Review Article
Volume 2 Issue 2 - 2018
Alzheimer's Disease: A Review
International Institute of Medicine and Science, California, USA
*Corresponding Author: Alain L Fymat, International Institute of Medicine and Science, California, USA.
Received: February 15, 2018; Published: March 03, 2018
Abstract
Over the past few decades, Alzheimer’s disease (AD), once considered a rare disorder, has emerged from obscurity to become a major public health problem impacting millions of older Americans and their families. The National Institute on Aging (NIA), the lead agency for AD research at the National Institutes of Health (NIH), launched its AD program in 1978, and since then, the study of this disease has become one of NIA’s top priorities. Several other NIH institutes also conduct and sponsor studies on AD. Other research organizations around the world, and many private-sector research, education, and advocacy groups contribute to these research efforts so that the study of AD is moving ahead rapidly. To better appreciate such efforts, I will initially provide a brief history of the major landmarks of the disease, and its precursors (subjective and mild cognitive impairments).
According to current belief, AD is caused by a disease that affects the brain. It is an irreversible, progressive brain disease that slowly destroys memory and thinking skills, eventually even the ability to carry out the simplest tasks. In most people with AD, symptoms first appear after age 60. In the absence of disease, the human brain often can function well into the 10th decade of life. After a brief survey of the healthy brain, the stages in the course of AD will be described. The various hypotheses (or theories) advanced for explaining the cause of the disease will then be elaborated upon,. The neuropathology and biochemistry of AD will then be briefly discussed in an attempt to unravel the mechanism of AD. The approach to the diagnosis of AD will be detailed as well as preventive methods, disease management and prognosis. Lastly, current research efforts and their treatment potential will be outlined.
Keywords: Agnosia; Alzheimer disease; Anosognosia; Apraxia; Cognitive impairment; Paraphasia; Protheopathy
Abbreviations: AA: Alzheimer's Association; Aβ: Amyloid Beta; ABP: Amyloid Beta Protein; ACE: Acetyl Cholin Esterase; Ach: Acetylcholine; AD: Alzheimer's Disease; ADDL: Amyloid-Derived Diffusible Ligands; ADRDA: AD and Related Disorders Association; AP: Amyloid Plaque; APA: American Psychiatric Association; ApoE: Apolipoprotein E; APP: Amyloid Precursor Protein; BAP: Beta Amyloid Peptide; BBB: Blood Brain Barrier; BDNF: Brain-Derived NF; CE: Choline Esterase; CIH: Calcium Ion Homeostasis; CSF: Cerebro-Spinal Fluid; CT: Computed Tomography; DHA: Docosa Hexaenoic acid (DA); DR: Death Receptor; DSM-MD: Diagnostic & Statistical Manual of Mental Disorders; EEG: Electro Encephalo- Graphy; EOFAD: Early Onset Familial AD; FAST: Functional Assessment Staging; FD: Frontotemporal Dementia; FDA: (U.S.) Food & Drug Administration; GWAS: Genome Wide Association Studies; HRT: Hormone Replacement Therapy; LBD: Lewy Body Dementia; LOSAD: Late Onset Sporadic AD; MAP: Microtubule-Associated Protein; MCI: Mild Cognitive Impairment; MMSE: Mini-Mental State Examination; MRI: Magnetic Resonance Imaging; NICE: (U.S.) National Institute for Health and Care Excellence; NINCDS: (U.S.) National Institute of Neurological and Communicative Disorders and Stroke; NF: Neurotropic Factors; NFT: Neuro Fibrillary Tangles; NSAID: Non-Steroidal Anti-Inflammatory Drug; PCD: Programmed Cell Death; PET: Positron Emission Tomography (to measure blood flow and glucose metabolism throughout the brain, producing a “map” of the active brain); PIB: Pittsburg Compound B (a PET compound); SCI: Subjective Cognitive Impairment; SP: Senile Plaques; SPECT: Single Photon Emission CT: VD: Vascular Dementia; WHO: World Health Organization
Disorders mentioned: Agnosia; Alzheimer's disease; Anosognosia; Apraxia; Bradycardia; Creutzfeldt-Jacobs disease; Mild cognitive impairment; Paraphasia; Pneumonia; Protheopathy (a protein misfolding disease).
Drugs listed: Acetylcholinesterase inhibitors (tacrine, rivastigmine, galantamine and donepezil); Apomorphine; Bapineuzumab (an antibody designed as identical to the naturally induced anti-amyloid antibody); Dimebon (an antihistamine); Donepezil (Aricept); Galantamine (Rasadyne); NMDA receptor agonist (Memantine); Huzerpine A (available over the counter); Memantine (Namenda); Methylthioninium chloride; Non-steroidal anti-inflammatory drugs; Rivastigmine (Exelon); Statins (cholesterol-lowering drugs).
Brief History
Ancient times: Old age was associated with dementia, a broad term referring to a decline in cognitive function to the extent that it interferes with daily life and activities.
1901, German psychiatrist, Dr Alois Alzheimer, identified the first case of what became known as Alzheimer's disease (AD) in a fifty-year-old woman he called Auguste D whom he followed until 1906 when she died. He then reported publicly on the case (1-3).
1901-1906: Eleven cases similar to the one reported by Alzheimer were reported in the medical literature [4].
1907: After suppressing some of the clinical (delusions and hallucinations) and pathological (arteriosclerotic changes) features contained in the original report of Auguste D, Emil Kraepelin described August D.'s disease as a distinctive disease [5]. He also named AD as presenile dementia(a subtype of senile dementia).
1907-1977: AD's diagnosis was reserved for individuals between the ages of 45 and 65 who developed symptoms of dementia.
1977: AD is diagnosed independent of age when patients present with a characteristic common symptom pattern, disease course, and neuropathology.
1984: The (U.S.) National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer's disease and Related Disorders Association (ADRDA, now known as the Alzheimer's Association, AA) issued Alzheimer's diagnostic criteria.
1991: Amyloid hypothesis was proposed.
2000: Prevalence of AD in the U.S. was estimated to be 1.6% both overall and in the 65–74 age group, with the rate increasing to 19% in the 75–84 group and to 42% in the greater than 84 group. Prevalence rates in less developed regions are lower.
2000-2010: Only one of the 244 experimental Alzheimer's drugs tested, only one (memantine) was approved by the (U.S.) Food & Drug Administration (FDA).
2003: The FDA approved the drug memantine.
2005: The World Health Organization (WHO) estimated that 0.379% of people worldwide had dementia, and that the prevalence would increase to 0.441% in 2015 and to 0.556% in 2030.
2006: Another study estimated that 0.40% of the world population (range 0.17–0.89%; absolute number 26.6 million, range 11.4–59.4 million) were afflicted by AD, and that the prevalence rate would triple and the absolute number would quadruple by 2050.
2007: The NINCDS-ADRDA diagnostic criteria are extensively updated.
2009: A new disease theory suggests that a close relative of the β-amyloid protein, and not necessarily the Ρ-amyloid itself, may be a major culprit in the disease.
2015: Approximately 29.8 million people worldwide have been diagnosed with AD.
The Healthy Brain
To understand AD, it is important to know a bit about the brain, how it works, and what happens during aging. The brain is a remarkable organ that allows us to carry out every element of our daily lives, manages many body functions, and directs all the functions we carry out consciously because of the complicated mix of chemical and electrical processes that take place in our brains.
The brain is made of about 100 billion nerve cells (called neurons) separated by about 100 trillion synapses, and several other cell types such as glial cells that help neurons survive and function. Glial cells hold neurons in place, provide them with nutrients, rid the brain of damaged cells and other cellular debris, and provide insulation to neurons in the brain and spinal cord. In fact, the brain has many more (~ 10 times more) glial cells than neurons. Another essential feature of the brain is its enormous network of blood vessels, receiving 20% of the body’s blood supply even though it is only about 2% of the body’s weight, Also, ~ 400 billion tiny blood vessels, or capillaries , carry oxygen, glucose (the brain’s principal source of energy), nutrients, and hormones to brain cells so they can do their work, and also carry away waste products.
The brain has many component parts, each of which is responsible for particular functions:
- The cerebral hemispheres: They account for ~ 85% of the brain's weight. The neurons in them are connected by the corpus callosum (thick bundles of nerve cell fibers). The left hemisphere appears to focus on details whereas the right hemisphere focuses on broad background. The cerebral cortex is the outer layer of these hemispheres, controlling voluntary movement, and regulating cognitive functions. The hemispheres have four lobes, each of which having different roles: the frontal lobe (for executive functions like thinking, organizing, planning, and problem solving, as well as memory, attention, and movement); the parietal lobe sitting behind the frontal lobe (for perception and integration of stimuli from the senses); the occipital lobe at the back of the brain (for vision); and the temporal lobe running along the side of the brain under the frontal and parietal lobes (for senses of smell, taste, and sound, and the formation and storage of memories).
- The cerebellum: Located beneath the occipital lobe and having also two hemispheres. It plays roles in balance and coordination, and motor learning.
- The brain stem: Located at the base of the brain, it connects the spinal cord with the rest of the brain. Its functions are crucial to survival (heart rate, blood pressure, breathing, sleep and dreaming).
- The limbic system: Lying deep inside the cerebral hemispheres, it links the brain stem with the higher reasoning elements of the cerebral cortex. It plays a key role in memory, emotion, and instinctive behaviors. It includes: the amygdala(for strong emotions such as fear), the hippocampus (for learning and short-term memories and their conversion into long-term memories for storage in other brain areas; the thalamus (for sensory and limbic information); and the hypothalamus(for monitoring activities such as body temperature, food intake, and the body’s internal clock).
