Case Report
Volume 2 Issue 3 - 2018
A Rare Case of Aicardi Syndrome in a Female African
1Department of Neurosurgery, University of Zimbabwe, Zimbabwe
2Department of Medicine, University of Zimbabwe, Zimbabwe
3Department of Pediatrics, University of Zimbabwe, Zimbabwe
2Department of Medicine, University of Zimbabwe, Zimbabwe
3Department of Pediatrics, University of Zimbabwe, Zimbabwe
*Corresponding Author: Serge Eddy Mba, University of Zimbabwe, Department of Neurosurgery, Harare, Zimbabwe.
Received: March 21, 2018; Published: April 04, 2018
Abstract
Aicardi syndrome is a rare genetic disorder that occurs in the pediatric population; the diagnosis is often made by the presence of corpus callosum agenesis/dysgenesis on MRI or CT scan, chorioretinal lacunae on fundoscopy and the presence of seizures. The pathogenesis of the disease remains incompletely elucidated. We report a case of Aicardi syndrome diagnosed in a 7 month old female African child who presented with chronic protracted seizures for 5 months. The case highlights the importance of clinical acumen and the high index of suspicion that the clinician should bear in order to inform his diagnosis and provide adequate and comprehensive management.
Keywords: Aicardi; Chorioretinal lacunae; Corpus callosum agenesis; Infantile spasm
Abbreviations: EEG: Electro-Encephalogram); MRI: Magnetic Resonance Imaging; CT scan: Computed Tomography; TAED1: Transcriptional Enhancer factor-1
Introduction
Aicardi syndrome is a rare X-linked cerebro-retinal genetic disorder affecting mainly children. The disease was initially described by Krause in 1946 [1] but the term Aicardi syndrome was latter coined by the French neurologist and epileptologist Jean Aicardi in 1965. Patient often first present to the ophthalmologist with various degree of visual deficit on the background of repeated seizures. To the author’s knowledge, to date, this is the first case reported in Africa in more than half a century.
Case Report
We report a case of a 7 month old female patient from Harare, Zimbabwe, who presented with a 5 month history of seizures and poor vision. This was a previously well child with no remarkable perinatal history, born at term through a normal vertex delivery at a local hospital. No history of maternal infection was recorded and the child’s immunization was up to date.
She was being breastfed and was gaining weight appropriately when suddenly at the age of two months she started developing focalized repetitive jerky movements of a tonic-clonic nature that seemed unprovoked, affecting predominantly the left upper and lower limbs. Seizures would last ten to fifteen seconds at time and she would have more than twenty such episodes in a day.
Mother also noticed that the child was no longer fixating nor following objects; as the condition deteriorated, patient was taken to a pediatrician who managed the patient on sodium valproate and ordered an Electro-Encephalogram and a MRI scan of the brain. On examination, the child was fully conscious, haemo dynamically stable with no dysmorphic features, normal cardiovascular, respiratory and abdominal examination.
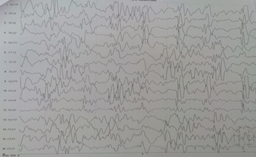
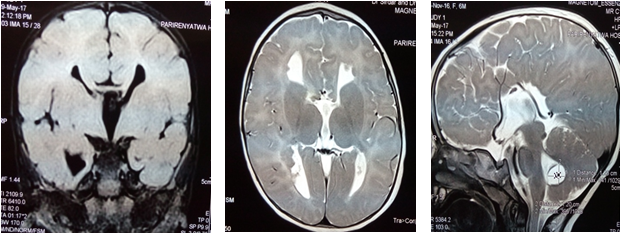
On cranial nerve examination, the child was unable to visually tract objects either through the horizontal or vertical plane, but all other cranial nerves were intact. EEG showed high voltage epileptiform waves suggestive of infantile spasm. An MRI of the brain showed complete agenesis of the corpus callosum, absence of the septum pellucidum as well as a posterior fossa Dandy-Walker cyst.
Upon referral to the neurosurgical team, a fundoscopy was done that showed retinal lacunae in both retinas and a diagnosis of Aicardi syndrome was made based on the presence of –seizures, - corpus callosum agenesis and – presence of retinal lacunae. Seizures were controlled on one single medication, phenobarbitone at 4 mg/kg/day. Mother was provided counselling and child was discharged from hospital after 10 days. On review in the outpatient department, the seizures had become very infrequent.
Discussion
Aicardi syndrome has a worldwide age-adjusted prevalence of 0.63 per 100.000 females [2]. It affects mainly children but the age range varies from 1 month to 42 years. The incidence rate per live birth for the Unites States and the Netherlands were 1 per 105 000 and 1 per 93 000, respectively [3]. It affects mainly females although scanty cases have been reported in males with Klinefelter syndrome [4].
The exact pathomechanism of the disease remains unknown. Many authors postulate the theory that the disease originates from de novo X chromosome mutation [5], this is despite the fact that other studies have failed to identify any pathogenic variants on chromosome X associated with the disease [6]. The TEAD1 1 mutation may play a role in the development of chorioretinal complications associated with Aicardi syndrome [6].
The diagnosis of Aicardi syndrome is a clinical diagnosis comprising of a triad of: Infantile spasms, chorioretinal lacunaes and corpus callosum agenesis/dysgenesis [3]. Infantile spasms are the most characteristic types of seizures in Aicardi syndrome, and are usually the main presenting symptom. Children with Aicardi tend to have earlier onset seizures and have highest seizure frequency with more than 93% having more than one seizure a day and about 50% having more than 10 seizures a day [7].
