Research Article
Volume 2 Issue 1 - 2018
Evaluation of a Hypoglycemic and Hypolipidemic Activities of Methanolic Extract of Antidesma bunius (Linn.) (Family Euphorbiaceae) leaf.
1Department of Pharmacy, Southeast University, Dhaka, Bangladesh
2Department of Pharmacy, North south University, Dhaka, Bangladesh
3Consultant neurosurgeon, Gouri Devi Hospital and Research Institute Durgapur, MBBS, MS, General surgery, MCH neurosurgery from PGI Chandigarah), India
2Department of Pharmacy, North south University, Dhaka, Bangladesh
3Consultant neurosurgeon, Gouri Devi Hospital and Research Institute Durgapur, MBBS, MS, General surgery, MCH neurosurgery from PGI Chandigarah), India
*Corresponding Author: Shariful Islam, Department of Pharmacy, Southeast University, Banani, Dhaka-1213, Bangladesh.
Received: October 31, 2018; Published: December 14, 2018
Abstract
Background and Objective: Diabetes mellitus (DM), is a group of metabolic disorders of carbohydrate, protein and fat results in high blood sugar levels over a prolonged period of time. Quest for new and cost effective antidiabetic drugs with least side effects from medicinal sources is a great challenge to us in present time due to the high prevalence of the disease and cost of the currently available antidiabetic drugs.
Methods: Diabetes was induced in male Long Evan rats by the administration of single intra-peritoneal injection of alloxan monohydrate (150 mg/kg b.w.). Effect of oral administration of two different doses of methanolic extract (250 and 500 mg/kg b.w.), metformin (100 mg/kg b.w.) alone for 7 days were examined on 0th, 3th, 5th and 7th day of treatment to evaluate hypoglycemic and hypolipidemic activity. After 7 days of treatment, hypolipidemic effects were estimated by measuring serum biochemical parameters such as TC, TG, LDL, and HDL. The survival rate, body weight and organ weight were also measured.
Results: The leaves extract showed remarkable anti-diabetic property which was evaluated by the percentage of inhibition of the blood glucose level along with TG, TC, LDL and increase in HDL in high concentration of the plant extract compared to the metformin, a reference drug.
Conclusion: It can be concluded that methanolic extract of A. bunius leaves possesses good hypoglycemic, hypolipidemic activity. Hence, further studies are suggested to identify the exact bioactive compound(s) which may aid ongoing quest for cost effective antidiabetic drug from natural source.
Keywords: Antidiabetic; Methanolic extract; Antidesma bunius (Linn.); Alloxan, Hypolipidemic activity; Hypoglycemic activity
Abbreviation: DM: Diabetes mellitus; DMSO: Dimethyl sulfoxide
Introduction
Antidesma bunius (Family- Euphorbiaceae) is traditionally used as sudorific and in the treatment of snakebite, decoction is used to promote perspiration in febrile condition, juice of the plant is useful in the treatment of insomnia. Fresh juice of the fruits is used in the manufacturing of wine as an antioxidant. Roots and leaf are anthelminthic and also used in indigestion cough and stomachache. The seeds are used against round worms and threadworms, coughs, flatulence, intestinal colic and also used as pesticide [1]. Diabetes mellitus (DM), commonly mention to as diabetes, is a group of metabolic disorders of carbohydrate, protein and fat results in high blood sugar levels over a prolonged period of time (World Health Organization, 2014) [2]. Severe complicacy of DM results in diabetic ketoacidosis, nonketotic hyperosmolar hyperglycemic coma, or death [3].