The Several Types of Dementia
Dementia is a global cognitive decline in which many mental abilities are lost. Memory loss is often one of the earlier symptoms, which typically include difficulty with reading, writing, speaking, following a conversation, reasoning, calculating, organizing, and planning. There are many causes of dementia, including vascular dementia, frontotemporal dementia, Lewy body dementia, and others, but AD is the most common (see Table 1)
| Type | Cause(s) or Mark(s) | Symptoms |
| Vascular dementia (VD) (may overlap with AD) |
Reduced blood flow to the brain | Multiple small strokes |
| Frontotemporal dementia (FTD) (much less common than AD) |
Changes in behavior, memory problems, difficulty speaking | |
| Lewy body dementia (LBD) | Hallucinations, delusions, increased sleeping, REM behavioral disturbance, etc. | |
| Alzheimer dementia (AD) | Amyloid beta plaques and neurofibrillary tangles (?) | Memory loss, severe cognitive deficits |
Table 1: Some of the various forms of dementia including their cause(s) or mark(s) and their symptoms.
Introduction to Alzheimer's disease
Precursors
Two types of sequential impairments are precursors of AD;
Subjective cognitive impairment
Subjective cognitive impairment (SCI)is a worsening condition that is noticeable to the individual but, in standard neuropsychological testing, still falls in the normal range. Brain MRI may show some shrinkage. It may last a decade or two before the impairment progresses to the next stage.
Subjective cognitive impairment (SCI)is a worsening condition that is noticeable to the individual but, in standard neuropsychological testing, still falls in the normal range. Brain MRI may show some shrinkage. It may last a decade or two before the impairment progresses to the next stage.
Mild cognitive impairment
Mild cognitive impairment (MCI) typically follows SCI. Neuropsychological tests show that memory, organizing, speaking, calculating, planning, or other cognitive abilities are abnormal (even though the individual may still be able to perform daily activities). MCI does not inevitably progress to AD but, in many people, especially those in whom there is memory loss, AD will follow within a few years.
Mild cognitive impairment (MCI) typically follows SCI. Neuropsychological tests show that memory, organizing, speaking, calculating, planning, or other cognitive abilities are abnormal (even though the individual may still be able to perform daily activities). MCI does not inevitably progress to AD but, in many people, especially those in whom there is memory loss, AD will follow within a few years.
Alzheimer's disease
Starting slowly and worsening over time, Alzheimer's disease (AD) is a chronic neurodegenerative disease responsible for 60-70% of cases of dementia (a broad term referring to a decline in cognitive function to the extent that it interferes with daily life and activities). Of the ten most common causes of death in the U.S., AD is the only one for which there is no effective treatment. The most common early symptom is short-term memory loss progressing to language problems, disorientation, mood swings, loss of motivation, not managing self-care, and behavioral issues followed by withdrawal from family and society. Gradually, bodily functions are lost, ultimately leading to death. Although the speed of progression of the disease can vary, the average life expectancy following diagnosis is three to nine years.
The cause of AD is poorly understood with ~ 70% of the risk believed to be genetic (many genes are involved) and the remaining risk factors including a history of head injuries, depression, or hypertension. The disease process is associated with two formations: amyloid plaques and neurofibrillary tangles in the brain. A probable diagnosis is based on the history (including the family history) of the illness and cognitive technique aided with medical imaging and blood tests to rule out other possible causes. Initial symptoms are often (but erroneously) mistaken for normal aging. Pathological examination of brain tissue would be needed for a definite diagnosis but this is not possible for live patients. Mental and physical exercise and avoiding obesity may seem to decrease the risk of AD; however, evidence to support these recommendations is weak. There are no medications or supplements that decrease risk. Exercise programs may be beneficial with respect to daily living activities, thus potentially improving outcomes. Antipsychotics are common but not usually recommended for the treatment of behavioral problems or psychosis.
No treatments stop or reverse the progression of AD, though some may temporarily improve symptoms. However, some researchers have recently claimed that they have developed a program to prevent and reverse the cognitive decline of dementia [6]. Indeed, there is no treatment for preventing patients with subjective cognitive impairment (SCI) or mild cognitive impairment (MCI) from developing full-blown AD.
Stages in the Course of Alzheimer's disease
There is a progressive pattern of cognitive and functional impairment:
Pre-dementia
The first symptoms are often mistakenly attributed to aging or stress:
The first symptoms are often mistakenly attributed to aging or stress:
- Mild cognitive difficulties (evidenced by detailed neuropsychological testing up to eight years before the clinical diagnosis criteria), particularly short term memory loss;
- Subtle problems with executive functions (attentiveness, planning, flexibility, abstract reasoning);
- Impairments in semantic memory (memory of meanings, concept relationships);
- Apathy (a most persistent neuropsychiatric symptom throughout the course of the disease;
- Depressive symptoms, irritability and reduced awareness of subtle memory difficulties are also common.
Mild Cognitive Impairment (MCI)
Mild cognitive impairment (MCI) is a preclinical transitional stage of the disease between normal aging and dementia. It can present with a variety of symptoms and, when memory loss is the predominant symptom, it is termed "amnestic MCI" and is frequently seen as a prodromal stage (an early or premonitory symptom) of the disease.
Mild cognitive impairment (MCI) is a preclinical transitional stage of the disease between normal aging and dementia. It can present with a variety of symptoms and, when memory loss is the predominant symptom, it is termed "amnestic MCI" and is frequently seen as a prodromal stage (an early or premonitory symptom) of the disease.
Early disease
This stage is characterized by the increasing impairment or difficulties of:
This stage is characterized by the increasing impairment or difficulties of:
- Learning and memory;
- Language (shrinking vocabulary; decreased word fluency; impoverishment of oral and written language);
- Executive functions;
- Agnosia (perception);
- Apraxia (execution of movements; difficulties in writing, drawing, dressing, coordination, planning) that may be more prominent than memory problems.
It is important to note that AD does not affect all memory capacities equally among all those affected in:
- Episodic memory (older memories of the person);
- Semantic memory (facts learned);
- Implicit memory (how to do things, such as using a fork to eat or how to drink from a glass).
As the disease progresses, people with AD can often continue to perform many tasks independently, but may need assistance or supervision with the most cognitively demanding activities.
Moderate disease
This stage is characterized by:
This stage is characterized by:
- Progressive deterioration eventually hindering independence;
- Inability to perform most common activities of daily living;
- Paraphasia (speech difficulties due to an inability to recall vocabulary, incorrect word substitutions);
- Reading and writing skills are progressively lost;
- Complex motor sequences become less coordinated (so the risk of falling increases);
- Worsened memory problems (failure to recognize close relatives);
- Impaired long term memory, which was previously intact;
- Behavioral and neuropsychiatric changes become more prevalent.
Common manifestations include some or all of the following:
- Wandering;
- Irritability;
- Lability;
- Crying, outbursts of unpremeditated aggression;
- Resistance to caregiving;
- Sundowning;
- Illusionary misidentifications;
- Delusions;
- Anosognosia (lost insight of the disease process and limitations); and
- Urinary incontinence. Urinary incontinence can develop.
Advanced disease
The final stages of the disease are characterized by:
The final stages of the disease are characterized by:
- Complete dependence of the patient upon caregivers;
- Language reduction to simple phrases or even single words, eventually leading to complete loss of speech. However, despite the loss of verbal language abilities, people can often understand and return emotional signals;
- Although aggressiveness can still be present, extreme apathy and exhaustion are much more common;
- Ultimately, inability to perform even the simplest tasks independently;
- Bedridden and inability to feed oneself.
The cause of death is usually an external factor (infection of pressure ulcers, pneumonia, etc.), not the disease itself.
The New York University Medical Center's Aging and Dementia Research Centers have also developed a slightly different staging scale, the Functional Assessment Staging (FAST) scale for AD (see Figure 3).
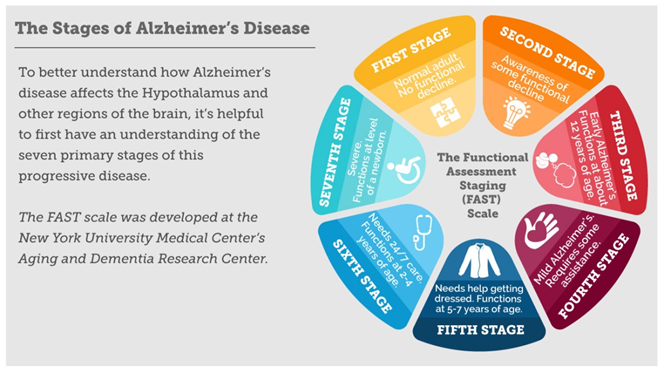
Figure 3: The FAST scale describing the stages of Alzheimer’s disease as developed
at the New York University Medical Center’s Aging and Dementia Research Centers.
