Research Article
Volume 2 Issue 1 - 2018
Urinary Tract Infection and Pattern of Antibiotic Sensitivity in Hospitalized Patients with Diabetes Mellitus in Myanmar
1Department of Endocrinology, University of Medicine 2, Yangon and Bahosi Private Hospital, Yangon, Myanmar
2Department of Medicine, Bahosi Private Hospital, Yangon, Myanmar
3Department of Microbiology, Bahosi Private Hospital, Yangon, Myanmar
2Department of Medicine, Bahosi Private Hospital, Yangon, Myanmar
3Department of Microbiology, Bahosi Private Hospital, Yangon, Myanmar
*Corresponding Author: Than Than Aye, Department of Endocrinology, University of Medicine 2, Yangon and Bahosi Private Hospital,
Yangon, Myanmar.
Received: August 07, 2018; Published: August 22, 2018
Abstract
Urinary tract infection (UTI) is one of the most common complications in diabetic patients, E.coli being the most prevalent organism. With the emergence of drug resistant E.coli, correct choice of antibiotic during septicemia due to UTI is important. The aim of this study is to determine the spectrum of organisms and its pattern of antibiotic sensitivity in diabetic patients with UTI.
A descriptive cross-sectional study was conducted from July 2014 to July 2015 in Bahosi Private Hospital in Yangon. The urine samples of diabetic patients with clinically suspected UTI were collected to identify organisms by microbiological cultures. Antibiotic sensitivity tests were carried out using different antibiotics. Culture samples with negative results were excluded. 210 positive culture samples were included. Statistical analysis was done using SPSS version 22.0.
E.coliwas the most frequent organism (60.5%) and it showed high resistance rates to commonly used antibiotics such as Ampicillin (93.7%), Ciprofloxacin (77.2%), Levofloxacin (76.8%), Tetracycline (76.4%), Cefazolin (73.2%), Ceftriaxone (72.6%), Cefepime (72.0%) and Co-trimoxazole (70.9%). E.coli was highly sensitive to Ertapenem, Imipenem, Meropenem, Amikacin and Nitrofurantoin. Of the remaining antibiotics, Piperacillin+Tazobactam and Co-amoxiclav had relatively low resistance rates.
E.coliis resistant to the majority of commonly used empirical antibiotics and sensitive to Carbapenems and Aminoglycosides. The awareness of this pattern of antibiotic sensitivity is essential for development of local guidelines for initial choice of antibiotics in diabetes patients with severe urinary tract infections.
Keywords: Urinary infection; Antibiotic sensitivity; Diabetes mellitus; E.coli
Abbreviations: Amp C: Class C Beta lactamase; CFU: Colony forming unit; E. Coli: Escherichia coli; ESBL: Extended-spectrum beta
lactamase; HbA1C: Glycated haemoglobin; UTI: Urniary tract infection
Introduction
The prevalence of type 2 diabetes mellitus is increasing worldwide. The number of adults in the world with diabetes has increased almost four times in less than four decades, from 108 million in 1980 to 422 million in 2014. [1] In Myanmar National Survey on the prevalence of diabetes and risk factors for non-communicable diseases conducted in 2013-2014 reported the prevalence of diabetes as 10.5% for the adult population aged between 25 and 65 years. [2]
Diabetes mellitus is a predisposing factor for the development of UTI as it alters host defense mechanism. Immunologic impairments, such as defective in migration and phagocytic properties of polymorph nuclear leucocytes, are occurred in diabetes mellitus patients. Neuropathy which is one of the complication of diabetes, impairs bladder emptying, favoring colonization of bacteria and a risk factor for recurrent urinary tract in fection. [3] The urinary tract is the principle site of infection in diabetic patients with complications. [4]
The most common organism of UTI in both diabetic and non-diabetic patients is E.coli. [5] Inappropriate and indiscriminate use of antibiotics has led to the emergence of multidrug-resistant E.coli. Increasing rates of resistance among E. coli is a growing concern in both developed and developing countries. [6] The spread of antimicrobial resistance is one of the major causes of failure in treatment of infectious diseases, resulting in increased morbidity, mortality and economic burden. [7]
In many parts of Myanmar, efficient facilities such as VITEK2 for culture of urine and testing of sensitivity to antimicrobials are not available, thus, leading to inadequate management of UTI in diabetic patients. Current status of causal organisms and resistance pattern of UTI in Myanmar is not available. Therefore, knowledge of causative agents and their sensitivity patterns will be beneficial by providing information on the best initial empirical therapy especially in septic emic patients. A wrong initial choice of antibiotic can lead to high mortality rate. Therefore, this study will be able to produce favorable effect on outcomes of patients and health-related expenditures. The aim of this study is to determine the spectrum of organisms and its pattern of antibiotic sensitivity in diabetic patients with UTI.
