Review Article
Volume 2 Issue 1 - 2017
Parkinson's Disease and other Movement Disorders: A Review
International Institute of Medicine and Science, California, USA
*Corresponding Author: Alain L Fymat, International Institute of Medicine and Science, California, USA.
Received: December 18, 2017; Published: December 23, 2017
Abstract
Parkinson's disease is a degenerative disorder of the central nervous system that belongs to a group of conditions called motor system disorders. It is both chronic and progressive. The disorders are the result of the loss of dopamine-producing brain cells. There are several symptoms associated with the disease; however, appearing in other diseases as well, not everyone who has one or more of these symptoms has Parkinson's disease. The disease usually affects people over the age of 60. There are currently no blood or laboratory tests and the diagnosis can be difficult. It usually affects people over the age of 60 and more males than females. While there is no cure today, medication and surgery can provide substantial improvement of the motor systems. After a brief history, I will discuss the nature of the disease, and review its signs and symptoms (motor and neuropsychiatric or non-motor). Motor symptoms include tremor, bradykinesia, rigidity, postural instability, and gait and posture disturbances. Other motor symptoms include the following: Depression, emotional changes, difficulty chewing and swallowing, speech changes, urinary problems or constipation, skin problems, sleep problems, orthostatic hypotension, muscle crams and dystonia, pain, fatigue and loss of energy, and sexual dysfunction. Neuropsychiatric symptoms include: Cognitive disturbances, dementia and other cognitive problems, impulse control disorders, behavior and mood alterations, punding, hallucinations or delusions, and alterations in the autonomous nervous system. I will then summarize the classification of the disease into five categories: Parkinsonism, idiopathic parkinsonism, atypical parkinsonism, and synucleiopathy and tauopathy as well as their effects, the regions affected and their characteristics.
I will subsequently address the causes of the disorder both genetic (including pathophysiology and brain cell death) and environmental (including the various forms of parkinsonism: post-encephalitic, drug-induced, toxin-induced, and dementia form). While there are currently no blood or laboratory tests that diagnose sporadic Parkinson disease, it is also difficult to diagnose accurately early on in the course of the disease. Thus, the diagnosis is based on medical history, a neurological examination and imaging scans to rule out certain other diseases. Staging of the disease will employ either the 5-stage scale of Hoehn-Yahr or/and the 4-stage scale of the Unified Parkinson's Diseases Rating Scale of the Movement Disorders Society. Prognosis will be succinctly discussed while devoting more effort on treatment either with drugs or/and surgery. Drug treatments will involve drugs that: increase the level of dopamine (the mainstay being Levodopa/Carbidopa), or mimic the presence of dopamine (agonists), or inhibit dopamine breakdown (with MAO-B inhibitors, COMT inhibitors), or decrease the reaction of acetylcholines (anticholinergics), as well as other drugs of unknown mechanism of action, and drugs that help control the non-motor symptoms of the disease. Surgery treatments include: lesional, deep brain stimulation, and intentional formation of lesions (pallidotomy, thalamotomy).
Few words will be said regarding complementary and supportive therapies, rehabilitation and palliative care, and prediction and prevention of the disease, and management. More will be said on parkinsonism from neurological disorders including: atherosclerotic, post-traumatic, and essential parkinsonism; tremors; normal pressure hydrocephalus; and parkinsonism accompanying other conditions. Other diseases and conditions resembling Parkinson's disease will also be covered such as multiple system atrophy; dementia with Lewy bodies; progressive supranuclear palsy; cortico-basal degeneration; and Parkinson-plus diseases. Obviously much more could be said on any of the above aspects. In particular, the several lines of current research in the disease, its different manifestations and associations are likewise extensive and have been appropriately reserved for a companion article.
Keywords: Alzheimer's disease; Dementia with Lewy bodies; Encephalitis; Encephalopathy; Idiopathic Parkinsonism; Juvenile Parkinsonism; Motor system disorders; Parkinsonism; Parkinsonism dementia; Parkinson's disease; Pseudo-Parkinsonism; Shy-Drager Syndrome; Synucleiopathy; Tauopathy; Vascular Parkinsonism
Abbreviations used: AD: Alzheimer's Disease; ALS: Amyotrophic Lateral Sclerosis (aka Lou Gehrig Disease); ANS: Autonomic Nervous System; ASP: Arterio-Sclerotic Parkinsonism; CBD: Cortico-Basal Degeneration; CJD: Creutzfeldt-Jakob Disease; CNS: Central Nervous System; COMT: Cayechol-O-Methyl Transferase; CT: Computed Tomography; CTE: Chronic Traumatic Encephalopathy; DBS: Deep Brain Stimulation; DLB: Dementia with Lewy Bodies; DDS: Dopamine Dysregulation Syndrome; DRG: Dementia Research Group; DRS: DysRegulation Syndrome; HD: Huntington's Disease; HIFU: High-Intensity Focused Ultrasound; HYS: Hoehn and Yahr Scale; L-dopa: Levodopa; LSVT: Lee Silverman Voice Treatment; MAO: Mono Amine Oxidase; MRI: Magnetic Resonance Imaging; MSA: Multiple System Atrophy; MSA-P: MSA-Parkinsonism; NIH: (U.S.) National Institutes of Health; NINDS: (U.S.) National Institute of Neurological Disorders and Stroke; NMS: Neuroleptic Malignant Syndrome; NPH: Normal Pressure Hydrocephalus; OCD: Obsessive Compulsive Disorders; OP: Orthostatic hypotension; OT: Occupational Therapy; PC: Palliative Care; PD: Parkinson's disease; PDD: Parkinson Disease Dementia; PET: Positron Emission Tomography; PMDS: (International) Parkinson and Movements Disorder Society; PS: Parkinson Syndrome; PSP: Progressive Supra nuclear Palsy; PTE: Post-Traumatic Encephalopathy; QSBBND: (U.K.) Queen Square Brain Bank for Neurological Disorders; RBD: REM Behavior Disorder; SDS: Shy-Drager Syndrome; Sympathetic Nervous System; SPECT: Single Photon Emission Computed Tomography; UPDRS: Unified Parkinson's Disease Rating Scale; WD: Wilson's Disease; yoPD: Young Onset PD.