Hypotheses for the Cause(s) of the Disease
Several competing hypotheses try to explain the cause of the disease, some of which may be responsible either singly or in combination:
Genetic modification
Based on twin and family studies, the genetic heritability of AD (and its memory components) is about 49-79%. It includes two varieties:
Based on twin and family studies, the genetic heritability of AD (and its memory components) is about 49-79%. It includes two varieties:
Early onset familial disease
Early onset familial AD (EOFAD): Around 0.1% of the cases are familial forms of autosomal (not sex-linked) dominant inheritance [7-8] with an onset before age 65. Most of autosomal dominant familial AD can be attributed to mutations in one of three genes: (1) those encoding amyloid precursor protein (APP), (2) presenilin 1 and (3) presinilin 2. Here, some of the mutations increase the production of a small protein called Aβ42 (the main component of senile plaques) while others merely alter the ratio Aβ42/ Aβ40 (the other major form) without increasing Aβ42 levels.
Early onset familial AD (EOFAD): Around 0.1% of the cases are familial forms of autosomal (not sex-linked) dominant inheritance [7-8] with an onset before age 65. Most of autosomal dominant familial AD can be attributed to mutations in one of three genes: (1) those encoding amyloid precursor protein (APP), (2) presenilin 1 and (3) presinilin 2. Here, some of the mutations increase the production of a small protein called Aβ42 (the main component of senile plaques) while others merely alter the ratio Aβ42/ Aβ40 (the other major form) without increasing Aβ42 levels.
Late onset sporadic disease
Late onset sporadic AD (LOSAD): This is the majority of cases of AD. Here, both environmental effects and genetic modifiers may act as risk factors. The best known genetic risk factor is the inheritance of the allele ε4 of the Apo lipoprotein E (or ApoE ε4). Between 40-80% of people with AD possess at least one ApoE ε4 allele. ApoE ε4 increases the risk of the disease by three times in heterozygotes and by 15 times in homozygotes [9]. This is the strongest known genetic risk factor. More recent genome-wide association studies (GWAS) have found 21 areas in genes that appear to affect the risk, including: ABCA7, BIN1, CASS4, CD2AP, CELF1, CLU, CR1, DRB5, EPHA1, FERMT2, HLA, INPP5D, MEF2C, MS4A, NME8, PICALM, PTK2B, SIC24A4, SORL1, TREM2, and ZCWPW1 [10-11].
Late onset sporadic AD (LOSAD): This is the majority of cases of AD. Here, both environmental effects and genetic modifiers may act as risk factors. The best known genetic risk factor is the inheritance of the allele ε4 of the Apo lipoprotein E (or ApoE ε4). Between 40-80% of people with AD possess at least one ApoE ε4 allele. ApoE ε4 increases the risk of the disease by three times in heterozygotes and by 15 times in homozygotes [9]. This is the strongest known genetic risk factor. More recent genome-wide association studies (GWAS) have found 21 areas in genes that appear to affect the risk, including: ABCA7, BIN1, CASS4, CD2AP, CELF1, CLU, CR1, DRB5, EPHA1, FERMT2, HLA, INPP5D, MEF2C, MS4A, NME8, PICALM, PTK2B, SIC24A4, SORL1, TREM2, and ZCWPW1 [10-11].
Mutations in the TEM2 gene have been associated with a 3-5 times higher risk of developing AD. A suggested mechanism of action is that when TREM2 is mutated, white blood cells in the brain are no longer able to control the amount of beta amyloid present [12].
Related to the genetic hypothesis is the “neurodevelopment hypothesis.” or “retrogenesis hypothesis”, it posits that as the fetus grows through a process of neurodevelopment beginning with neurulation and ending with myelination, the brains of people with AD go through a reverse neurodegeneration process starting with demyelination and death of axons (white matter) and ending with the death of grey matter. Likewise, as infants go through stages of cognitive development, the reverse process of progressive cognitive impairment occurs in adults [13].
Amyloid cascade hypothesis
The amyloid hypothesis postulates that extracellular amyloid beta (Aβ) deposits are the fundamental cause of the disease (14). There are four supports for this postulate: (1) The gene for APP is located on chromosome 21; (2) people with trisomy 21 (Down syndrome) who have an extra gene copy almost universally exhibit at least the earliest symptoms of AD by 40 years of age; and (3) a specific isoform of ApoE4 is a major genetic risk factor for AD. While apolipoproteins enhance the breakdown of beta amyloid, some isoforms (such as ApoE4) are not very effective at this task, leading to excess amyloid buildup in the brain; and (4) transgenic mice that express a mutant form of the human APP gene develop fibrillary amyloid plaques and Alzheimer's-like brain pathology with spatial learning deficits. On the basis of laboratory and animal studies, various compounds were designed to test the hypothesis on humans. Unfortunately, while these compounds performed as predicted, the AD patients on whom they were tested either got not better or incredibly got worse!
The amyloid hypothesis postulates that extracellular amyloid beta (Aβ) deposits are the fundamental cause of the disease (14). There are four supports for this postulate: (1) The gene for APP is located on chromosome 21; (2) people with trisomy 21 (Down syndrome) who have an extra gene copy almost universally exhibit at least the earliest symptoms of AD by 40 years of age; and (3) a specific isoform of ApoE4 is a major genetic risk factor for AD. While apolipoproteins enhance the breakdown of beta amyloid, some isoforms (such as ApoE4) are not very effective at this task, leading to excess amyloid buildup in the brain; and (4) transgenic mice that express a mutant form of the human APP gene develop fibrillary amyloid plaques and Alzheimer's-like brain pathology with spatial learning deficits. On the basis of laboratory and animal studies, various compounds were designed to test the hypothesis on humans. Unfortunately, while these compounds performed as predicted, the AD patients on whom they were tested either got not better or incredibly got worse!
An experimental vaccine [15] was found to clear the amyloid plaques in early human trials, but it did not have any significant effect on dementia, leading to suspect non-plaque Aβ oligomers (aggregates of many monomers) as the primary pathogenic form of Aβ. These toxic oligomers, also referred to as amyloid-derived diffusible ligands (ADDLs), bind to a surface receptor on neurons and change the structure of the synapse, thereby disrupting neuronal communication [16]. One receptor for Aβ oligomers may be the prion protein, the same protein that has been linked to mad cow disease and the related human condition (Creutzfeldt-Jacobs disease), thus potentially linking the underlying mechanism of these neurodegenerative disorders with AD [17].
An update of the amyloid hypothesis suggests that a close relative of the β-amyloid protein, and not necessarily the β-amyloid itself, may be a major culprit in the disease. The theory holds that an amyloid-related mechanism that prunes neuronal connections in the brain in the fast-growth phase of early life may be triggered by aging-related processes in later life to cause the neuronal withering of AD. N-APP, a fragment of APP from the peptide's N-terminus, is adjacent to β-amyloid and is cleaved from APP by one of the same enzymes. N-APP triggers the self-destruct pathway by binding to a neuronal receptor called death receptor 6 (DR6, also known as TNFRSF21). DR6 is highly expressed in the human brain regions most affected by Alzheimer's, so it is possible that the N-APP/DR6 pathway might be hijacked in the aging brain to cause damage. In this model, β-amyloid plays a complementary role, by depressing synaptic function.
Compatible with the amyloid hypothesis is the “fungal infection hypothesis”. This hypothesis was proposed by the microbiologist L. Carrasco when his group found statistical correlation between disseminated mycoses and AD. Further work revealed that fungal infection is present in different brain regions of AD patients, but not in the control individuals. A fungal infection explains the symptoms observed in AD patients. The slow progression of AD fits with the chronic nature of some systemic fungal infections, which can be asymptomatic and thus unnoticed and untreated. The fungal hypothesis is also compatible with some other established AD hypotheses, like the amyloid hypothesis, that can be explained as an immune system response to an infection in the CNS (as found by R. Moir and R. Tanzi in mouse and worm models of AD).
Tau Hypothesis
This hypothesis proposes that tau protein abnormalities initiate the disease cascade [18] wherein hyper phosphorylated tau begins to pair with other threads of tau eventually forming neurofibrillary tangles inside nerve cell bodies. When this occurs, the microtubules disintegrate, destroying the structure of the cell's cytoskeleton, which collapses the neuron's transport system. This may result first in malfunctions in biochemical communication between neurons and later in the death of the cells.
This hypothesis proposes that tau protein abnormalities initiate the disease cascade [18] wherein hyper phosphorylated tau begins to pair with other threads of tau eventually forming neurofibrillary tangles inside nerve cell bodies. When this occurs, the microtubules disintegrate, destroying the structure of the cell's cytoskeleton, which collapses the neuron's transport system. This may result first in malfunctions in biochemical communication between neurons and later in the death of the cells.
Toxicity Hypothesis
There is evidence that exposure to toxic elements (metals) may be a contributing factor to the development of Alzheimer's disease. The same holds but tentatively for air pollution exposure.
There is evidence that exposure to toxic elements (metals) may be a contributing factor to the development of Alzheimer's disease. The same holds but tentatively for air pollution exposure.
The INT Hypothesis
In his earlier publications and recent book [6], Professor Dale Bredesen of the University of California at Los Angeles posited that the cause of AD is three-pronged: (1) Inflammation (from infection, diet, or other causes); (2) Neurotrophy (from decline or shortage of supportive nutrients, hormones, and other brain-supporting molecules); and (3) Toxicity (from substances such as metals or biotoxins), from which the acronym derives. These would correspond to three different subtypes of AD. In this view, and excepting the genetic hypothesis, the multiple other hypotheses advanced so far would be merely risk (not causative) factors for AD. In particular, the amyloid cascade and tau hypotheses are protective responses of the brain from these three categories of metabolic threats. In a separate publication, more will be said about the INT hypothesis and its overarching seminal work.