Patients and Methods
A descriptive cross-sectional study was conducted at Bahosi Private Hospital during the period from July 2014 to July 2015. Hospitalized diabetic patients with clinically suspected UTI at the time of admission or acquired during their stay in the hospital, who gave informed consent were included in the study.
Diagnosis of diabetes was made according to WHO criteria with symptoms of hyperglycemia and fasting blood sugar ≥ 7mmol/l or random blood sugar ≥ 11.1mmol/l or HbA1C ≥ 48mmol/L (6.5%). Clinically suspected UTI was defined as the presence of at least two of the following complaints: frequency, urgency, nocturia, dysuria, incontinence, suprapubic pain, flank pain, cost vertebral angle tenderness, fever (38°C) and chills. [8]
After giving proper instructions, clean-catch mid-stream urine samples of diabetic patients with clinically suspected UTI were collected with screw-capped wide mouth plastic bottles. In Microbiology Laboratory, urine specimens were inoculated onto Blood agar and MacConkey agar with 0.01ml calibrated wire loop and incubated at 37°C for 18 hours. Significant bacteriuria is defined as urine culture grew > 105 CFU/ml of urine (CFU= colony forming unit). Culture samples with negative results were excluded.
A total of 210 positive urine cultures were included in the study. Positive urine cultures showing significant bacteriuria were identified by their characteristic appearance on their respective media, and confirmed by the pattern of biochemical reactions using VITEK2 identification. Antimicrobial susceptibility tests were carried out using different antibiotics. Statistical analysis was done using SPSS version 22.0.
Results
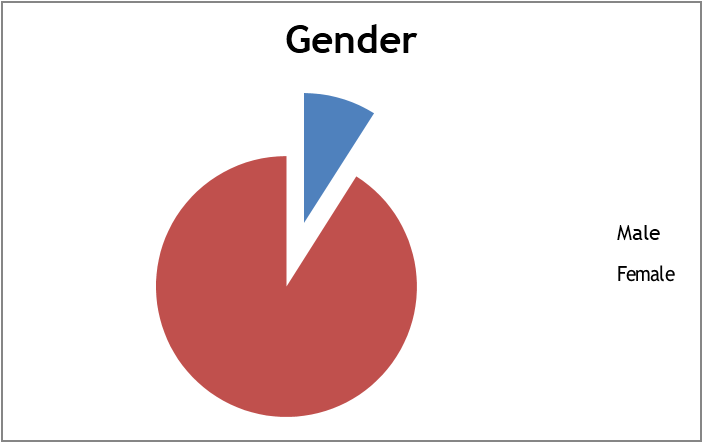
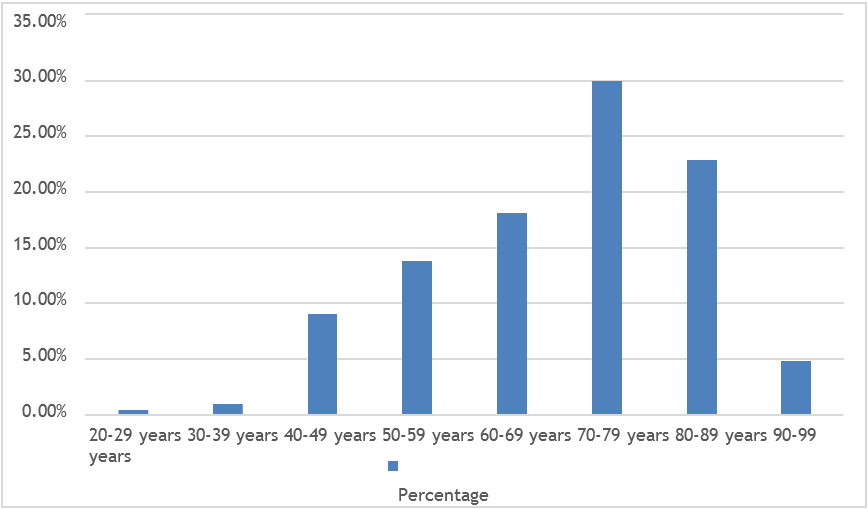
A total of 210 patients showing significant bacteriuria were included in the study. Out of them, 91.00% were females and 9.00% were males (Figure-1). Mean age was 70.32 years ± 14.01 SD (age ranging from 20 to 96 years) (Figure-2).
