in the chaperone network”. Cellular and Molecular Life Sciences 59.10 (2002): 1694-1697.
- G. Kappe., et al. “The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1-10”. Cell Stress Chaperones 8.1 (2003): 53-61.
- YE Kim., et al. “Molecular Chaperone Functions in Protein Folding and Proteostasis”. Annual Review of Biochemistry 82 (2013): 323-355.
- J. A. Barnes., et al. “Expression of inducible Hsp70 enhances the proliferation of MCF-7 breast cancer cells and protects against the cytotoxic effects of hyperthermia”. Cell Stress Chaperones 6.4 (2001): 316–325.
- CS Park., et al. “An immunohistochemical analysis of heat shock protein 70, p53, and estrogen receptor status in carcinoma of the uterine cervix”. Gynecologic Oncology 74.1 (1999): 53–60.
- D Whitley., et al. “Heat shock proteins: A review of the molecular chaperones”. Journal of Vascular Surgery 29.4 (1999): 748–751.
- H Saibil. “Chaperone machines for protein folding, unfolding and disaggregation”. Nature Reviews Molecular Cell Biology 14.10 (2013): 630–642.
- FU Hartl and M. Hayer-Hartl. “Converging concepts of protein folding in vitro and in vivo”. Nature Structural & Molecular Biology 16.6 (2009): 574–581.
- J Verghese., et al. “Biology of the Heat Shock Response and Protein Chaperones: Budding Yeast (Saccharomyces cerevisiae) as a Model System”. Microbiology and Molecular Biology Reviews MMBR 76.2 (2012): 115–158.
- Braakman and DN Hebert. “Protein Folding in the Endoplasmic Reticulum”. Cold Spring Harbor Perspectives in Biology 5.5 (2013): a013201
- MH Al-Whaibi. “Plant heat-shock proteins: A mini review”. Journal of King Saud University – Science 23.2 (2011): 139–150.
- Y Tutar, “Inhibition of Heat Shock Protein 70 and 90 (Hsp70 and Hsp90) in Target Spesific Cancer Treatment,” Advanced Techniques in Biology & Medicine 3.2 (2015): 2379.
- KM Flaherty., et al. “Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein”. Nature 346.6285 (1990): 623–628.
- KM Flaherty., et al. “Similarity of the three-dimensional structures of actin and the ATPase fragment of a 70-kDa heat shock cognate protein”. Proceedings of the National Academy of Sciences 88.11 (1991): 5041–5045.
- S Corrao., et al. “Hsp10 nuclear localization and changes in lung cells response to cigarette smoke suggest novel roles for this chaperonin”. Open Biology 4.10 (2014):
- J Acunzo., et al. “Hsp27 as a therapeutic target in cancers”. Current Drug Targets 15.4 (2014): 423–431.
- Mitra A., et al. “Multi-faceted role of HSP40 in cancer”. Clinical & Experimental Metastasis 26.6 (2009): 559-567.
- W-W Tong., et al. “The tumor promoting roles of HSP60 and HIF2α in gastric cancer cells”. Tumor Biology 37.7 (2016): 9849–9854.
- SK Calderwood and L Neckers. “Hsp90 in Cancer : Transcriptional Roles in the Nucleus”. Advances in Cancer Research 129 (2016): 89-106.
- K Berthenet., et al. “HSP110 promotes colorectal cancer growth through STAT3 activation”. Oncogene 36.16 (2017): 2328–2336.
- TE Merchant., et al. “Brain tumors across the age spectrum: biology, therapy, and late effects”. Seminars in Radiation Oncology 20.1 (2010): 58–66.
- LA Torre., et al. “Global cancer statistics, 2012”. CA: A Cancer Journal for Clinicians 65.2 (2015): 87–108.
- RL Siegel., et al. “Cancer statistics, 2016”. CA: A Cancer Journal for Clinicians 66.1 (2016): 7–30.
- DR Ciocca., et al. “Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: an update”. Archives of Toxicology 87.1 (2013): 19–48.
- H Wang., et al. “Heat shock proteins at the crossroads between cancer and Alzheimer’s disease”. BioMed Research International (2014):239164.
- GA Alexıou., et al. “Expression of Heat Shock Proteins in Brain Tumors Beyin Tümörlerinde Isı Şok Proteinlerinin Ekspresyonu”. Turkish Neurosurgery 24.5 (2014): 745–749.
- MW Graner., et al. “The Heat Shock Response and Chaperones/Heat Shock Proteins in Brain Tumors: Surface Expression, Release, and Possible Immune Consequences,” Journal of Neuroscience 27.42 (2007): 11214 -11227.
- S Bellyei., et al. “Inhibition of cell death by a novel 16.2 kD heat shock protein predominantly via Hsp90 mediated lipid rafts stabilization and Akt activation pathway”. Apoptosis: A Review of Programmed Cell Death 12.1(. 2007): 97–112.
- S Bellyei., et al. “Preventing apoptotic cell death by a novel small heat shock protein”. European Journal of CellBiology 86.3 (2007): 161-171.
- MR Mirzaei., et al. “Down-regulation of HSP40 gene family following OCT4B1 suppression in human tumor cell lines”. Iranian Journal of Basic Medical Sciences 19.2 (2016): 187–193.
- Z Bromberg and Y. Weiss. “The Role of the Membrane-Initiated Heat Shock Response in Cancer”. Frontiers in Molecular Biosciences 3 (2016):
- LN Chien., et al. “Comparative Brain and Central Nervous System Tumor Incidence and Survival between the United States and Taiwan Based on Population-Based Registry”. Frontiers in Public Health 4 (2016): 151.
- J Wu., et al. “Heat Shock Proteins and Cancer”. Trends in Pharmacological Sciences 38.3 (2017): 226–256.
- SW Van Gool. “Brain Tumor Immunotherapy: What have We Learned so Far?” Frontiers in Oncology 5 (2015):
- GA Alexiou., et al. “Expression of heat shock proteins in medulloblastoma” Journal of Neurosurgery: Pediatrics 12.5 (2013): 452–457.
- S Chatterjee and TF Burns. “Targeting Heat Shock Proteins in Cancer: A Promising Therapeutic Approach”. International Journal of Molecular Sciences 18.9 (2017):
- S Schaefer., et al. “The small-molecule kinase inhibitor D11 counteracts 17-AAG-mediated up-regulation of HSP70 in brain cancer cells”. PLOS ONE 12.5 (2017): e0177706.
- K Jhaveri and S Modi. “Chapter Fifteen - HSP90 Inhibitors for Cancer Therapy and Overcoming Drug Resistance”. Advances in Pharmacology 65 (2012): 471–517.
- CR Schultz., et al. “Inhibition of HSP27 alone or in combination with pAKT inhibition as therapeutic approaches to target SPARC-induced glioma cell survival”. Molecular Cancer 11 (2012):20.
- MPD Hatfield and S Lovas. “Role of Hsp70 in cancer growth and survival”. Protein & Peptide Letters 19.6 (2012): 616–624.
Citation:
Sadeeq Muhammad Sheshe., et al. “Heat Shock Proteins in Brain Cancer: A mini Review”. Holistic Approaches in Oncotherapy
1.1 (2017): 16-22.
Copyright: © 2017 Sadeeq Muhammad Sheshe., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.