In his earlier publications and recent book [6], Professor Dale Bredesen of the University of California at Los Angeles posited that the cause of AD is three-pronged: (1) Inflammation (from infection, diet, or other causes); (2) Neurotrophy (from decline or shortage of supportive nutrients, hormones, and other brain-supporting molecules); and (3) Toxicity (from substances such as metals or biotoxins), from which the acronym derives. These would correspond to three different subtypes of AD. In this view, and excepting the genetic hypothesis, the multiple other hypotheses advanced so far would be merely risk (not causative) factors for AD. In particular, the amyloid cascade and tau hypotheses are protective responses of the brain from these three categories of metabolic threats. In a separate publication, more will be said about the INT hypothesis and its overarching seminal work.
Other Hypotheses or Risk Factors
Neurovascular Hypothesis
This hypothesis states that poor functioning of the blood brain barrier (BBB) may be involved [19]. The cellular homeostasis of bio metals (such as ionic copper; iron and zinc) is disrupted in AD, though it remains unclear whether this is produced by or causes the changes in proteins. These ions affect and are affected by tau, APP and ApoE, and their dysregulation may cause oxidative stress that may contribute to the pathology. However, this link remains controversial.
This hypothesis states that poor functioning of the blood brain barrier (BBB) may be involved [19]. The cellular homeostasis of bio metals (such as ionic copper; iron and zinc) is disrupted in AD, though it remains unclear whether this is produced by or causes the changes in proteins. These ions affect and are affected by tau, APP and ApoE, and their dysregulation may cause oxidative stress that may contribute to the pathology. However, this link remains controversial.
Neuroinflammation Hypothesis
Systemic markers of the innate immune system are risk factors for late-onset AD [20].
Systemic markers of the innate immune system are risk factors for late-onset AD [20].
Cholinergic hypothesis
This is the oldest hypothesis that proposes that AD is caused by reduced synthesis of the neurotransmitter acetylcholine [21]. It has not received widespread support, largely because medications intended to treat acetylcholine deficiency have not been very effective [22]. Other cholinergic effects have also been proposed leading to generalized neuro inflammation.
This is the oldest hypothesis that proposes that AD is caused by reduced synthesis of the neurotransmitter acetylcholine [21]. It has not received widespread support, largely because medications intended to treat acetylcholine deficiency have not been very effective [22]. Other cholinergic effects have also been proposed leading to generalized neuro inflammation.
Cardiovascular Hypothesis
Although cardiovascular risk factors, such as hypercholosterolemia, hypertension, diabetes, and smoking are associated with a higher risk of onset and course of AD, the corresponding treatment drugs have had no effect on AD.
Although cardiovascular risk factors, such as hypercholosterolemia, hypertension, diabetes, and smoking are associated with a higher risk of onset and course of AD, the corresponding treatment drugs have had no effect on AD.
Smoking
Smoking is a significant AD risk factor but it is unclear whether smoking alone could cause AD.
Smoking is a significant AD risk factor but it is unclear whether smoking alone could cause AD.
Gum disease infection
An infection with Spirochetes (a bacterium) may cause dementia and may be involved in the pathogenesis of Alzheimer's disease.
An infection with Spirochetes (a bacterium) may cause dementia and may be involved in the pathogenesis of Alzheimer's disease.
Dysfunction of Oligodendrocytes
Dysfunction of oligodendrocytes and their associated myelin during aging contributes to axon damage, which then causes amyloid production and tau hyper-phosphorylation as a side effect.
Dysfunction of oligodendrocytes and their associated myelin during aging contributes to axon damage, which then causes amyloid production and tau hyper-phosphorylation as a side effect.
Neuropathology of Alzheimer's Disease
AD is characterized by loss of neurons and synapses in the cerebral cortex and certain subcortical regions. This loss results in gross atrophy of the affected regions, including degeneration in the temporal lobe and parietal lobe, and parts of the frontal cortex and cingulate gyrus. Degeneration is also present in brainstem nuclei like the locus coeruleus. Studies utilizing magnetic resonance imaging (MRI) and positron emission tomography (PET) have documented reductions in the size of specific brain regions in people with AD as they progressed from mild cognitive impairment to AD, and in comparison with similar images from healthy older adults (Figure 4).
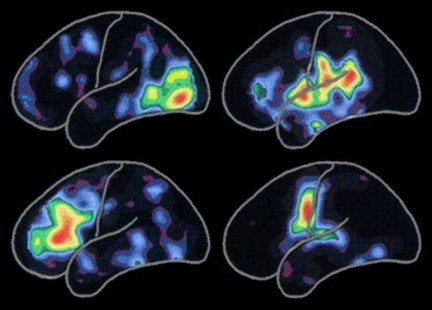
Figure 4: Positron Emission Tomography (PET) images illustrating
Alzheimer’s disease effects over several portions of the brain.
Both amyloid plaques (AP) and neurofibrillary tangles (NFT) are clearly visible by microscopy in brains of those afflicted by AD: AP are dense, mostly insoluble deposits of beta-amyloid peptide (BAP) and cellular material outside and around neurons, and NFT are aggregates of the microtubule-associated protein tau which has become hyperphosphorylated and accumulates inside the cells themselves. Although many older individuals develop some AP and NFT as a consequence of aging, the brains of people with AD have a greater number of them in specific brain regions such as the temporal lobe. Lewy bodies are not rare in the brains of people with AD (Figures 5 and Figure 6).
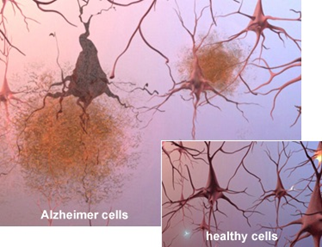
Figure 5: Microscope slides of healthy and Alzheimer cells showing
plaques and neurofibrillary tangles.
Biochemistry of Alzheimer's disease
AD has been identified as a protein misfolding disease (protheopathy) caused by plaque accumulation of abnormally folded amyloid beta protein (ABP) and tau protein in the brain. Plaques are made up of Aβ (small peptides, amino-acids in length), a fragment from the larger APP, itself a transmembrane protein that penetrates through the neuron's membrane. APP is critical to neuron growth, survival, and post-injury repair. In AD, gamma secretase and beta secretase act together in a proteolytic process which causes APP to be divided into smaller fragments. One of these fragments gives rise to fibrils of amyloid beta, which then form clumps that deposit outside neurons in dense formations known as senile plaques (SP).
AD is also considered a tauopathy due to abnormal aggregation of the tau protein. Every neuron has a cytoskeleton, an internal support structure partly made up of structures called microtubules. These microtubules act like tracks, guiding nutrients and molecules from the body of the cell to the ends of the axon and back. A protein called tau stabilizes the microtubules when phosphorylated, and is therefore called a microtubule-associated protein (MAP). In AD, tau undergoes chemical changes, becoming hyperphosphorylated; it then begins to pair with other threads, creating NFT and disintegrating the neuron's transport system.
Disease Mechanism
Exactly how disturbances of production and aggregation of the BAP give rise to the pathology of AD is not known. The amyloid hypothesis traditionally points to the accumulation of BAPs as the central event triggering neuron degeneration. Accumulation of aggregated amyloid fibrils, which are believed to be the toxic form of the protein responsible for disrupting the cell's calcium ion homeostasis (CIH), induces programmed cell death (PCD, apoptosis).
It is also known that Aβ selectively builds up in the mitochondria in the cells of AD's affected brains, and it also inhibits certain enzyme functions and the utilization of glucose by the neurons. Various inflammatory processes and cytokines may also have a role in the pathology of AD. Inflammation is a general marker of tissue damage in any disease, and may be either secondary to tissue damage in AD or a marker of an immunological response. There is increasing evidence of a strong interaction between the neurons and the immunological mechanisms in the brain. Obesity and systemic inflammation may interfere with immunological processes which promote disease progression.
Alterations in the distribution of different neurotrophic factors (NF) and in the expression of their receptors such as the brain-derived neurotrophic factor (BDNF) have been described in AD.
Diagnosis
Diagnostic Factors
AD is usually diagnosed based on three factors [23]:
AD is usually diagnosed based on three factors [23]:
- The person's medical history;
- The history from relatives; and
- The behavioral observations.
Supportive factors are:
- The presence of characteristic neurological and neuropsychological features; and
- The absence of alternative conditions.
Supportive Imaging
Advanced medical imaging with computed tomography, MRI, PET and single photon emission computed tomography (SPECT) can be used to help exclude other cerebral pathologies or subtypes of dementia. Moreover, they may predict conversion from prodromal stages (mild cognitive impairment) to AD. Assessment of intellectual functioning, including memory testing can further characterize the state of the disease. Diagnostic criteria exist to ease and standardize the diagnostic process for practicing physicians. The diagnosis can be confirmed with very high accuracy post-mortem when brain material is available and can be examined histologically.
Advanced medical imaging with computed tomography, MRI, PET and single photon emission computed tomography (SPECT) can be used to help exclude other cerebral pathologies or subtypes of dementia. Moreover, they may predict conversion from prodromal stages (mild cognitive impairment) to AD. Assessment of intellectual functioning, including memory testing can further characterize the state of the disease. Diagnostic criteria exist to ease and standardize the diagnostic process for practicing physicians. The diagnosis can be confirmed with very high accuracy post-mortem when brain material is available and can be examined histologically.