Escherichia coli was the most frequent organism isolated, accounting for 60.5% (127 cases), followed by Klebsiella pneumoniae 13.8% (29 cases) and Enterococci species 12.9% (27 cases). Pseudomonas species such as Pseudomonas aeruginosa, Pseudomonas stutzeri and Burkholderia cepacia, were positive in 3.5% of cases. Other rare organisms such as Citrobacter froundii, Kocuria kristinaeand Kluyvera intermedia grew in one case each. (Figure-2)
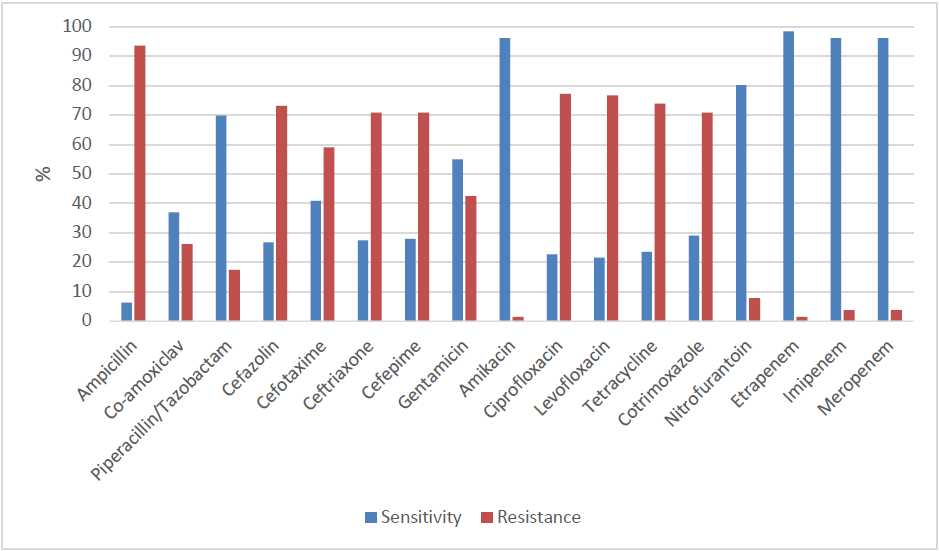
E.colishowed high resistance rates to Ampicillin (93.7%), Ciprofloxacin (77.2%), Levofloxacin (76.8%), Tetracycline (76.4%), Cefazolin (73.2%), Ceftriaxone (72.6%), Cefepime (72.0%), Aztreonam (72.0%), and Co-trimoxazole (70.9%). (Figure-3)
E. Coli was found out to be sensitive to Ertapenem (98.4%), Imipenem (96.1%), Meropenem (96.1%), Amikacin (96.1%) and Nitrofurantoin (80.3%). (Figure-3) Of the remaining antibiotics, Piperacillin+Tazobactam and Co-amoxiclav had relatively low resistance rates with 17.50% and 26.0% respectively.
| Organism | Frequency | Percent |
| E. coli | 127 | 60.5 |
| Klebsiella pneumoniae | 29 | 13.8 |
| Enterococci spp | 27 | 12.9 |
| Proteus mirabilis | 3 | 1.4 |
| Citrobacter froundii | 2 | 1.0 |
| Staphylococcus hominis | 1 | 0.5 |
| Streptococcus agalactiae | 3 | 1.4 |
| Enterobacter cloacae | 1 | 0.5 |
| Serratia marcescens | 2 | 1.0 |
| Klebsiella oxytoca | 1 | 0.5 |
| Staphylococcus agalactiae | 1 | 0.5 |
| Kluyvera intermedia | 1 | 0.5 |
| Moraxella group | 1 | 0.5 |
| Staphylococcus aureus | 3 | 1.4 |
| Pseudomonas aeruginosa | 4 | 1.9 |
| Burkholderia cepaia | 2 | 1.0 |
| Pseudomonas stutzeri | 1 | 0.5 |
| Kcuria Kristinae | 1 | 0.5 |
Table 1: Prevalence of UTI.