Chronic or Serious long-term complicacy of DM involve kidney disease, stroke, cardiovascular disease, foot ulcers, and destruction to the eyes [4]. In 2015, approximately 415 million people affected by diabetes worldwide [5], among them type 2 DM making up about 90% of the cases [6,7]. This studies also suggests that 8.3% of the adult population affected by Type 1 DM [8]. With equal rates in both women and men [8]. Laterally, the prevalence of type 1 DM is also continuously increasing to that of type 2 DM worldwide [9-11]. The amount of diabetic people in world is anticipated to increase to 366 million in 2030 [12]. Circumscribe almost 80% of the population associated with DM in under-developed and developing countries people [13]. Search out for new antidiabetic drugs with minimum or no side effects from medicinal parts is a demanding to us because in present available drugs for diabetics, insulin or oral hypoglycemic agents have one or more potential side effects and most of them are expensive. We have shown that methanolic extracts of A. bunius had antidiabetic activity in alloxan-induced diabetic animal model. Our experiment was designed to compare the hypoglycemic and lipid lowering activities of methanolic extract of A. bunius leaf alone with metformin in alloxan induced diabetic rats. Antioxidants protect human body against free radicals by scavenging them [14]. Free radicals are liable for devastation of protein, as well as that lipid and DNA by oxidative process during aerobic metabolism in the biological system [15], lead to many degenerative disease and long-term or chronic diseases together with atherosclerosis, carcinogenesis, diabetes mellitus, ischemic cardiac disease, immunocompromised and neurodegenerative diseases etc [16].
Materials and Methods
Collection of plant materials
The leaves of Antidesma bunius linn. Were collected from Lawchapra, Bakshiganj, Jamalpur, Bangladesh in August, 2016, and identified by an expert taxonomist. A voucher specimen was submitted to the national herbarium, Mirpur, Dhaka, Bangladesh. Accession number: DACB 43490.
The leaves of Antidesma bunius linn. Were collected from Lawchapra, Bakshiganj, Jamalpur, Bangladesh in August, 2016, and identified by an expert taxonomist. A voucher specimen was submitted to the national herbarium, Mirpur, Dhaka, Bangladesh. Accession number: DACB 43490.
Preparation of extract
In cold extraction process powdered plant materials are submerged in a suitable solvent or solvent system in an air tight flat bottom container for seven days, with occasional shaking and stirring. The major portion of plant materials will be dissolved in the solvent. Solvent is then separated from dispersed materials and evaporated to get desired extract. Approximately 400gm of powder were soaked in 1.7 liter of methanol for about 14 days at room temperature with occasional shaking. After 14 days the solution was filtered using cotton filter and Whitman’s filter paper. The filtrates (methanolic extract) obtained were evaporated first using rotary evaporator & then placed in a water- bath until dried or being semisolid. It rendered a gummy or semisolid concentrates and were designated as crude extracts of methanol. Finally, the extract was stored at room temperature.
In cold extraction process powdered plant materials are submerged in a suitable solvent or solvent system in an air tight flat bottom container for seven days, with occasional shaking and stirring. The major portion of plant materials will be dissolved in the solvent. Solvent is then separated from dispersed materials and evaporated to get desired extract. Approximately 400gm of powder were soaked in 1.7 liter of methanol for about 14 days at room temperature with occasional shaking. After 14 days the solution was filtered using cotton filter and Whitman’s filter paper. The filtrates (methanolic extract) obtained were evaporated first using rotary evaporator & then placed in a water- bath until dried or being semisolid. It rendered a gummy or semisolid concentrates and were designated as crude extracts of methanol. Finally, the extract was stored at room temperature.
Drugs and chemicals
Alloxan (Hydrate, Lobachemie PVT. LTD.), metformin (COMET tablet, Square Pharmaceuticals Ltd, Bangladesh), Blood samples analyzed for blood glucose content by using Ez Smart 168 (Tyson Bioresearch, Inc. Chu-Nan, Taiwan) glucose test meter. Total cholesterol (TC), triglyceride (TG), low density lipoprotein (LDL), high density lipoprotein (HDL) kits were purchased from Human Gesellschaft fur Bio-chemical mbH-Wiesbaden, Germany. Saline water was purchased from local market. Other chemicals were obtained from local sources and were of analytical grade.