Criteria
The (U.S.) National Institute of Neurological and Communicable Disorders and Stroke (NINCDS) and the Alzheimer's disease and Related Disorders Association (ADRDA, now known simply as the Alzheimer' Association, AA) issued diagnostic criteria (24) that are the most currently used. Extensively updated in 2007, these criteria require that the presence of cognitive impairment and a suspected dementia syndrome be confirmed by neuropsychological testing before a clinical diagnosis of possible or probable AD be made. A histopathologic confirmation including a microscpic examination of brain tissue is required for a definitive diagnosis. Good statistical reliability and validity have been shown between the diagnostic criteria and definitive histopathological confirmation.
The (U.S.) National Institute of Neurological and Communicable Disorders and Stroke (NINCDS) and the Alzheimer's disease and Related Disorders Association (ADRDA, now known simply as the Alzheimer' Association, AA) issued diagnostic criteria (24) that are the most currently used. Extensively updated in 2007, these criteria require that the presence of cognitive impairment and a suspected dementia syndrome be confirmed by neuropsychological testing before a clinical diagnosis of possible or probable AD be made. A histopathologic confirmation including a microscpic examination of brain tissue is required for a definitive diagnosis. Good statistical reliability and validity have been shown between the diagnostic criteria and definitive histopathological confirmation.
Eight cognitive domains are most commonly impaired in AD:
- Memory;
- Language;
- Perceptual skills;
- Attention;
- Constructive abilities;
- Orientation;
- Problem solving; and
- Functional abilities.
These domains are equivalent to the NINCDS-ADRDA Alzheimer's Criteria as listed in the Diagnostic & Statistical Manual of Mental Disorders (DSM-IV-TR) published by the American Psychiatric Association (APA) [25-28].
Testing Techniques
There are four tests that are administered for the diagnosis of AD:
There are four tests that are administered for the diagnosis of AD:
Neuropsychological
These tests including the mini-mental state examination (MMSE) are widely used to evaluate the cognitive impairments needed for diagnosis. However, more comprehensive test arrays are necessary for high reliability of results, particularly in the earliest stages of the disease.
These tests including the mini-mental state examination (MMSE) are widely used to evaluate the cognitive impairments needed for diagnosis. However, more comprehensive test arrays are necessary for high reliability of results, particularly in the earliest stages of the disease.
Neurological Examination
In early AD, this examination will usually provide normal results, except for obvious cognitive impairment, which may not differ from that resulting from other diseases processes, including other causes of dementia. Further neurological examinations are crucial in the differential diagnosis of AD and other diseases (26). Interviews with family members are also utilized in the assessment of the disease. Caregivers can supply important information on the daily living abilities, as well as on the decrease, over time, of the person's mental function.
In early AD, this examination will usually provide normal results, except for obvious cognitive impairment, which may not differ from that resulting from other diseases processes, including other causes of dementia. Further neurological examinations are crucial in the differential diagnosis of AD and other diseases (26). Interviews with family members are also utilized in the assessment of the disease. Caregivers can supply important information on the daily living abilities, as well as on the decrease, over time, of the person's mental function.
Supplemental Testing
It provides extra information on some features of the disease or is used to rule out other diagnoses. Thus: Blood tests can identify other causes for dementia than AD; thyroid function tests; B12 assessment; ruling out syphilis; ruling out metabolic problems (including tests for kidney function, electrolyte levels and for diabetes); assessing levels of heavy metals (e.g. lead, mercury) and anemia; ruling out delirium.
It provides extra information on some features of the disease or is used to rule out other diagnoses. Thus: Blood tests can identify other causes for dementia than AD; thyroid function tests; B12 assessment; ruling out syphilis; ruling out metabolic problems (including tests for kidney function, electrolyte levels and for diabetes); assessing levels of heavy metals (e.g. lead, mercury) and anemia; ruling out delirium.
Psychological Testing
These tests are employed for depression, since depression can either be concurrent with AD (an early sign of cognitive impairment) or even be the cause.
These tests are employed for depression, since depression can either be concurrent with AD (an early sign of cognitive impairment) or even be the cause.
- C-PIB-PET scan: It is not recommended to be used as an early diagnostic tool or for predicting the development of AD when patients show signs of mild cognitive impairment (MCI).
- 18F-FDG PET scans: It is not supported by evidence as a single test for identifying patients who may develop AD.
Prevention
At present, there is no definitive evidence to support that any particular measure is effective in preventing AD. Global studies of measures to prevent or delay the onset of AD have often produced inconsistent results:
Modifiable Factors
Epidemiological studies have proposed relationships between certain modifiable factors, such as diet, cardiovascular risk, pharmaceutical products, or intellectual activities among others, and a population's likelihood of developing AD. Only further research, including clinical trials, will reveal whether these factors can help to prevent AD (30-33).
Epidemiological studies have proposed relationships between certain modifiable factors, such as diet, cardiovascular risk, pharmaceutical products, or intellectual activities among others, and a population's likelihood of developing AD. Only further research, including clinical trials, will reveal whether these factors can help to prevent AD (30-33).
Lifestyle
Engaging in intellectual activities (reading, playing board games, completing crossword puzzles, playing musical instruments, regular social interactions) show a reduced risk for AD. This is compatible with the cognitive reserve theory, which states that some life experiences result in more efficient neural functioning, providing the individual a cognitive reserve that delays the onset of dementia manifestations (34).
Engaging in intellectual activities (reading, playing board games, completing crossword puzzles, playing musical instruments, regular social interactions) show a reduced risk for AD. This is compatible with the cognitive reserve theory, which states that some life experiences result in more efficient neural functioning, providing the individual a cognitive reserve that delays the onset of dementia manifestations (34).
Education
Education delays the onset of AD's syndrome without changing the duration of the disease.
Education delays the onset of AD's syndrome without changing the duration of the disease.
Learning a second language
Even later in life, it seems to delay getting AD.
Even later in life, it seems to delay getting AD.
Physical activity
This is also associated with a reduced risk of AD. Physical exercise is associated with a decreased rate of dementia and is also effective in reducing symptom severity in those who are already afflicted by the disease.
This is also associated with a reduced risk of AD. Physical exercise is associated with a decreased rate of dementia and is also effective in reducing symptom severity in those who are already afflicted by the disease.
Healthy diet (Japanese, Mediterranean)
Conclusions on dietary components have at times been difficult to ascertain as results have differed between population-based studies and randomised controlled trials. Nonetheless, a healthy diet lowers the risk of AD and improves outcomes in those with AD. A diet high in saturated fats and simple carbohydrates (mono- and di-saccharide) increases the risk. The Mediterranean diet's beneficial cardiovascular effect has been proposed as the mechanism of action (30-33).
Conclusions on dietary components have at times been difficult to ascertain as results have differed between population-based studies and randomised controlled trials. Nonetheless, a healthy diet lowers the risk of AD and improves outcomes in those with AD. A diet high in saturated fats and simple carbohydrates (mono- and di-saccharide) increases the risk. The Mediterranean diet's beneficial cardiovascular effect has been proposed as the mechanism of action (30-33).
Foods containing flavonoids (e.g., cocoa, tea,..):
May decrease the risk of AD.
May decrease the risk of AD.
Light-to-moderate use of alcohol (particularly red wine)
There is limited evidence that it is associated with a lower risk of AD.
There is limited evidence that it is associated with a lower risk of AD.
Caffeine
There is tentative evidence for a protective effect.
There is tentative evidence for a protective effect.
Vitamins and minerals
No consistent evidence of any benefit, including for vitamin A,B12 and E, the alpha-tocopherol form of vitamin E, selenium, zinc, and folic acid with or without vitamin B12.
No consistent evidence of any benefit, including for vitamin A,B12 and E, the alpha-tocopherol form of vitamin E, selenium, zinc, and folic acid with or without vitamin B12.
Supplements
Omega-3 fatty acid supplements from plants and fish, and dietary docosahexaenoic acid (DHA), do not appear to benefit people with mild to moderate AD.
Omega-3 fatty acid supplements from plants and fish, and dietary docosahexaenoic acid (DHA), do not appear to benefit people with mild to moderate AD.
Spices
No benefit shown in humans despite a tentative evidence of benefit in animals. Likewise, there is no convincing evidence that ginkgo has any positive effect on cognitive impairment and dementia.
No benefit shown in humans despite a tentative evidence of benefit in animals. Likewise, there is no convincing evidence that ginkgo has any positive effect on cognitive impairment and dementia.
Cannabinoids
No concrete evidence in improving the symptoms of AD or dementia;
No concrete evidence in improving the symptoms of AD or dementia;
Disease Management
There is presently no cure for AD; available treatments offer relatively small symptomatic benefit but remain palliative in nature. Current treatments can be divided into pharmaceutical, psychosocial and caregiving.
Pharmaceutical Treatment
Off-label drugs
Statins (which are cholesterol-lowering drugs) have not been effective in preventing or improving the course of the disease. Non-steroidal anti-inflammatory drugs (NSAIDs) can reduce the likelihood of developing AD and reduce inflammation-related amyloid plaques. Hormone replacement therapy may, however, increase the risk of dementia.
Off-label drugs
Statins (which are cholesterol-lowering drugs) have not been effective in preventing or improving the course of the disease. Non-steroidal anti-inflammatory drugs (NSAIDs) can reduce the likelihood of developing AD and reduce inflammation-related amyloid plaques. Hormone replacement therapy may, however, increase the risk of dementia.