Disorders cited: Alzheimer's disease; Amyotrophic lateral sclerosis (Lou Gehrig's disease); Apraxia; Arterio-sclerotic parkinsonism (aka pseudo-parkinsonism; vascular parkinsonism); Atypical Parkinsonism; Bradykinesia; Chronic traumatic encephalopathy; Corticobasal degeneration; Creutzfeldt-Jacob disease; Dementia; Dementia with Lewy bodies; Dopamine dysregulation syndrome; Dysphagia; Dystonia; Encephalitis lethargica; Equine encephalomyelitis (western, eastern, Japanese B encephalitis); Gastroparesis; Gastrostomy; Huntington's disease; Idiopathic Parkinsonism; Juvenile parkinsonism; Multiple system atrophy (regular; with Parkinsonism symptoms; with poor coordination); Myoclonus; Normal Pressure Hydrocephalus; Obsessive compulsive disorders; Orthostatic hypotension; Palsy; Parkinson's Disease; Parkinson syndrome; Parkinsonism; Parkinsonism-dementia complex of Guam; Post-traumatic encephalopathy; Progressive supranuclear palsy; Psychosis; Reserpine; Shy-Drager Syndrome; Synucleiopathy; Tauopathy; (Gilles de la) Tourette syndrome; Wilson's disease;
Drugs listed: Amantadine (an anti-viral); Amytriptyline (an anti-depressant); Anticholinergics (Trihexyphenidyl; Apomorphine (a dopamine agonist); Benserazide (a dopa inhibitor); Benzotropine (an anticholinergics); Bromocriptine; Cabergoline; Carbidopa (a dopa inhibitor); Chlorpromazine (an anti-psychotic); Cholinisterase inhibitors; Clozapine (an atypical antipsychotic); Donepezil (an anti-dementia); Dopamine agonists (Apomorphine; Bromocriptine; Duodopa; Entacapone); Entacapone (a COMT inhibitor); Ethopropazine (an anticholinergics); Fluorocortisone (an anti-orthostatic hypotension); Fluoxetine (an anti-depressant); Haloperidol (a typical anti-psychotic drug); Levodopa (L-dopa); Lisuride; MAOI-Inhibitors (Rasagiline; Safinamide; Selegiline aka Depenyl); Memantine; Meperidine (a sedative); Metoclopramide (an anti-stomach disorders drug); Modafinil; Pergolide; Piridebil; Pramipexole (a dopamine agonist); Quetiapine (an atypical anti-psychotic drug); Reserpine (an anti-high blood pressure); Rivastigmine (helps treat dementia); Ropinirole (a dopamine agonist); Rosagiline (an MAO inhibitor); Rotigotine (a dopamine agonist); Safinamide (an MAO inhibitor); Selegiline (an MAO inhibitor); Tolcapone (a COMT inhibitor); Trihexphenidyl (an anticholinergics); Valproate.
Introduction
Parkinson's disease (PD) is a degenerative disorder of the central nervous system (CNS) that belongs to a group of conditions called motor system (or movement) disorders. It is both chronic (it persists over a long period of time) and progressive (its symptoms grow worse over time). The disorders are the result of the loss of dopamine-producing brain cells. As nerve cells (neurons) in parts of the brain become impaired or die, four primary symptoms appear: (1) tremor, or trembling in hands, arms, legs, jaw, and face; (2) rigidity, or stiffness of the limbs or trunk of the body; (3) bradykinesia, or slowness of movement; and (4) postural instability, or impaired balance and coordination. As these symptoms become more pronounced, patients may have difficulty walking, talking, or completing other simple tasks. Other symptoms may include: (5) depression and other emotional changes; (6) difficulty in swallowing, chewing, and speaking; (7) urinary problems or constipation; (8) skin problems; and (9) sleep disruptions. The symptoms may begin to interfere with daily activities. However, these symptoms appear in other diseases as well so that not everyone with one or more of these symptoms has PD.