Alloxan (Hydrate, Lobachemie PVT. LTD.), metformin (COMET tablet, Square Pharmaceuticals Ltd, Bangladesh), Blood samples analyzed for blood glucose content by using Ez Smart 168 (Tyson Bioresearch, Inc. Chu-Nan, Taiwan) glucose test meter. Total cholesterol (TC), triglyceride (TG), low density lipoprotein (LDL), high density lipoprotein (HDL) kits were purchased from Human Gesellschaft fur Bio-chemical mbH-Wiesbaden, Germany. Saline water was purchased from local market. Other chemicals were obtained from local sources and were of analytical grade.
Antidiabetic Activity Test
Experimental animal
Albino rats of either sex, aged 4-5 weeks, obtained from the Animal Resource Branch of the International Centre for Diarrhoeal Diseases and Research, Bangladesh (ICDDR, B) were used for the experiment. They were housed in standard polypropylene cages and kept under controlled room temperature (25 ± 20C; relative humidity 60-70%) in a 12h light-dark cycle were used for the study and animals were fed ICDDR, B formulated rodent food and water. The protocol of the study was approved by the institutional animal ethics committee.
Experimental animal
Albino rats of either sex, aged 4-5 weeks, obtained from the Animal Resource Branch of the International Centre for Diarrhoeal Diseases and Research, Bangladesh (ICDDR, B) were used for the experiment. They were housed in standard polypropylene cages and kept under controlled room temperature (25 ± 20C; relative humidity 60-70%) in a 12h light-dark cycle were used for the study and animals were fed ICDDR, B formulated rodent food and water. The protocol of the study was approved by the institutional animal ethics committee.
Experimental Design
Albino rats were randomly assigned into group I, II, III, IV, V; 4 rats are selected in each group for the respective one week treatment for the determination of blood glucose, lipid profile test studies.
Albino rats were randomly assigned into group I, II, III, IV, V; 4 rats are selected in each group for the respective one week treatment for the determination of blood glucose, lipid profile test studies.
Group I: Control; only food and water ad libitum were orally administered to rats.
Group II: Diabetic Control; alloxan monohydrate 150 mg/kg b.w. was administered intraperitoneally to rats.
Group III: Diabetic + metformin, 100 mg/kg b.w.
Group IV: Diabetic + Plant Extract, 250 mg/kg b.w.
Group V: Diabetic + Plant Extract, 500 mg/kg b.w.
Group II: Diabetic Control; alloxan monohydrate 150 mg/kg b.w. was administered intraperitoneally to rats.
Group III: Diabetic + metformin, 100 mg/kg b.w.
Group IV: Diabetic + Plant Extract, 250 mg/kg b.w.
Group V: Diabetic + Plant Extract, 500 mg/kg b.w.
Preparation of dosage of standard drug and plant extract
Metformin was in white crystal form and freshly soluble in water. The solution of metformin was prepared by dissolving with dimethyl sulfoxide (DMSO) and based on literature searches the doses of metformin were selected which were 100 mg/kg b.w. respectively and was administered intra-peritoneal route to rats at 0.5 ml drug.
Metformin was in white crystal form and freshly soluble in water. The solution of metformin was prepared by dissolving with dimethyl sulfoxide (DMSO) and based on literature searches the doses of metformin were selected which were 100 mg/kg b.w. respectively and was administered intra-peritoneal route to rats at 0.5 ml drug.
Plant extract was semisolid and very slightly soluble in water. The dosage was prepared in suspension using saline water. In order to administer the crude extract at dose of 250mg/kg and 500mg/kg body-weight of rat, required amount of A. bunius extract was measured [17,18]. Then the extract was dissolved in 2 ml of DMSO (Di-methyl sulfoxide). Small amount of suspending agents (Tween-80) was added for proper mixing. The final volume of each suspension was made 2ml. Test drug was given at the amount of 0.5ml in intra-peritoneal route to each rat [19].
Induction of Diabetes
Firstly, body weight of each rats were measured. Then required amount of alloxan was measured according to the body weight by following the dose of 150 mg of alloxan per 1000 gm of body weight [20,21]. Then calculated amount of alloxan was dissolved in 0.5 ml of sterile normal saline water. Group II-V animals were allowed to fast for 12 hours were rendered diabetic by injection intraperitoneally a freshly prepared solution of alloxan (150mg/kg/b.w.) in saline water after overnight and drink 10% glucose solution to overcome drug induced hypoglycemia. After 24 hours blood glucose content was measured by using glucose monitoring system by the blood sample which was collected from the tail vein of the rats. Animals got the blood glucose levels above 7.0 mmol/L was selected for the study.