Discussion
Urinary tract infections are imposing a substantial financial burden on society. [9,10] UTI in diabetic patients can lead to life-threatening sepsis. Women are at increased risk of UTI than men due to several factors such as shorter urethra, close proximity to anal opening, certain types of birth control (diaphragms), and menopause. [11] In this study, patients who had clinically suspected UTI and showed significant bacteriuria were included, and we found out that there were more women than men.
In our study, the most prevalent organism was E.coli (60.5%). This agrees with the studies in Nepal (64.5%) [12], Bangladesh (61.8%) [13] And in North America (56.9%). [14] The second uropathogen is Klebsiella pneumonia (13.8%), whereas, the third most common organism is Enterococci spp (12.9%). Our study is Comparable to the study in Italy in which E.coli was the most common organism, followed by Klebsiella and Enterococcus. [15]
Extended-spectrum beta lactamase (ESBL) producing bacteria exhibiting resistance to most of the antibiotics, is increasing around the world. [16] This study shows that E.coli is resistant to most of the commonly used antibiotics. Cotrimoxazole is said to be the drug of choice in treating UTI. [17] However, our study shows that E.coli is resistant to Cotrimoxazole (70.9%). It is stated that Quinolones are one of the best therapeutic options for UTI. [18] Nevertheless, in the present study, the resistant rate to Quinolones is high (77%). It can also be seen that there is a high resistance to Cephalosporin groups (70%) in our study. This indicates organisms are ESBL and Amp C producers. [18]
The susceptibility of Nitrofurantoin and Amikacin is satisfactory with sensitivity rates of 80.3% and 96.1% respectively. Carbapenems are the final treatment options for various kinds of infections. [19] E.colihas the highest sensitivity rates to Carbapenems, indicating that there are not many carbapenemase-producing strains in this study.
Myanmar is a developing country. Although UTI is one of the most common causes of morbidity, there is a lack of sufficient data of causal organisms and their antibiotic sensitivity pattern among Myanmar population. Multiple courses of antibiotics introducing to the diabetic patients with the recurrent UTI could be of the main reasons of the appearance of multi-drug resistant organisms. Keeping the emergence of antimicrobial resistant organisms in mind, antibiotic therapy should be thoughtful, rational and prudent. [20] Empirical antibiotic therapy is often given to UTI. However, because of the evolving antibiotic resistance phenomenon, regular monitoring of resistance patterns is crucial to improve guidelines for empirical antibiotic therapy. [21,22] In non-life-threatening cases, antibiotics should be commenced according to the culture and sensitivity results. This would not only reduce the unnecessary use of antibiotics but also halt the dissemination of antibiotic resistant strains. In case of emergency, high potency drugs should be considered, as there is not enough time to wait for the culture and sensitivity results. Since antibiotic resistance is associated with the indiscriminate use of antibiotics which are easily available as over-the-counter drugs in developing countries like Myanmar, this study will be able to highlight the importance of the use of antibiotics, and will be an evidence to exert a stimulus to the stake holders regarding the health care in Myanmar to take further actions. The main limitations of this study were that the previous antibiotics usage, the current treatment of diabetes, the other co morbidities apart from diabetic mellitus, and the immune status of the patients were not taken into account.
Conclusion
Overall, the most prevalent organism is E.coli and it is resistant to various antibiotics including Ampicillin, Cephalosporin’s, Quinolones and Cotrimoxazole. This alarming fact should incite policy makers to formulate an antibiotic policy for the rational use of antibiotics, especially in areas where there is no facility to perform culture and sensitivity. Diabetic patients are at high risk of developing UTI, thus continued surveillance of the resistance rates of uropathogen to different antibiotics is needed to ensure appropriate treatment of UTI.
Conflict of Interest
We declare that there is no conflict of interest.
We declare that there is no conflict of interest.
Acknowledgement
We would like to express our gratitude towards the administrative team of Bahosi Private Hospital, and consultant microbiologists, medical technicians, and the staffs at the microbiology laboratory for their sincere support for the research project. We also thank to the patients who volunteered for the project.
We would like to express our gratitude towards the administrative team of Bahosi Private Hospital, and consultant microbiologists, medical technicians, and the staffs at the microbiology laboratory for their sincere support for the research project. We also thank to the patients who volunteered for the project.
References
- WHO. Global report on diabetes. World Health Organization, Geneva (2016).