PD is the second most common neurological disorder after Alzheimer's diseas (AD). It affects approximately seven million people globally and one million people in the United States. The proportion in a population at a given time is about 0.3% in industrialized countries. PD is more common in the elderly and rates rise from 1% in those over 60 years of age to 4% of the population over 80. The mean age of onset is around 60 years, although 5–10% of cases, classified as young onset PD, begin between the ages of 20 and 50. PD may be less prevalent in those of African and Asian ancestry, although this finding is disputed. Some studies have proposed that it is more common in men than women, but others failed to detect any differences between the two sexes. The number of new cases per year of PD is between 8 and 18 per 100,000 person–years. Many risk factors and protective factors have been proposed, sometimes in relation to theories concerning possible mechanisms of the disease, however, none have been conclusively related to PD by empirical evidence. When epidemiological studies have been carried out in order to test the relationship between a given factor and PD, they have often been flawed and their results have in some cases been contradictory. The most frequently replicated relationships are an increased risk of PD in those exposed to pesticides, and a reduced risk in smokers.
Early symptoms are subtle and occur gradually. In some people, the disease progresses more quickly than in others. There are currently no blood or laboratory tests that have been proven to help in diagnosing sporadic PD and the disease can be difficult to diagnose accurately. It is therefore based on medical history and a neurological examination. Brain scans or laboratory tests may rule out other diseases. In 2013, PD resulted in about 103,000 deaths globally, up from 44,000 deaths in 1990. The death rate increased from an average of 1.5 to 1.8 per 100,000 during that time. In 2015, PD affected 6.2 million people and resulted in about 117,400 deaths globally. In the over-60 population, about 1% are affected. Males are more often affected than females. When it is seen in people before the age of 50, it is called young-onset PD (yoPD). The average life expectancy following diagnosis is between 7 and 14 years. Public awareness campaigns include World Parkinson's Day (on the birthday of James Parkinson, 11 April) and the use of a red tulip as the symbol of the disease. No cure for PD exists today, but research is ongoing and medications or surgery can often provide substantial improvement of the motor symptoms.
PD is the most common form of parkinsonism, in which disorders of other causes produce features and symptoms that closely resemble Parkinson’s disease. While most forms of parkinsonism have no known cause, there are cases in which the cause is known or suspected or where the symptoms result from another disorder.
A Brief History
Several early sources, including an Egyptian papyrus, an Ayurvedic medical treatise, the Bible, and Galen's writings describe symptoms resembling those of PD. After Galen, there are no references unambiguously related to PD until the 17th century.
In the 17th and 18th centuries: Several authors, including Sylvius, Gaubius, Hunter and Chomel wrote about elements of the disease.
In 1817: The English doctor James Parkinson published An Essay on the Shaking Palsy in which he reported the first detailed description of six cases of paralysis agitans. He described the characteristic resting tremor, abnormal posture and gait, paralysis and diminished muscle strength, and the way that the disease progresses over time.
Between 1868 and 1881: Early neurologists, including Trousseau, Gowers, Kinnier, and most notably Jean-Martin Charcot, made further additions to the knowledge of the disease. These years were a landmark in the understanding of the disease. Among other advances, Charcot made the distinction between rigidity, weakness and bradykinesia and also championed the renaming of the disease in honor of James Parkinson.
In 1911: Casimir Funk synthesized the first anti-PD drug, Levodopa, but it received little attention until the mid-20th century. It dramatically reduced the anticholinergics and surgery (lesioning of the corticospinal pathway of some of the basal ganglia structures), which theretofore were the only treatments.
In 1912: Frederic Lewy described microscopic particles in affected brains, later named "Lewy bodies".
!n 1919: Konstantin Tetiakoff reported that the substantia nigra was the main cerebral structure affected in Parkinson's disease.
In 1938: Rolf Hassler confirmed Tetiakoff's findings by further studies.
In the 1950s: The underlying biochemical changes in the brain were identified due largely to the work of Arvid Carlsson on the neurotransmitter dopamine.
In 1957: Founding of the National Parkinson Foundation by William Black.
In 1961: Founding of the American Parkinson Association.
In 1967: Levodopa entered clinical practice and brought about a revolution in the management of PD.
In 1967: Publication of the Hoehn and Yahr scale for staging Parkinson's disease.
In 1973: Neurologist Oliver Sacks published Awakenings, an account of his work in the late 1960s with surviving post-encephalitic patients in a New York hospital. Using the then-experimental drug Levodopa, he was able to temporarily "awaken" these individuals from their statue-like state.
Late 1980s: Alim Louis Benabid and colleagues at Grenoble, France, introduced deep brain stimulation (DBS) as a possible treatment.
In 1981: The James Parkinson Tulip cultivar was registered by a Dutch horticulturalist.
In 1992: Founding of the European Parkinson Association.
In 1997: Olen Hornykiewicz elucidated the role of dopamine on PD.
In 1997: Spillantini, Trojanowski, Goerdert and others found that alpha-synuclein was the main component of Lewy bodies.