Firstly, body weight of each rats were measured. Then required amount of alloxan was measured according to the body weight by following the dose of 150 mg of alloxan per 1000 gm of body weight [20,21]. Then calculated amount of alloxan was dissolved in 0.5 ml of sterile normal saline water. Group II-V animals were allowed to fast for 12 hours were rendered diabetic by injection intraperitoneally a freshly prepared solution of alloxan (150mg/kg/b.w.) in saline water after overnight and drink 10% glucose solution to overcome drug induced hypoglycemia. After 24 hours blood glucose content was measured by using glucose monitoring system by the blood sample which was collected from the tail vein of the rats. Animals got the blood glucose levels above 7.0 mmol/L was selected for the study.
Results
Hypoglycemic Activity
The parameters of blood glucose (BG) level, TG, TC, LDL Cholesterol, HDL Cholesterol and body weight were measured after one week of drug treatment to study the effect of methanolic extract of A. bunius leaves (250 & 500 mg/kg BW), Metformin (100mg/kg BW) were applied in alloxan- induced diabetic rats. Metformin was used as standard antidiabetic agent. In order to determine hypoglycemic activity blood samples are collected from tail-veins of rats and tested for blood glucose level by glucometer at 0th, 3rd,5th and 7th day of treatment. Following chart shows the effect of methanolic extracts of the leaves of A. bunius on blood glucose levels of Long Evan rats after one week of continuous treatment. Sequential injection of alloxan caused a significant increase in blood glucose concentration in all group of rats compared with their respective baseline blood glucose and to control values. At all-time points, blood glucose concentration remain unchanged in normal rats treated with distilled water. However, oral administration of the plant extracts (250 & 500mg/kg) as well as metformin (100mg/kg) to diabetic rats decreased in blood glucose concentrations. The blood glucose concentration decrease in case of metformin (66.46%) and A. bunius extract of 250 and 500mg/kg (29.57% and 62.8%) respectively.
The parameters of blood glucose (BG) level, TG, TC, LDL Cholesterol, HDL Cholesterol and body weight were measured after one week of drug treatment to study the effect of methanolic extract of A. bunius leaves (250 & 500 mg/kg BW), Metformin (100mg/kg BW) were applied in alloxan- induced diabetic rats. Metformin was used as standard antidiabetic agent. In order to determine hypoglycemic activity blood samples are collected from tail-veins of rats and tested for blood glucose level by glucometer at 0th, 3rd,5th and 7th day of treatment. Following chart shows the effect of methanolic extracts of the leaves of A. bunius on blood glucose levels of Long Evan rats after one week of continuous treatment. Sequential injection of alloxan caused a significant increase in blood glucose concentration in all group of rats compared with their respective baseline blood glucose and to control values. At all-time points, blood glucose concentration remain unchanged in normal rats treated with distilled water. However, oral administration of the plant extracts (250 & 500mg/kg) as well as metformin (100mg/kg) to diabetic rats decreased in blood glucose concentrations. The blood glucose concentration decrease in case of metformin (66.46%) and A. bunius extract of 250 and 500mg/kg (29.57% and 62.8%) respectively.