Label drugs
Six medications are currently used to treat the cognitive problems of AD of which five are Acetylcholinesterase (ACE) inhibitors (tacrine, rivastigmine, galantamine, donepezil, and Huperzine A) and the fifth one is an NMDA receptor agonist (memantine). However, no medication has been clearly shown to delay or halt the progression of the disease and the benefit from their use is small.
Six medications are currently used to treat the cognitive problems of AD of which five are Acetylcholinesterase (ACE) inhibitors (tacrine, rivastigmine, galantamine, donepezil, and Huperzine A) and the fifth one is an NMDA receptor agonist (memantine). However, no medication has been clearly shown to delay or halt the progression of the disease and the benefit from their use is small.
- ACE inhibitors: Reduction in the activity of the cholinergic neurons is a well-known feature of AD. ACE inhibitors are employed to reduce the rate at which acetylcholine (Ach) is broken down, thereby increasing the concentration of ACh in the brain and combating the loss of ACh caused by the death of cholinergic neurons. There is evidence for the efficacy of these medications in mild to moderate Alzheimer's disease and in the advanced stage. Their use in MCI has not shown any effect in a delay of the onset of AD.
In other words, the cholinesterase (CE) inhibitor keeps CE (a particular enzyme) from destroying Ach (a type of brain chemical called a neurotransmitter). Since AD is characterized by a reduction in Ach, blocking CE that breaks down Ach, allows more Ach to remain in the synapses. However, while this works, there are important caveats: (1) blocking the breakdown of Ach does not affect the cause or progression of AD. The disease therefore still progresses; (2) responding to the inhibition of Ach, the brain produces more CE which, in turn, limits the drug efficacy.
The most common side effects are nausea and vomiting, both of which are linked to cholinergic excess. These side effects arise in approximately 10–20% of users, are mild to moderate in severity, and can be managed by slowly adjusting medication doses. Less common secondary effects include muscle cramps, decreased heart rate (bradycardia), decreased appetite, weight loss, and increased gastric acid production.
- NMDA receptor agonist:Memantine was first used as an anti-influenza agent. It acts on the glutamatergic system by blocking NMDA receptors and inhibiting their overstimulation by glutamate. Glutamate is an excitatory neurotransmitter of the nervous system; excessive amounts in the brain can lead to cell death through a process called excitotoxicity, which consists of the overstimulation of glutamate receptors. (Note: Excitotoxicity occurs not only in AD but also in other neurological diseases such as Parkinson's and multiple sclerosis.) Memantine has a small benefit in the treatment of AD. Reported adverse events with this drug are infrequent and mild, including hallucinations, confusion, dizziness, headache and fatigue. The combination of memantine and donepezil is of clinically marginal effectiveness.
- In other words, memantine inhibits the transmission of brain signals between neurons via the neurotransmitter glutamate. Inhibiting that transmission reduces glutamate's excitotoxic effect (meaning the toxic effects associated with neuronal activation) and so may initially impair cognitive function. Like for ACE inhibitors, memantine does not get at the underlying cause of AD or stop the progress of AD and even less cure it.
- Atypical antipsychotics: Antipsychotics are modestly useful in reducing aggression and psychosis in people with AD, but their advantages are offset by serious adverse effects such as stroke, movement difficulties, cognitive decline and, when used for a long term, increased mortality. While promising, Huzerpine A requires further evidence before its use can be recommended.
Psychosocial interventions
Adjuncts to pharmaceutical treatments, psychosocial interventions are classified as oriented within behavior, emotion, cognition, and stimulation approaches.
Adjuncts to pharmaceutical treatments, psychosocial interventions are classified as oriented within behavior, emotion, cognition, and stimulation approaches.
- Behavioral interventions: They have not improved overall functioning, but they can help reduce some derivative behavioral problems such as incontinence.
- Emotion-oriented interventions: These include several therapies: reminiscence, validation, supportive, sensory integration, and simulated presence. A Cochrane review has found no evidence to support the usefulness of these therapies.
- Cognition-oriented treatments: These include reality orientation and cognitive retraining (that is the reduction of cognitive deficits). Both have shown some efficacy improving cognitive capacities, although in some studies these effects were transient and negative effects such as frustration have also been reported.
- Stimulation-oriented treatments: They include art, music, pet therapies, exercise, and any other kind of recreational activities. They have shown modest support for improving behavior, mood and, to a lesser extent, function. Nevertheless, as important as these effects are, the main support for the use of stimulation therapies is the change in the person's routine.
Caregiving
Given that AD has presently no cure and renders people incapable of tending to their own needs, caregiving is essentially “the” treatment and must therefore be carefully managed over the course of the disease.
Given that AD has presently no cure and renders people incapable of tending to their own needs, caregiving is essentially “the” treatment and must therefore be carefully managed over the course of the disease.
During the early and moderate stages, modifications to the living environment and lifestyle can increase patient's safety and reduce caretaker burden including: adherence to simplified routines, placing of safety locks, labelling household items. If eating becomes problematic, food will need to be prepared in smaller pieces or even pureed. When swallowing difficulties arise, use of feeding tubes may be required. The use of physical restraints is rarely indicated in any stage of the disease, although there are situations when they are necessary to prevent harm to the person with AD or their caregivers.
As the disease progresses, different medical issues can appear, such as oral and dental disease. Pressure ulcers, malnutrition, hygiene problems, infections (respiratory, skin, eyes). Careful management can prevent them, while professional treatment is needed when they do arise.
During the final stages of the disease, treatment is centered on relieving discomfort until death, often with the help of a hospice facility.
Prognosis
The early stages of AD are difficult to diagnose. A definitive diagnosis is usually made once cognitive impairment compromises daily living activities, although the person may still be living independently. The symptoms will progress from mild cognitive problems, such as memory loss through increasing stages of cognitive and non-cognitive disturbances, eliminating any possibility of independent living, especially in the late stages of the disease. Life expectancy of people with AD is less. Following diagnosis it typically ranges from three to ten years.
Fewer than 3% of people live more than fourteen years. Disease features significantly associated with reduced survival are an increased severity of cognitive impairment, decreased functional level, history of falls, and disturbances in the neurological examination. Other coincident diseases such as heart problems, diabetes or history of alcohol abuse are also related with shortened survival. While the earlier the age at onset, the higher the total survival years, life expectancy is particularly reduced when compared to the healthy population among those who are younger. Men have a less favorable survival prognosis than women. Pneumonia and dehydration are the most frequent immediate causes of death brought by AD, while cancer is a less frequent cause of death than in the general population.
Research Directions
Emphasis in Alzheimer's research has been placed on diagnosing the condition before symptoms begin. A number of biochemical tests have been developed to attempt earlier detection. One such test involves the analysis of CSF for beta-amyloid or tau proteins, both total tau protein and phosphorylated tau181P protein concentrations.
Vaccination
An example of such a vaccine under investigation was ACC-001, although the trials were suspended in 2008. Another similar agent is Bapineuzumab, an antibody designed as identical to the naturally induced anti-amyloid antibody [35].
An example of such a vaccine under investigation was ACC-001, although the trials were suspended in 2008. Another similar agent is Bapineuzumab, an antibody designed as identical to the naturally induced anti-amyloid antibody [35].
Putative immunological therapies
Unlike preventative vaccination, putative immunological therapies would be used to treat people already diagnosed. They are based on the concept of training the immune system to recognize, attack, and reverse the deposition of amyloid, thereby altering the course of the disease. However, immunotherapeutic agents have been found to cause some concerning adverse drug reactions, such as amyloid-related imaging abnormalities [36, 37].
Unlike preventative vaccination, putative immunological therapies would be used to treat people already diagnosed. They are based on the concept of training the immune system to recognize, attack, and reverse the deposition of amyloid, thereby altering the course of the disease. However, immunotherapeutic agents have been found to cause some concerning adverse drug reactions, such as amyloid-related imaging abnormalities [36, 37].
Neuroprotective agents
Other approaches are Neuroprotective agents, such as AL-108, and metal-protein interaction attenuation agents, such as PBT2. A TNFα receptor-blocking fusion protein etarnacept has shown encouraging results.
Other approaches are Neuroprotective agents, such as AL-108, and metal-protein interaction attenuation agents, such as PBT2. A TNFα receptor-blocking fusion protein etarnacept has shown encouraging results.
Putative pharmaceutical therapies
As of 2014, the safety and efficacy of more than 400 pharmaceutical treatments had been or were being investigated in over 1,500 clinical trials worldwide, and approximately a quarter of these compounds are in Phase III trials, the last step prior to review by regulatory agencies. On the other hand, in the decade 2002–2012, 244 compounds were assessed in Phase I, Phase II, or Phase III trials, and only one of these (memantine) received FDA approval (though others were still in the pipeline).
As of 2014, the safety and efficacy of more than 400 pharmaceutical treatments had been or were being investigated in over 1,500 clinical trials worldwide, and approximately a quarter of these compounds are in Phase III trials, the last step prior to review by regulatory agencies. On the other hand, in the decade 2002–2012, 244 compounds were assessed in Phase I, Phase II, or Phase III trials, and only one of these (memantine) received FDA approval (though others were still in the pipeline).
- Antiviral medication: The herpes simplex virus HSV-1 has been found in the same areas as amyloid plaques. This suggested the possibility that AD could be treated or prevented with antiviral medication. Studies of antivirals in cell cultures have shown promising results.