In the 1990s: Researchers at the (U.S.) National Institutes of Health (NIH) and other institutions studied the genetic profiles of a large Italian family and three Greek families with familial PD and found that their disease was related to a mutation in this gene.
In the late 1990s: A second alpha-synuclein mutation was found in a German family with PD. These findings prompted studies of the role of alpha-synuclein in PD, which led to the discovery that Lewy bodies seen in all cases of PD contain alpha-synuclein protein. This discovery revealed the link between hereditary and sporadic forms of the disease.
In 2003: Studying inherited PD, researchers discovered that the disease in one large family was caused by a triplication of the normal alpha-synuclein gene on one copy of chromosome 4, which caused people in the affected family to produce too much of the normal alpha-synuclein. The study also showed that an excess of the normal form of synuclein could result in PD, just as the abnormal form does.
In May 2006: The FDA approved the drug Rasagiline to be used along with Levodopa for patients with advanced PD or as a single-drug treatment for early PD.
In March 2017, the FDA approved the drug Safinamide tablets as an add-on treatment for individuals with PD who are currently taking Levodopa/Carbidopa and experiencing "off" episodes (when the person's medications are not working well, causing an increase in PD symptoms).
11 April of every year, the birthday of James Parkinson, is World Parkinson Day.
Nature of The Disease
PD is a long-term degenerative disorder of the CNS that mainly affects the motor system. It is a common, disabling and currently incurable neurodegenerative condition that affects over 2% of people over the age of 75. Tremendous progress has been made in recent years in understanding better its possible causes. This has been principally driven by genetic discoveries of the genes/molecules that determine a higher risk factor for developing the disease. We now have the opportunity to harness these discoveries into a more complete understanding of neurodegeneration (cell death) and dysfunction in this disease and to fully characterize the common clinical traits so that PD treatment can be realized.
PD is a long-term degenerative disorder of the CNS that mainly affects the motor system. It is a common, disabling and currently incurable neurodegenerative condition that affects over 2% of people over the age of 75. Tremendous progress has been made in recent years in understanding better its possible causes. This has been principally driven by genetic discoveries of the genes/molecules that determine a higher risk factor for developing the disease. We now have the opportunity to harness these discoveries into a more complete understanding of neurodegeneration (cell death) and dysfunction in this disease and to fully characterize the common clinical traits so that PD treatment can be realized.
The symptoms generally come on slowly over time. Early in the disease, the most obvious symptoms are shaking, rigidity, slowness of movement, and difficulty walking.. Thinking and behavioral problems may also occur. In the advanced stages, dementia (a general mental deterioration due to organic or psychological factors) becomes common. Depression and anxiety are also common, occurring in more than a third of people with PD. Other symptoms include sensory, sleep, and emotional problems [1-3]. The main motor symptoms are collectively called "parkinsonism", or "Parkinson syndrome" (PS).
The cause of PD is generally unknown but, like for many other diseases, it is believed to involve both genetic and environmental factors. Those with a family member affected are more likely to get the disease themselves. There is also an increased risk in people exposed to certain pesticides and among those who have had prior head injuries, while there is a reduced risk in tobacco smokers and those who drink coffee or tea [4]. The motor symptoms of the disease result from the death of cells in the substantia nigra, a region of the midbrain. This results in not enough dopamine in these areas. The reason for this cell death is poorly understood, but involves the build-up of proteins into Lewy bodies in the neurons. Diagnosis of typical cases is mainly based on symptoms, with tests such as neuroimaging being used to rule out other diseases.
There is no cure for PD, with treatment directed at improving symptoms. Initial treatment is typically with the anti-Parkinson medication Levodopa (L-dopa) with dopamine agonists being used once Levodopa becomes less effective. As the disease progresses and neurons continue to be lost, these medications become less effective while at the same time they produce a complication marked by involuntary writhing movements. Diet and some forms of rehabilitation have shown some effectiveness at improving symptoms [5,6]. Surgery to place microelectrodes for deep brain stimulation (DBS) has been used to reduce motor symptoms in severe cases where drugs are ineffective. Evidence for treatments for the non-movement-related symptoms of PD, such as sleep disturbances and emotional problems, is less strong (Figure 1).
Signs and Symptoms
The most recognizable symptoms in PD are movement ("motor") related. Non-motor symptoms, which include autonomic dysfunction, neuropsychiatric problems (mood, cognition, behavior or thought alterations), and sensory (especially altered sense of smell) and sleep difficulties, are also common. Some of these non-motor symptoms may be present at the time of diagnosis. Table 1 summarizes the corresponding symptoms.
Parkinson disease signs and symptoms
| Symptoms | Expressions |
Motor:
|
|
Non-motor disturbances:
|
|
Table 1: Parkinson's disease signs and symptoms.
Motor symptoms
The motor symptoms of PD are the result of reduced dopamine production in the brain's basal ganglia. Four primary motor symptoms are considered cardinal in PD: tremor, slowness of movement (bradykinesia), rigidity, postural instability; however, a fifth one: gait and posture disturbances could also be added [7-8].