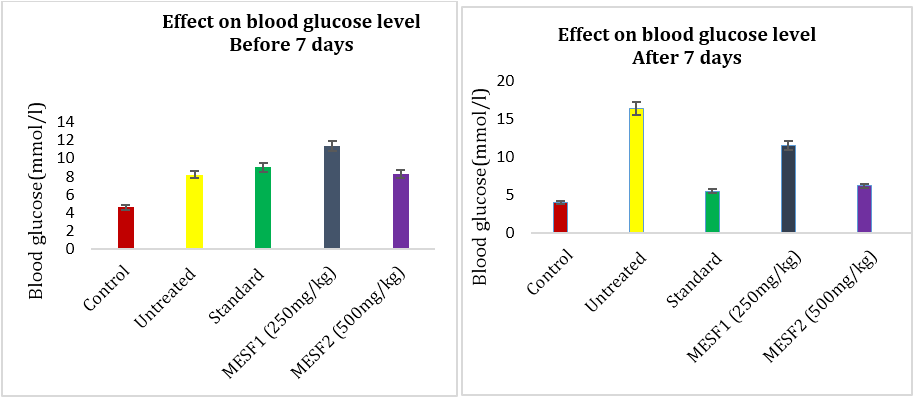
Figure 1 and 2: Effect of A. bunius extracts & metformin on blood glucose level in alloxan induced diabetic rats.
| Groups | Fasting Blood glucose level /(mol/l) | ||||
| Days of treatment | |||||
| At the time of grouping | Day 0 | Day 3 | Day 5 | Day 7 | |
| Control | 4.3 ± 0.27 | 4.6 ± 0.28 | 3.9 ± 0.20 | 3.7 ± 0.23 | 4.0 ± 0.22 |
| Untreated diabetic | 4.6 ± 0.34 | 8.2 ± 0.37 | 9.1 ± 0.32 | 13.4 ± 0.39 | 16.4 ± 0.37 |
| Diabetic+ Metformin (100mg/kg) |
5.13 ± 0.32 | 9.0 ± 0.311 | 6.21 ± 0.37 | 4.4 ± 0.41 | 5.5 ± 0.34 |
| Diabetic+ Antidesma bunius (250mg/kg) | 6.2±0.28 | 11.4±0.36 | 11.5±0.32 | 12.15±0.30 | 11.55±0.32 |
| Diabetic+ Antidesma bunius (500mg/kg) | 5.3±0.31 | 8.3±0.41 | 8.0±0.22 | 6.4±0.37 | 6.2±0.35 |
Table 1: Effect of Antidesma bunius on blood glucose levels in alloxan induced diabetic rats.
Effect of A. bunius on lipid profile
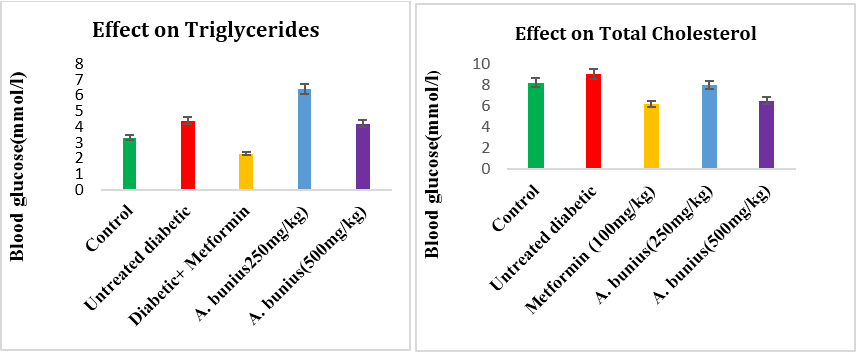
Showed that the effect of the A. bunius extract on TG, TC, HDL, LDL in alloxan induced diabetic rats. After alloxan induced, the result showed that TG, TC, LDL increased while HDL decreased compared to control rats. The administration of A. bunius extracts (250 & 500mg/kg) and metformin (100mg/kg) decreased TG, TC, LDL levels and significantly increased HDL levels.
Showed that the effect of the A. bunius extract on TG, TC, HDL, LDL in alloxan induced diabetic rats. After alloxan induced, the result showed that TG, TC, LDL increased while HDL decreased compared to control rats. The administration of A. bunius extracts (250 & 500mg/kg) and metformin (100mg/kg) decreased TG, TC, LDL levels and significantly increased HDL levels.