The early onset of seizures could be due to the fact that the final shape and structure of the corpus callosum is completed by 20 weeks post conception and absence of the said corpus callosum is linked to early repeated seizures [8]. Developmental corpus callosum dysgenesis or agenesis is not only linked to the seizures previously mentioned but also form an important diagnostic feature in Aicardi syndrome [9]; it is thought to interfere with the overall efficiency of auditory and visual learning and memory, to the effect that children with corpus callosum agenesis will present with impairment in verbal performance [10].
The hallmark finding that complete the triad is chorioretinal lacunae which are described as well circumscribed full thickness defects limited to the pigment epithelium and choroid of the eye [11]. These lacunae are responsible for the decrease or absent visual acuity associated with Aicardi syndrome.
The clinician has at his disposal therefore a variety of tools:
- EEG (Figure 1) that show characteristic infantile spasm wave patterns; typically the EEG may show some repetitive high voltage epileptiform synchronous and symmetrical poly spikes, sharp discharge followed by relative flattening hypoarrythmia of infantile spasm
- MRI scan is the radiologic investigation of choice (Figure 2) and shows the absence of the septum pellucidum, absence of the genu and splenium of the corpus callosum; at the posterior fossa, there is also a cyst at the inferior aspect of the fourth ventricle akin to a Dandy Walker cyst. The Ammon’s horn of the hippocampus is incompletely formed.
- Fundoscopy is an important investigation as it demonstrates the chorioretinal lacunae (Figure 3) with clarity and help assess visual pathway.
Management of Aicardi syndrome is mainly symptomatic and seeks to address seizure control. Medical management consists of antiseizure medication either as monotherapy or a combination of various classes of medication [3]. Surgical management is usually reserved for refractory cases and is often palliative in nature; it takes the form of either corpus callosotomy or vagus nerve stimulator implantation [12] or a combination thereof [7]. Despite those advances, children are rarely if ever seizure free [13]. The prognosis of Aicardi syndrome is generally poor and genetic counselling is almost always required [14]
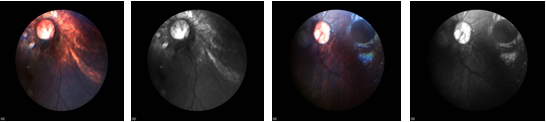
Figure 3: Right eye: multiple round chorioretinal lacunae in the posterior pole, temporal to the disc
Left eye: optic disc pallor, fewer lacunaes Normal anterior segment in both eyes.
Left eye: optic disc pallor, fewer lacunaes Normal anterior segment in both eyes.
Conclusion
Aicardi syndrome is a rare genetic disorder with a complex etiology. To make a diagnosis requires a high index of suspicion and available diagnostic tools. Although difficult to treat, the debilitating spasms can be managed either medically with anti-seizure medication or surgically with corpus callosotomy when required. Despite these advances, a great deal of research need to be done to fully elucidate and shed further light on this complex condition.
Acknowledgements
We acknowledge the contribution of Saiko Mangombe who assisted the authors by taking high quality fundoscopy photographs of our patient.
We acknowledge the contribution of Saiko Mangombe who assisted the authors by taking high quality fundoscopy photographs of our patient.
Conflict of interest
The authors declare no conflict.
The authors declare no conflict.
References
- Krause AC. “Congenital encephalo-ophthalmic dysplasia”. Archives of Ophthalmology 36.4 (1946): 387-444.
- Kroner BL., et al. “New incidence, prevalence and survival of Aicardi syndrome from 408 cases”. Journal of Child Neurology 23.5 (2008): 531-535.
- Lund C., et al. “Aicardi Syndrome: An Epidemiologic and Clinical Study in Norway”. Pediatric Neurology 52.2 (2015): 182-186.
- Shetty J., et al. “Aicardi syndrome in a 47 XXY male e A variable developmental phenotype” European Journal of Pediatric Neurology 18.4 (2014): 529–531.
- Fruhman G., et al. “Ophthalmologic findings in Aicardi syndrome”. JAAPOS 16 (2012): 238-241.
- Bibiana k., et al. “Independent variant analysis of TEAD1 and OCEL1 in 38 Aicardi syndrome patients”. Molecular Genetics & Genomic Medicine 5.2 (2017): 117–121.
- Govil-Dalela T., et al. “Agenesis of the Corpus Callosum and Aicardi Syndrome: a neuroimaging and clinical comparison”. Pediatric Neurology 68 (2017): 44–48.
- Raybaud C. “The corpus callosum, the other great forebrain commissures, and the septum pellucidum: anatomy, development, and malformation”. Neuroradiology 52 (2010): 447-477.
- Paul KL., et al. “Learning and memory in individuals with agenesis of the corpus callosum”. Neuropsychologia 86 (2016): 183–192.
- Hinkley BNL., et al. “The contribution of the corpus callosum to language lateralisation”. The Journal of Neuroscience 36.16 (2016): 4522– 4533.
- Chappaz A., et al. “Iris cyst in a child with Aicardi syndrome: a novel association”. Journal of AAPOS20.5 (2016): 451–452.
- Kasasbeh SA., et al. “Palliative epilepsy surgery in Aicardi syndrome: A case series and review of literature”. Child's Nervous System 30.3 (2013): 497-503
- Guillamón E., et al. “Combination of corpus callosotomy and vagus nerve stimulation in the treatment of refractory epilepsy”. European Neurology 71.1-2 (2014): 65-74.
- Laguado-Herrera YV., et al. “[Aicardi syndrome with Dandy-Walker type malformation]”. Revista De Neurologia 61.2 (2015): 71-74.
Citation:
Serge Eddy Mba., et al. “A Rare Case of Aicardi Syndrome in a Female African”. Current Opinions in Neurological Science 2.3
(2018): 458-462.
Copyright: © 2018 Serge Eddy Mba., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.