The motor symptoms of PD are the result of reduced dopamine production in the brain's basal ganglia. Four primary motor symptoms are considered cardinal in PD: tremor, slowness of movement (bradykinesia), rigidity, postural instability; however, a fifth one: gait and posture disturbances could also be added [7-8].
- Tremor: The most common presenting sign is a coarse slow tremor of the hand at rest, which disappears during voluntary movement of the affected arm and in the deeper stages of sleep. It typically appears in only one hand, eventually affecting both hands as the disease progresses. Frequency of PD tremor is between 4 and 6 Hertz (cycles per second). A feature of tremor is pill-rolling, the tendency of the index finger and thumb to touch and perform together a circular movement. The term derives from the similarity between the movement of people with PD and the early pharmaceutical technique of manually making pills [9].
- Bradykinesia (slowness of movement): It is found in every case of PD, and is due to disturbances in motor planning of movement initiation, and associated with difficulties along the whole course of the movement process, from planning to initiation to execution of a movement. Performance of sequential and simultaneous movement is impaired. Bradykinesia is the most handicapping symptom of Parkinson’s disease leading to difficulties with everyday tasks such as dressing, feeding, and bathing. It leads to particular difficulties in carrying out two independent motor activities at the same time and can be made worse by emotional stress or concurrent illnesses. Paradoxically patients with Parkinson's disease can often ride a bicycle or climb stairs more easily than walk on a level. While most physicians may readily notice bradykinesia, formal assessment requires a patient to do repetitive movements with their fingers and feet.
- Rigidity: This is stiffness and resistance to limb movements caused by increased muscle tone, an excessive and continuous contraction of muscles. In parkinsonism, the rigidity can be uniform ("lead-pipe rigidity") or ratchety ("cogwheel rigidity"). The combination of tremor and increased tone is considered to be at the origin of cogwheel rigidity. Rigidity may be associated with joints pain; such pain being a frequent initial manifestation of the disease. In early stages of PD, rigidity is often asymmetrical and it tends to affect the neck and shoulder muscles prior to the muscles of the face and extremities. With the progression of the disease, rigidity typically affects the whole body and reduces the ability to move.
- Postural instability: It is typical in the later stages of the disease, leading to impaired balance and frequent falls, and secondarily to bone fractures, loss of confidence, and reduced mobility. Instability is often absent in the initial stages, especially in younger people, especially prior to the development of bilateral symptoms. Up to 40% of people diagnosed with PD may experience falls and around 10% may have falls weekly, with the number of falls being related to the severity of PD.
- Gait and posture disturbances: There are other recognized motor signs and symptoms including festination (rapid shuffling steps and a forward-flexed posture when walking with no flexed arm swing). Freezing of gait (brief arrests when the feet seem to get stuck to the floor, especially on turning or changing direction), a slurred monotonous quiet voice, mask-like facial expression, and handwriting that gets smaller and smaller are other common signs.
PD does not affect everyone the same way, and the rate of progression and the particular symptoms differ among individuals. PD symptoms typically begin on one side of the body. However, the disease eventually affects both sides. Even after the disease involves both sides of the body, the symptoms are often less severe on one side than on the other.
Early symptoms of PD may be subtle and occur gradually. Affected people may feel mild tremors or have difficulty getting out of a chair. Activities may take longer to complete than in the past and individuals may note some stiffness in addition to slowness. They may notice that they speak too softly or that their handwriting is slow and looks cramped or small. This very early period may last a long time before the more classical and obvious motor (movement) symptoms appear.
As the disease progresses, the symptoms of PD may begin to interfere with daily activities. Affected individuals may not be able to hold utensils steady or they may find that the shaking makes reading a newspaper difficult. People with PD often develop a so-called parkinsonian gait that includes a tendency to lean forward, taking small quick steps as if hurrying (or festination), and reduced swinging in one or both arms. They may have trouble initiating movement (start hesitation), and they may stop suddenly as they walk (freezing).
A number of other symptoms may accompany PD, and some can be treated with medication or physical therapy:
- Depression: This common disorder may appear early in the course of the disease, even before other symptoms are noticed. Some people lose their motivation and become dependent on family members. Fortunately, depression typically can be treated successfully with antidepressant medications such as Amytriptyline or Fluoxetine.
- Emotional changes:Some people with PD become fearful and insecure, while others may become irritable or uncharacteristically pessimistic.
- Difficulty with swallowing and chewing:Muscles used in swallowing may work less efficiently in later stages of the disease: Food and saliva may collect in the mouth and back of the throat, which can result in choking or drooling. These problems may also make it difficult to get adequate nutrition. Speech-language therapists, occupational therapists, and dietitians can often help with these problems.
- Speech changes:About half of all individuals with PD have speech difficulties that may be characterized as speaking too softly or in a monotone voice. Some may hesitate before speaking, slur, or speak too fast. A speech therapist may be able to help these individuals reduce some of these problems.
- Urinary problems or constipation:In some people with PD, bladder and bowel problems can occur due to the improper functioning of the autonomic nervous system (ANS), which is responsible for regulating smooth muscle activity. Medications can effectively treat some of these symptoms.