The TG level decreased in case of metformin (47.72%) and A. bunius extract of 250 & 500 mg/kg (-45.45% and 4.54 %) respectively compared to untreated diabetic group rats. The TC level decreased in Case of metformin (31.11%) and A. bunius extract of 250 & 500 mg/kg (11.11% and 27.78%) respectively compared to untreated diabetic group rats. The LDL-C levels decreased in case of metformin (78.84%) and A. bunius extract of 250 & 500 mg/kg (48.07% and 63.47%) respectively compared to untreated diabetic group rats. The HDL-C C levels increased in case of metformin (-75.47%) and A. bunius extract of 250 & 500 mg/kg (-45.28% and -69.81%) respectively compared to untreated diabetic group rats.
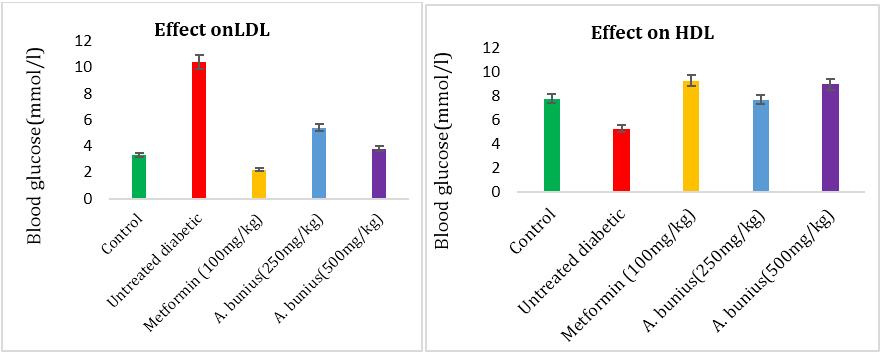
Figure 5 and 6:
Figure 3, 4, 5 and 6: Effect of A. bunius extract on TG, TC, LDL and HDL level in alloxan induced diabetic rats. Data as mean± SEM; n=4 in each group.
Figure 3, 4, 5 and 6: Effect of A. bunius extract on TG, TC, LDL and HDL level in alloxan induced diabetic rats. Data as mean± SEM; n=4 in each group.
| Group | Body weight (g) | Organ weight (mg/g) | (mmol/l) Lipid profile | |||||
| Initial | Final | % variation | Liver | TG | TC | LDL-C | HDL-C | |
| Control | 75 | 93 | -24 | 4.73 | 3.3 ± 0.30 | 8.2 ± 0.37 | 3.3 ± 0.32 | 7.8 ± 0.10 |
| Untreated diabetic | 97 | 90 | 7.21 | 4.15 | 4.4 ± 0.31 | 9.0 ± 0.16 | 10.4 ± 0.31 | 5.3 ± 0.30 |
| Diabetic+ Metformin (100mg/kg) |
82 | 94 | -14.63 | 4.49 | 2.3 ± 0.30 | 6.2 ± 0.20 | 2.2 ± 0.20 | 9.3 ± 0.31 |
| Diabetic + Antidesma bunius (250mg/kg) | 104 | 93 | 10.33 | 5.02 | 6.4 ± 0.31 | 8.0 ± 0.24 | 5.4 ± 0.41 | 7.7 ± 0.16 |
| Diabetic + Antidesma bunius (500mg/kg) | 99 | 90 | 9.49 | 4.21 | 4.2 ± 0.20 | 6.5 ± 0.18 | 3.8 ± 0.06 | 9.0 ± 0.20 |
Table 2: Effect of A. bunius on lipid profile in alloxan induced diabetic rats.
Discussion
Diabetes is the third killer of mankind after cardiovascular and cancer, because of its high prevalence, mortality and morbidity [21]. The chronic or serious long-term hyperglycemia of diabetes is associated with long term damage, failure and dysfunction of various organs [22]. Alloxan has been seen to cause a monolithic reduction of the β-cells of the islets of Langerhans which induce hyperglycemic [23]. A large number of medicinal plant have been tested for their anti-diabetic and anti-hyperlipidemic activity, many remain to be scientifically established. Administration of plant extracts, dose-dependent reduction in blood glucose levels in rat was observed. There was a remarkable fall in the blood glucose levels at higher dose of 500 mg/kg of plant extract compared to 250 mg/kg of plant extract. Then that the lower dose of plant extract at 250 mg/kg body weight showed slightly reduction of blood glucose levels in diabetic rats, but the reduction of blood glucose was significant at a dose of 450 mg/kg body weight.