Treating the underlying disease pathology
- Reduction of beta-amyloid levels: This is a common target of compounds (such as apomorphine) under investigation. Immunotherapy or vaccination for the amyloid protein is one treatment modality under study.
- Inhibiting tau aggregation: In 2008, two separate clinical trials showed positive results in modifying the course of disease in mild to moderate AD with methylthioninium chloride and dimebon (an antihistamine). Unfortunately, work with methylthioninium chloride showed that bioavailability of methylthioninium from the gut was affected by feeding and by stomach acidity, leading to unexpectedly variable dosing. Further, the consecutive phase-III trial of dimebon failed to show positive effects in the primary and secondary endpoints. A new stabilized formulation, as the prodrug LMTX was in phase-III trials (in 2014).
Meditation
Preliminary research on the effects of meditation on retrieving memory and cognitive functions have been encouraging. A 2015 review suggests that mindfulness = based interventions may prevent or delay the onset of MCI and AD.
Preliminary research on the effects of meditation on retrieving memory and cognitive functions have been encouraging. A 2015 review suggests that mindfulness = based interventions may prevent or delay the onset of MCI and AD.
Possible transmission between people
Rare cases of possible transmission between people are being studied, e.g., to growth hormone patients.
Rare cases of possible transmission between people are being studied, e.g., to growth hormone patients.
Anti-fungal infection of AD brain
Fungal infection of AD brains has also been described (see above the corresponding hypothesis discussed above).
Fungal infection of AD brains has also been described (see above the corresponding hypothesis discussed above).
Approaches based on improved imaging methods
- Magnetic resonance imaging (MRI) with volumetric: Combined with certain numerical programs (Neuroreader and NeuroQuant), MRI with volumetric provides percentile scores comparing the patient with other patients of a similar age with regard to shrinkage of the brain and brain regions.
- Single photon emission computed tomography (SPECT): Of the many medical imaging techniques available, SPECT appears to be superior in differentiating AD Alzheimer's disease from other types of dementia, giving a greater level of accuracy compared with mental testing and medical history analysis. Advances have led to the proposal of new diagnostic criteria [38,39].
- Positron emission tomography: PET scanning can be used in five different instances:
- With the radiopharmaceutical called florbetapir (containing the longer-lasting radionuclide fluorine-18) to diagnose AD. It was given FDA approval for this use [38, 39];
- To reliably determine the levels of Aβ deposition by the measurement of Aβ levels in the CNS. Using immunosuppression and mass spectrometry, Nakamura., et al. (2018) [40] have used as plasma biomarkers the ratios [APP699/Aβ1-42, Aβ1-40 /Aβ1-42] and a composite score to reliably predict individual deposition levels of Aβ in the brain. These results highlight the potential use of plasma biomarkers to predict Aβ burden;
- When the diagnosis is difficult or uncertain such as, for example, distinguishing between fronto-temporal dementia and AD. In the latter case, FDG-PET shows a characteristic pattern of reduced glucose metabolism in the temporal and parietal regions, often including the posterior cingulate an d precuneus, which are often impaired in AD;
- Amyloid-PET scans to show amyloid accumulation in the brain, which may occur without AD and conversely. Ongoing studies aim to determine whether a positive amyloid PET scan in the absence of symptoms will be helpful in AD diagnosis. However, the pattern of amyloid accumulation does not correlate well with the brain regions displaying symptoms. Note that this scan can detect relatively large collections of amyloid, but it does not reveal whether the amyloid is present in the blood vessels, and cannot look at relatively rapid changes in single amyloid plaques. Amyloid in blood vessels can, in rare case, lead to hemorrhages.
- Tau-PET scans tend to show abnormalities that correspond more closely with symptoms.
- PiB-PET: This amyloid-imaging modality remains investigational. It is likely to be used in conjunction with other markers rather than as an alternative. Volumetric MRI can detect changes in the size of brain regions. Measuring those regions that atrophy during AD's progress is showing promise as a diagnostic indicator. It may prove less expensive than other imaging methods currently under study. The imaging agent Florbetapil can help to detect Alzheimer's brain plaques, but will require additional clinical research before it can be made available commercially [41,42].
- CSF testing: This may be helpful in the diagnosis of AD. Here, Aβ-42 shows a characteristic reduction in the CSF and an increase in the total tau and phospho-tau.
- Electroencephalography (EEG): Can be helpful in evidencing non convulsive seizure activity, which occurs in ~ 5% of AD patients.
Novel tests on the horizon for cognitive decline assessment
There are three such novel tests under study:
There are three such novel tests under study:
- Neural exosomes: Professor Edward J. Goetzl and his colleagues at the University of California at San Francisco have come up with a blood test that evidences AD in neural exosomes (tiny fragments of cells and materials expelled from cells that circulate in the blood). These exosomes include increases in Aβ-42 (the main one associated with AD), phosphorylated tau, cathepin D (a protease that is increased in exosomes), RESY (indicating levels of trophic support), and phosphorylation ratio of IRS-1 (indicating insuline resistance). The test can also reveal insulin resistance and many other critical parameters of brain biochemistry. Not only will this test assess cognitive decline, but also the type of AD (1, 2 or 3) and, most importantly, whether the treatment program is effective or needs adjustments. This approach also has the potential “to assess neurotransmitter pathways, hormonal signaling, trophic factor signaling, vitamin effects on neural function, trauma effects, vascular compromise, therapeutic responses, and many more biochemical signatures in the brain” [43].
- Retinal imaging: Retinal imaging can overcome the limitations of the PET-amyloid imaging approach. It has the following advantages: (a) It provides an early evaluation and assessment of risk for cognitive decline. Although, as described earlier, Aβ in the brain can be imaged with a PET scan, only relatively large accumulations of amyloid can be imaged. In particular, whether the amyloid is in the blood vessels cannot be revealed; (b) it can identify many (often hundreds of small plaques; (c) it can map the location of each plaque; (d) it can potentially reveal whether the amyloid affects the retinal blood vessels (and, by extension, the brain's vessels as well) in addition to neurons and synapses themselves; (e) it can accurately assess the effectiveness of treatment; and (f) it is much less expensive than a PETscan (44).
- Eye movement tracking:Damage to the mesial temporal lobe deep in the brain occurs early in AD. It impairs the ability to remember and recognize novelties in one's environment. An object recognition imaging test has been developed that takes advantage of this feature but tracking eye movements, thus detecting impairment of the hippocampus and nearby structures [45].
Conclusions
Alzheimer's disease (AD) is a chronic neurodegenerative disease of poorly understood cause. Several theories (hypotheses) have been propounded for its cause: genetic (early onset familial disease, late onset sporadic disease), cholinergic, amyloid, fungal infection, tau, neurovascular, neuroinflammation, neurodevelopment, cardiovascular, gum disease infection, dysfunction of oligodendrocytes, and others related to lifestyle, diet, and the environment. Such a wide array of hypotheses is by itself indicative of our lack of understanding and knowledge of the disease notwithstanding the fact that the disease has been identified since 1901 and despite the considerable number of publications (in excess of 50,000) that deal with the subject. There are no known treatments to stop or reverse the progression of AD, though some may temporarily improve symptoms. However, some researchers have recently claimed that they have developed a program to prevent and reverse the cognitive decline of dementia.
AD is characterized by loss of neurons and synapses in various brain parts and brain size shrinking in advanced cases, as documented by several MRI and PET studies, and microscope studies that evidence both amyloid plaques (dense, mostly insoluble deposits of beta-amyloid peptide and cellular material outside and around neurons) and neurofibrillary tangles (aggregates of the microtubule-associated protein tau which has become hyperphosphorylated and accumulates inside the cells themselves), and Lewy bodies which are not rare in the brains of people with AD. Exactly how disturbances of production and aggregation of the beta amyloid plaques give rise to the pathology of AD is not known.
The early stages of AD are difficult to diagnose. The diagnosis is based on three factors (the person's medical history, the history from relatives, and the behavioral observations) supported by other factors (such as the presence of characteristic neurological and neuropsychological features, and the absence of alternative conditions) and medical imaging techniques (MRI, PET, SPECT). A definitive diagnosis is usually made once cognitive impairment compromises daily living activities. The symptoms will progress from mild cognitive problems through increasing stages of cognitive and non-cognitive disturbances. Life expectancy of people with AD typically ranges from three to ten years from diagnosis. Criteria, testing techniques (neuropsychological, neurological, and psychological) are well established. At present, there is no definitive evidence to support that any particular measure is effective in preventing AD. Global studies of measures to prevent or delay the onset of AD have often produced inconsistent results. Like wise modifiable factors (lifestyle, education, learning a second language, physical activity, healthy diet, alcohol, caffeine, vitamins and minerals, supplements, spices, cannabinoids, etc.) have limited (and for some no) effect. Adjuncts to pharmaceutical treatments, psychosocial interventions are oriented towards behavior, emotion, cognition, and stimulation.
Given that AD has presently no cure and renders people incapable of tending to their own needs, caregiving is essentially “the” treatment and must therefore be carefully managed over the course of the disease.