- Skin problems:In PD, the skin on the face may become oily, particularly on the forehead and at the sides of the nose. The scalp may become oily too, resulting in dandruff. In other cases, the skin can become very dry. Standard treatments for skin problems can help.
- Sleep problems: Sleep problems are common in PD and can be worsened by medications. Symptoms can manifest as difficulty staying asleep at night, restless sleep, nightmares and emotional dreams, and daytime drowsiness (including sudden sleep attacks resembling narcolepsy) or sudden sleep onset during the day. Another common problem is “REM behavior disorder (RBD)” in which people act out their dreams, potentially resulting in injury to themselves or their bed partners. Such symptoms may begin years before the development of motor or cognitive features of PD or DLB. The medications used to treat PD may contribute to some of these sleep issues. Many of these problems respond to specific therapies.
- Orthostatic hypotension: Orthostatic hypotension (OH) is a sudden drop in blood pressure when a person stands up from a lying-down or seated position. This may cause dizziness, lightheadedness, and, in extreme cases, loss of balance or fainting. Studies have suggested that, in PD, this problem results from a loss of nerve endings in the sympathetic nervous system that controls heart rate, blood pressure, and other automatic functions in the body. The medications used to treat PD may also contribute to this symptom. Orthostatic hypotension may improve by increasing salt intake. Physicians treating the disorder may also reduce anti-hypertension drug dosage or by prescribing medications such as Fluorocortisone.
- Muscle cramps and dystonia: The rigidity and lack of normal movement associated with PD often causes muscle cramps, especially in the legs and toes. Massage, stretching, and applying heat may help with these cramps. PD can also be associated with dystonia-sustained muscle contractions that cause forced or twisted positions. Dystonia in PD is often caused by fluctuations in the body's level of dopamine. Management strategies may involve adjusting medications.
- Pain:Many people with PD develop aching muscles and joints because of the rigidity and abnormal postures often associated with the disease. Treatment with Levodopa and other dopaminergic drugs often alleviates these pains to some extent. Certain exercises may help.
- Fatigue and loss of energy: Many people with PD often have fatigue, especially late in the day. Fatigue may be associated with depression or sleep disorders, but it may also result from muscle stress or from overdoing activity when the person feels well. Fatigue may also result from akinesia-trouble initiating or carrying out movement. Exercise, good sleep habits, staying mentally active, and not forcing too many activities in a short time may help to alleviate fatigue.
- Sexual dysfunction: Because of its effects on nerve signals from the brain, PD may cause sexual dysfunction. PD-related depression or use of certain medications may also cause decreased sex drive and other problems. People should discuss these issues with their physician as they may be treatable.
- Hallucinations, delusions, and other psychotic symptoms:They can be caused by the drugs prescribed for PD. Reducing PD medications dosages or changing medications may be necessary if hallucinations occur. If such measures are not effective, doctors sometimes prescribe drugs called atypical antipsychotics, which include Clozapine and Quetiapine. The typical antipsychotic drugs, which include Haloperidol, worsen the motor symptoms of PD and should not be used.
Neuropsychiatric symptoms
PD can also cause neuro-psychiatric disturbances, which can range from mild to severe. These include disorders of cognition, mood, behavior, and thought.
PD can also cause neuro-psychiatric disturbances, which can range from mild to severe. These include disorders of cognition, mood, behavior, and thought.
- Cognitive disturbances: They can occur in the early stages of the disease and sometimes prior to diagnosis, and increase in prevalence with duration of the disease. The most common cognitive deficit in PD is executive dysfunction, which can include problems with planning, cognitive flexibility, abstract thinking, rule acquisition, inhibiting inappropriate actions, initiating appropriate actions, working memory, and control of attention. Other cognitive difficulties include slowed cognitive processing speed, impaired recall and impaired perception and estimation of time. Nevertheless, improvement appears when recall is aided by cues. Visuospatial difficulties are also part of the disease, seen for example when the individual is asked to perform tests of facial recognition and perception of the orientation of drawn lines. A person with PD has two to six times the risk of dementia compared to the general population.
- Dementia or other cognitive problems:Some people with PD may develop memory problems and slow thinking. Cognitive problems become more severe in late stages of PD, and a diagnosis of Parkinson’s disease dementia (PDD) may be given. Memory, social judgment, language, reasoning, or other mental skills may be affected. There is currently no way to halt PD dementia, but drugs such as Rivastigmine, Donepezil, or Memantine may help. The medications used to treat the motor symptoms of PD may cause confusion and hallucinations. The prevalence of dementia increases with age and, to a lesser degree, duration of the disease [10].
- Impulse control disorders: These include pathological gambling, compulsive sexual behavior, binge eating, compulsive shopping and reckless generosity can be caused by medication, particularly orally active dopamine agonists. The dopamine dysregulation syndrome (DRS) - with wanting of medication leading to overusage – is a rare complication of Levodopa use [11].