Conclusion
Diabetes patient in our country is increasing day by day and now it’s become one of the major issue of death for the human being. It also arises multiple diseases if it is not controlled properly. Though there are several drugs and insulin are available to control this disease, but scientists are still looking for natural remedies due to adverse effects associated with those drugs and insulin. In this study, we have shown the beneficial effects of A. bunius leaves extract and compared it with the standard drug metformin. Results of this study indicates that higher doses (500mg/kg) of extract showed better potency than lower dose (250mg/kg). The outcome of this study also suggests that High doses of extract works like standard to reduce the blood glucose level, TG, TC, LDL and increases the HDL level. Finally, we can concluded that this leaves extract of A. bunius plants has anti-diabetic property with minimal amount of side effects. Further studies are required to determine the functional sites of this components, which are responsible for the beneficial effects and to identify it’s mechanism of action. Hence, it is worthwhile to isolate and elucidate the bioactive principles that are responsible for the better activity that is underway.
Acknowledgments
The authors are grateful to the Department of Pharmacy, Southeast University, Bangladesh, for their support during the research and also thankful to Bangladesh National Herbarium, Dhaka, Bangladesh for their identification.
The authors are grateful to the Department of Pharmacy, Southeast University, Bangladesh, for their support during the research and also thankful to Bangladesh National Herbarium, Dhaka, Bangladesh for their identification.
Consent
It is not applicable.
It is not applicable.
Ethical approval
The protocol of the experiment was approved by the animal ethics committee of the Department of Pharmacy, Southeast University, Dhaka, Bangladesh. The animals care and health were maintained according to the guidelines of National Institutes of Health.
The protocol of the experiment was approved by the animal ethics committee of the Department of Pharmacy, Southeast University, Dhaka, Bangladesh. The animals care and health were maintained according to the guidelines of National Institutes of Health.
Conflict of interest statement
We declare that we have no conflict of interest.
We declare that we have no conflict of interest.
References
- Morton, J. In: Fruits of warm climates. Julia F. Morton, Miami, FL. (1987): 210-212.
- "About diabetes". World Health Organization. Archived from the original on 31 March 2014.
- Kitabchi AE m et al. "Hyperglycemic crises in adult patients with diabetes”. Diabetes Care 32.7 (2009): 1335-1343.
- "Diabetes Fact sheet N°312". WHO. October 2013. Archived from the original on 26 August 2013.
- "Update 2015". IDF. International Diabetes Federation. p. 13. Archived from the original on 2016-03-22.
- Williams textbook of endocrinology (12th ed.). Philadelphia: Elsevier/Saunders. pp. 1371–1435. ISBN 978-1-4377-0324-5.
- Shi Yuankai and Hu Frank B. "The global implications of diabetes and cancer". The Lancet 383.9933 (2014): 1947-1948.
- American Diabetes Association. “Standards of Medical Care for Patients with Diabetes Mellitus”. Diabetes Care 26 (2003): 533-550.
- International Diabetes Federation. International Diabetes Federation Diabetes Atlas. Brussels (2006).
- Negrato CA., et al. “Temporal Trends in Incidence of Type 1 Diabetes between 1986 and 2006 in Brazil”. Journal of Endocrinological Investigation 33.6 (2010): 373-377.
- Wild S Roglic G and Green A. “Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030”. Diabetes Care 27.5 (2004): 1047-1053.
- International Diabetes Federation (IDF) (2012) Country Estimates Table 2011. IDF Diabetes Atlas.
- Oke JM and Hamburger MO. “Screening of some Nigerian medicinal plants for antioxidant activity using 2, 2, diphenyl-picryl-hydrazyl radical”. African Journal of Biomedical Research 5.1.2 (2002): 77-79.