- Latt., et al. “Report on National Survey of Diabetes Mellitus and Risk Factors for Non-Communicable Diseases in Myanmar 2014”. Ministry of Health (2014).
- Saleem M and Daniel B. “Prevalence of Urinary Tract Infection among Patients with Diabetes in Bangalore City”. International Journal of Emerging Sciences 1.2 (2011): 133-142.
- Sahib AKY. “Study of ciprofloxacin resistant Escherichia coli (CREC) in type 2 diabetic patients with symptomatic urinary tract infections”. Iraqi journal of community medicine 21.1 (2008): 58-63.
- Bonadio ., et al. “The influence of diabetes mellitus on the spectrum of uropathogens and the antimicrobial resistance in elderly adult patients with urinary tract infection”. BMC Infectious Diseases 6 (2006): 6-54.
- El Kholy A., et al. “Antimicrobial resistance in Cairo, Egypt 1999-2000: a survey of five hospitals”. Journal of Antimicrobial Chemotherapy51.3 (2003): 625-630.
- Kumar Y., et al. “Antibiogram and characterization of resistance markers among E. coli Isolates from urinary tract infections”. Journal of Infection in Developing Countries7.7 (2013): 513-519.
- Yismaw G., et al “Urinary Tract Infection: Bacterial etiologies, drug resistance profile and associated risk factors in diabetic patients attending Gondar University Hospital, Gondar, Ethiopia”. European Journal of Experimental Biology 2.4 (2012): 889- 898.
- European Association of Urology. Guidelines on Urological Infections, March (2015): 6.
- Ipe DS., et al. “Asymptomatic bacteriuria: prevalence rates of causal microorganisms, etiology of infection in different patient populations, and recent advances in molecular detection”. FEMS Microbiology Letters 346.1(2013): 1-10.
- Mayo clinic. July 23, 2015. Urinary Tract Infection (UTI).
- Acharya D., et al. “Diabetes mellitus and Urinary Tract Infection: Spectrum of Uropathogens and their Antibiotic Sensitivity Pattern”. Journal of Manmohan Memorial Institute of Health Sciences1.4 (2015): 24-28.
- Saber MH., et al. “The Pattern of Organism Causing Urinary Tract Infection in Diabetic and Non Diabetic Patients in Bangladesh”. Bangladesh Journal of Medical Microbiology 4.1 (2010): 6-8.
- Zhanel GG Hisanaga TL., et al. “Antibiotic resistance in outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Aliance (NAUTICA)”. International journal of antimicrobial agents 26.5 (2005): 380-388.
- Magliano E., et al. “Gender and Age-Dependent Etiology of Community- Acquired Urinary Tract Infections”. The Scientific World Journal 2012 (2012).
- Oteo J., et al. “Extended-spectrum [beta]-lactamase producing Escherichia coli: changing epidemiology and clinical impact”. Current Opinion in Infectious Diseases 23.4 (2010): 320-326.
- Longmore M., et al. Oxford Handbook of Clinical Medicine. 9th Edition, New York. Oxford University Press Inc (2014).
- Pragash DS., et al. “Study of Uropathogens among Type II Diabetic Patients and Their Antimicrobial Resistance Pattern among Rural South Indian Population”. Scholars Journal of Applied Medical Sciences 2.2 (2014): 589-591.
- James AK., et al. “Trends in Antimicrobial Resistance among Urinary Tract Infection Isolates of Escherichia coli from Female Outpatients in the United States”. Antimicrobial Agents Chemotherapy 46.8 (2002): 2540-2545.
- Abbo LM and Hooton TM. “Antimicrobial Stewardship and Urinary Tract Infections”. Antibiotics3.2 (2014): 174-192.
- National Committee for Clinical Laboratory Standards Performance standards for antimicrobial disc susceptibility tests. 7th ed. M2-A7. Wayne, Pennsylvania, USA: NCCLS (2000).
- Grude N., et al. “Urinary tract infections in Norway: bacterial etiology and susceptibility, a restrospectiv study of clinical isolates”. Clinical Microbiology and Infection7.10 (2001): 543-547.
Citation:
Than Than Aye., et al. “Urinary Tract Infection and Pattern of Antibiotic Sensitivity in Hospitalized Patients with Diabetes
Mellitus in Myanmar”. Archives of Endocrinology and Diabetes Care 2.1 (2018): 147-153.
Copyright: © 2018 Than Than Aye., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.