- Behavior and mood alterations: They are more common in PD without cognitive impairment than in the general population, and are usually present in PD with dementia. The most frequent mood difficulties are depression, apathy, and anxiety. Establishing the diagnosis of depression is complicated by the fact that the body language of depression may masquerade as PD including a sad expressionless anxious face, a hang dog appearance, slow movement, and monotonous speech. Up to 30% of people with PD may experience symptoms of anxiety, ranging from a generalized anxiety disorder to social phobia, panic disorders and obsessive compulsive disorders (OCD). They contribute to impaired quality of life and increased severity of motor symptoms such as on/off fluctuations or freezing episodes.
- Punding: Here, complicated repetitive aimless stereotyped behaviors occuring for many hours is another disturbance caused by anti-Parkinson medication.
- Hallucinations or delusions: They occur in approximately 50% of people with PD over the course of the illness, and may herald the emergence of dementia. These range from minor hallucinations – "sense of passage" (something quickly passing beside the person) or "sense of presence" (the perception of something/someone standing just to the side or behind the person) – to full blown vivid, formed visual hallucinations and paranoid ideation. Auditory hallucinations are uncommon in PD, and are rarely described as voices. It is now believed that psychosis is an integral part of the disease. A psychosis with delusions and associated delirium is a recognized complication of anti-Parkinson drug treatment and may also be caused by urinary tract infections (as frequently occurs in the fragile elderly), but drugs and infection are not the only factors, and underlying brain pathology or changes in neurotransmitters or their receptors (e.g., acetylcholine, serotonin) are also thought to play a role in psychosis in PD (12-14).
- In addition to neuropsychiatric and motor symptoms, PD can impair other functions.
- Alterations in the autonomous nervous system: They can lead to orthostatic hypotension (OH, low blood pressure upon standing) discussed earlier, oily skin and excessive sweating, urinary incontinence, and altered sexual function. Constipation and impaired stomach emptying (gastric dysmotility) can be severe enough to cause discomfort and even endanger health. Changes in perception may include an impaired sense of smell, disturbed vision, pain, and paresthesia (tingling and numbness). All of these symptoms can occur years before diagnosis of the disease.
Figure 2 illustrates the brain regions affected by Parkinson's disease.
Classification
Table 2 summarizes the classification of Parkinson's disease and its variations.
| Disorder type | Features | Region(s) affected | Characteristics |
| Parkinsonism (Parkinson syndrome) or bradykinesia | Causes:
|
Main motor systems |
|
| Idiopathic parkinsonism | Most common form of parkinsonism Cause: None identifiable |
||
| Atypical parkinsonism (Parkinson-plus syndromes) |
|
||
| Parkinson | Long term degenerative disorder of the central nervous system Causes:
|
Motor system: cells death in the substantia nigra |
|
| Synucleiopathy | Example: Dementia with Lewy bodies | Abnormal accumulation of alpha-synuclein protein in the brain |
Table 2: Features, region(s) affected and symptoms of Parkinson's disease and its variations.
The movement difficulties found in PD are called "parkinsonism" and a number of different disorders feature parkinsonism. "Parkinsonism" is defined as bradykinesia, that is slowness in initiating voluntary movements, with progressive reduction in speed and range of repetitive actions such as voluntary finger-tapping in combination with one of three other physical signs: muscular rigidity, tremor at rest, and postural instability [7].
PD is the most common form of parkinsonism and is sometimes called "idiopathic parkinsonism", meaning parkinsonism with no identifiable cause. Identifiable causes of parkinsonism include toxins, infections, side effects of drugs, metabolic derangement, and brain lesions such as strokes. Several neurodegenerative disorders also may present with parkinsonism and are sometimes referred to as "atypical parkinsonism" or “Parkinson plus syndromes” (illnesses with parkinsonism plus some other features distinguishing them from PD). They include multiple system atrophy, progressive supranuclear palsy, corticobasal degeneration, and dementia with Lewy bodies (DLB).
Scientists sometimes refer to PD as a “synucleiopathy” (due to an abnormal accumulation of alpha-synuclein protein in the brain) to distinguish it from other neurodegenerative diseases, such as “Tauopathy” or Alzheimer's disease (AD) where the brain accumulates tau protein. Considerable clinical and pathological overlap exists between tauopathies and synucleinopathies. In contrast to PD, AD presents most commonly with memory loss, but the cardinal signs of PD (slowness, tremor, stiffness, and postural instability) are not normal features of AD.
DLB is another synucleinopathy and it has close pathological similarities with PD, especially with the subset of PD cases with dementia. The relationship between PD and DLB is complex and incompletely understood. They may represent parts of a continuum with variable distinguishing clinical and pathological features or they may prove to be separate diseases.
Causes of the Disease
Research indicates that PD is the product of a complex interaction of genetic and environmental factors.
Research indicates that PD is the product of a complex interaction of genetic and environmental factors.
Genetic causes
Around 15% of individuals with PD have a first-degree relative who has the disease, and 5–10% of people with PD are known to have forms of the disease that occur because of a mutation in one of several specific genes. Harboring one of these gene mutations may not lead to the disease; susceptibility factors put the individual at an increased risk, often in combination with other risk factors, which also affect age of onset, severity and progression [15].