- Kalaiselvi M., et al. “In vitro and In vivo antitumor activity of Jasminum sambac (Linn) Ait oleaceae flower against Dalton’s ascites lymphoma induced Swiss albino mice”. International Journal of Pharmacy and Pharmaceutical Sciences 4.1 (2012): 144-147.
- Stanner A., et al., “A review of the epidemiological evidence for the ‘antioxidant hypothesis”. Public Health Nutrition 7.3 (2004): 407-422.
- El-Tantawy WH., et al. “Investigation of antidiabetic action of Antidesma bunius extract in type 1 diabetes”. Archives of Physiology and Biochemistry 121.3 (2015: 116-122.
- Khatune NA., et al. “Antidiabetic, antihyperlipidemic and antioxidant properties of ethanol extract of Grewia asiatica Linn. Bark in alloxan-induced diabetic rats”. BMC Complementary and Alternative Medicine 18 (2016).
- Stephen Irudayaraj S., et al. “Antidiabetic and antioxidant activities of Toddalia asiatica (L.) Lam. leaves in streptozotocin induced diabetic rats”. Journal of Ethnopharmacology 143.2 (2012): 515-523.
- El-Gamal EK. “Therapeutic Benefits of Garlic against Alloxan-Induced Diabetic in Rats”. Journal of Medical Science And clinical Research 5.2 (2017).
- FJ Alarcon-Aguilar., et al. “Hypoglycemic activity of root water decoction, sesquierpenoids, and one Polysaccharide fraction from Psacalium decompositum in mice”. Journal of Ethno Pharmacology 69.3 (2000): 207-215.
- Prieto P., et al. “Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E”. Analytical biochemistry 269.2 (1999): 337-341.
- Choi HY., et al. “Application of flow injection—chemiluminescence to the study of radical scavenging activity in plants”. Phototherapy Research 14.4 (2000): 250-253.
- Desmarchelier C., et al. “Antioxidant and prooxidant activities in aqueous extracts of Argentine plants”. International journal of pharmacognosy 35.2 (1997): 116-120.
- Li WL., et al. “Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus”. Journal of Ethno pharmacology 92.1 (2004): 1-21.
- Lyra R., et al. “Prevention of type 2 diabetes mellitus”. Arq Bras Endorical Metabo 50 (2006): 239-249.
- M Goldner and G Gomori. “Alloxan induced diabetes”. Journal of Endocrinology 33 (1943): 297-299.
- Elya B., et al. “Antidiabetic activity test by inhibition of α-Glucosidase and phytochemical screening from the most active fraction of Buni (Antidesma bunius L.) stem barks and leaves”. International Journal of PharmTech Research 4.4 (2012): 1667-1671.
- Kumar S., et al. “Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves”. BMC Research Notes 7 (2014): 560.
- Christova-Bagdassrian VL., et al. “Total phenolics and total flavonoids, nitrate contents and microbiological tests in dry extract of Bulgarian White birch leaves (Betula pendula)”. International Journal of Advanced Research 2.6 (2014): 668-674.
- Khan RA., et al. “Assessment of flavonoids contents and in vitro antioxidant activity of Launaea procumbens”. Chemistry Central Journal 6.1 (2012): 43.
- Oktay M., et al. “Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts”. LWT - Food Science and Technology 36.2 (2003): 263-271.
- Baumann J., et al. “Prostaglandin synthetase inhibiting O2 radical scavenging properties of some flavonoids and related phenolic compounds. Deutsche Pharmakologische Gesellschaft abstracts of the 20th spring meeting, Naunyn-Schmiedebergs abstract no: R27 cited”. Archives of Pharmacal Research 307 (1979): R1–77.
- khatoon M., et al. “Estimation of total phenol and in vitro antioxidant activity of Albizia procera leaves”. BMC Research Notes 6 (2013): 121.
Citation:
Shariful Islam., et al. “Evaluation of a Hypoglycemic and Hypolipidemic Activities of Methanolic Extract of Antidesma bunius
(Linn.) (Family Euphorbiaceae) leaf.” Archives of Endocrinology and Diabetes Care 2.1 (2018): 176-183.
Copyright: © 2018 Shariful Islam., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.