Emphasis in Alzheimer's research has been placed on diagnosing the condition before symptoms begin. A number of biochemical tests have been developed to attempt earlier detection. One such test involves the analysis of the cerebrospinal fluid for beta-amyloid or tau proteins (both total tau protein and phosphorylated tau181P protein concentrations). Other approaches involve preventive vaccination (e.g., with bapineuzumab, an antibody designed as identical to the naturally induced anti-amyloid antibody); putative immunological therapies (based on the concept of training the immune system to recognize, attack, and reverse the deposition of amyloid, thereby altering the course of the disease). However, immunotherapeutic agents have been found to cause some concerning adverse drug reactions; Neuroprotective agents (e.g., Al-108, PBT2 and TNFα receptor-blocking fusion protein etarnacept); putative pharmaceutical therapies among the more than 400 pharmaceutical treatments having been investigated or in advanced clinical trials; treating the underlying disease pathology such as by reduction of beta-amyloid levels (e.g., by Apo morphine, investigational immunotherapy, or vaccination) and inhibiting tau aggregation (e.g., with methylthioninium chloride and dimebon). Still other methodologies involve meditation, and anti-fungal infection of the brain. In addition, other approaches based on improvements in imaging techniques (SPECT, PET, and PiB-PET) are also investigated.
Selected References
Since the original report and description of the disease by Dr. Alzheimer, in excess of 50,000 publications have appeared on the various aspects of the disease. It would of course be impossible to review all these publications. The references below are only a limited selection of the most recent publications.
- Alzheimer A. “Über eine eigenartige Erkrankung der Hirnrinde [About a peculiar disease of the cerebral cortex]”. Allgemeine Zeitschrift für Psychiatrie und Psychisch- Gerichtlich Medizin 64.1–2 (1907): 146–148.
- Alzheimer A. “About a Peculiar Disease of the Cerebral Cortex”. Alzheimer Disease and Associated Disorders 1.1 (1987): 3–8.
- Berrios G. “Alzheimer's disease: A conceptual history”. International Journal of Geriatric Psychiatry 5.6 (1990): 355–365.
- Ballard C., et al. “Alzheimer's disease”. Lancet 377.9770 (2011): 1019–1031.
- Kraepelin E. “Clinical Psychiatry: A Textbook for Students and Physicians (Reprint). (Translated by Diefendorf A. Ross. Kessinger Publishing. (2007): 568.
- Bredesen D. “The end of Alzheimer's: The first program to prevent and reverse the cognitive decline of dementia”. Vermillion publishers (U.K.) and Avery publishers (U.S.) (2017): 307.
- Margaret Gatz., et al. “Role of genes and environments for explaining Alzheimer disease”. Archives of General Psychiatry 63.2 (2006): 168–174.
- Stephen C. Waring and Roger N. Rosenberg. “Genome-wide association studies in Alzheimer disease”. Archives of Neurology 65.3 (2008): 329–334.
- Kaj Blennow., et al. “Alzheimer's disease”. The Lancet 368.9533 (2006): 387–403.
- Lambert JC., et al. “Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease”. Nature Genetics 45.12 (2013):1452–1458.
- Robert W. Mahley., et al. “Apolipoprotein E4: A causative factor and therapeutic target in neuropathology including Alzheimer.s disease” Proceedings of the U.S. National Academy of Sciences 103.15 (2006): 5644–5651.
- Rita Guerreiro., et al. “TREM2 variants in Alzheimer's disease” The New England Journal of Medicine 368.2 (2012): 117–127.
- Eikelenboom P., et al. “Neuroinflammation: An early event in both the history and pathogenesis of Alzheimer's disease”. Neuro-Degenerative Diseases 7.1–3 (2010): 38–41.
- John Hardy and David Allsop. “Amyloid deposition as the central event in the etiology of Alzheimer's disease”. Trends in Pharmacological Sciences 12.10 (1991): 383–388.
- “Long-term effects of Aβ42 immunization in Alzheimer's disease: Follow-up of a randomized, placebo-controlled Phase I trial”. Lancet 372.9634 (2008): 216–223.
- Pascale N. Lacor., et al.“Aß oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease”. The Journal of Neuroscience 27.4 (2007): 796–807.
- Juha Laurén., et al. “Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers”. Nature 457.7233 (2009):1128–1132.
- Amritpal Mudher and Simon Lovestone. “Alzheimer's disease: Do tauists and baptists finally shake hands?” Trends in Neurosciences 25.1(2002): 22–26.
- Deane R and Zlokovic BV. "Role of the blood-brain barrier in the pathogenesis of Alzheimer's disease". Current Alzheimer research 4.2 (2007): 191–197.
- Kluger A., et al. “Retrogenesis: Clinical, physiologic, and pathologic mechanisms in brain aging, Alzheimer's disease and other dementing processes”. European Archives of Psychiatry and Clinical Neuroscience 249.3 (1999): 28–36.
- Paul T Francisa., et al. “The cholinergic hypothesis of Alzheimer's disease: A review of progress”. Journal of Neurology, Neurosurgery, and Psychiatry 66.2 (1999):137–147.
- Martorana A., et al. “Beyond the cholinergic hypothesis: Do current drugs work in Alzheimer's disease?” CNS Neuroscience & Therapeutics 16.4 (2010): 235–245.
- (U.S.) National Institute on Aging. “About Alzheimer's Disease's Symptoms”. (2012).
- National Institute of Neurological and Communicative Disorders and Stroke – Alzheimer's Disease (NINCDS) - Alzheimer's Disease and Related Disorders Association (ADRDA) Work Group Report under the Auspices of Department of Health and Human Services Task Force on Alzheimer's Disease (1984), Neurology 34.7: 939–944.
- American Psychiatric Association. “Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR (4th Edition Text Revision ed.). Washington, DC: American Psychiatric Association. (2000)
- G.Waldemar., et al. “Recommendations for the Diagnosis and Management of Alzheimer's Disease and Other Disorders Associated with Dementia: EFNS Guideline”. European Journal of Neurology 14.1 (2007): e1–26.
- World Health Organization (WHO). Dementia Fact Sheet No. 362. (2015).
- (U.S.) National Institute for Health and Care Excellence (NICE). “Dementia Diagnosis and Assessment”. (2014):
- Daviglus ML., et al. “NIH state-of-the-science conference: Preventing Alzheimer's disease and cognitive decline”. (2010)
- Kawas CH. “Medications and diet: Protective factors for AD?” Alzheimer Disease and Associated Disorders 20(3 Suppl 2) (2006): S89–96.
- Luchsinger JA and Mayeux R. “Dietary factors and Alzheimer's disease”. Lancet Neurology3.10 (2004): 579–587.
- José A. Luchsinger., et al. “Diet and Alzheimer's disease”. Current Neurology and Neuroscience Reports 7.5 (2007): 366–372.
- Solfrizzi Vincenzo., et al. “Lifestyle-related factors in predementia and dementia syndromes”. Expert Review of Neurotherapeutics 8.1 (2008): 133–58.
- Hawkes Cheryl A and McLaurin JoAnne. “Immunotherapy as treatment for Alzheimer's disease”. Expert Review of Neurotherapeutics 7.11 (2007):1535–1548.
- Wischik CM., et al. “Tau-aggregation inhibitor therapy for Alzheimer's disease”. Biochemical Pharmacology 88.4 (2014): 529-539.
- Szekely C A., et al. “Prevention of Alzheimer's Disease”. International Review of Psychiatry 19.6 (2007): 693-706.
- Solomon B. “Clinical immunologic approaches for the treatment of Alzheimer's disease”.Expert Opinion on Investigational Drugs 16.6 (2007): 819–828.
- Desikan., et al. “Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer's disease”. Brain 132 (2009): 2048-2057.
- Rebekah Moan. “MRI software accurately IDs preclinical Alzheimer.s disease”. Diagnostic Imaging (2009).
- Nakamura., et al. “High performance plasma amyloid-β biomarkers for Alzheimer's disease”. Nature 554 .7690 (2018): 33.
- Zhang S., et al. “(11) C-PIB-PET for the early diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI)”. The Cochrane Database of Systematic Reviews 7 (2014): CD010386.
- Smailagic N., et al. “18F-FDG PET for the early diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI)”. The Cochrane Database of Systematic Reviews 1 (2015): CD010632.
- Goetzl EJ (2013-15). “Biomarkers and diagnostic methods for Alzheimer's disease an d other neurodegenerative disorders”. U.S. Patent 20150119278 A1.
- Verdooner SR. “Apparatus and method for identifying one or more amyloid beta plaques in a plurality of discrete OCT retinal layers”. U.S. Patent 9854963 (2018).
- Neurotrack. “A new brain health app warns users of memory decline”. (2016)
Citation:
Alain L Fymat. “Alzheimer’s Disease: A Review”. Current Opinions in Neurological Science 2.2 (2018): 415-436.
Copyright: © 2018 Alain L Fymat. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























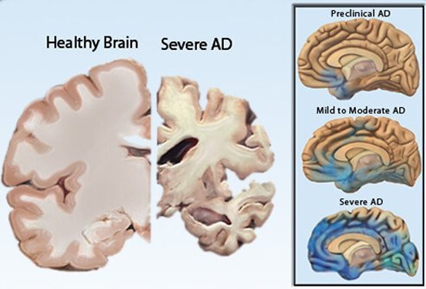
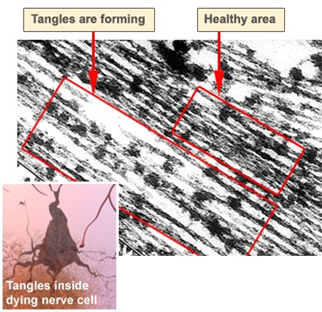
 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.