Around 15% of individuals with PD have a first-degree relative who has the disease, and 5–10% of people with PD are known to have forms of the disease that occur because of a mutation in one of several specific genes. Harboring one of these gene mutations may not lead to the disease; susceptibility factors put the individual at an increased risk, often in combination with other risk factors, which also affect age of onset, severity and progression [15].
Scientists have identified several genetic mutations associated with PD (see Table 3), including the alpha-synuclein gene, and many other genes. Studying the genes responsible for inherited cases of PD can help understand both inherited and sporadic cases. The same genes and proteins that are altered in inherited cases may also be altered in sporadic cases by environmental toxins or other factors. Discovering genes will help identify new ways of treating PD. The first to be identified was alpha-synuclein followed by parkin, DJ-1, PINK1, and LRRK2.
Genes implicated in the development of PD include SNCA, LRRK2, GBA, PRKN, PINK1, PARK7, VPS35, EIF4G1, DNAJC13, CHCHD2, DJ-1 and Parkin (see Table 3).
| Gene mutations | Features | Gene mutations | Features |
| SNCA |
|
PARK7 | |
| LRRK2 |
|
VPS35 | |
| GBA |
|
EIF4GI | |
| PRKN | DNAJC13 | ||
| PINK1 |
|
CHCHD2 | |
| DJ1 |
|
Parkin | Translated into a protein that normally helps cells break down and recycle proteins |
Table 1: Genes implicated in Parkinson’s disease.
Several Parkinson-related genes are involved in the function of lysosomes, organelles that digest cellular waste products. It has been suggested that some cases of PD may be caused by lysosome dysfunctions that reduce the ability of cells to break down alpha-synuclein.
Pathophysiology
The main pathological characteristics of PD are cell death in the brain's basal ganglia (affecting up to 70% of the dopamine secreting neurons in the substantia nigra pars compacta by the end of life) and the presence of Lewy bodies (accumulations of the protein alpha-synuclein) in many of the remaining neurons. This loss of neurons is accompanied by the death of astrocytes (star-shaped glialcells) and a significant increase in the number of microglia (another type of glial cell) in the substantia nigra [16].
The main pathological characteristics of PD are cell death in the brain's basal ganglia (affecting up to 70% of the dopamine secreting neurons in the substantia nigra pars compacta by the end of life) and the presence of Lewy bodies (accumulations of the protein alpha-synuclein) in many of the remaining neurons. This loss of neurons is accompanied by the death of astrocytes (star-shaped glialcells) and a significant increase in the number of microglia (another type of glial cell) in the substantia nigra [16].
There are five major pathways in the brain connecting other brain areas with the basal ganglia. These are known as (1) the motor, (2) oculo-motor, (3) associative, (4) limbic and (5) orbitofrontal circuits, with names indicating the main projection area of each circuit. All of them are affected in PD, and their disruption explains many of the symptoms of the disease, since these circuits are involved in a wide variety of functions, including movement, attention and learning. Scientifically, the motor circuit has been examined the most intensively. Dopamine acts to facilitate this release of inhibition, so high levels of dopamine function tend to promote motor activity, while low levels of dopamine function, such as occur in PD, demand greater exertions of effort for any given movement. The net effect of dopamine depletion is to produce hypokinesia, an overall reduction in motor output. Conversely, drugs that are used to treat PD may produce excessive dopamine activity, allowing motor systems to be activated at inappropriate times and thereby producing dyskinesias.
Brain cell death
Several mechanisms have been hypothesized that may cause brain cells to be lost:
Several mechanisms have been hypothesized that may cause brain cells to be lost:
- Abnormal accumulation of the protein alpha-synuclein bound to ubiquitinin damaging cells: This insoluble protein accumulates inside neurons forming inclusions called Lewy bodies. According to the Braak staging (a classification of the disease based on pathological findings), Lewy bodies first appear in the olfactory bulb, medulla oblongata and pontine tegmentum. At this stage, individuals may be asymptomatic or may have early non-motor symptoms (such as loss of sense of smell, or some sleep or automatic dysfunction). As the disease progresses, Lewy bodies develop in the substantia nigra, areas of the midbrain and basal forebrain and, finally, the neocortex. These brain sites are the main places of neuronal degeneration in PD. However, Lewy bodies may not cause cell death and they may be protective (with the abnormal protein sequestered or walled off). Other forms of alpha-synuclein (e.g., oligomers) that are not aggregated in Lewy bodies and Lewy neurites may actually be the toxic forms of the protein. In people with dementia, a generalized presence of Lewy bodies is common in cortical areas. Neurofibrillary tangles and senile plaques, characteristic of Alzheimer's disease, are not common unless the person is demented.
- Other cell-death mechanisms: These include: proteosomal and lysosomal system dysfunction and reduced mitochondrial activity. Iron accumulation in the substantia nigra is typically observed in conjunction with the protein inclusions. It may be related to oxidative stress, protein aggregation and neuronal death, but the mechanisms are not fully understood.
PD occurs when nerve cells, or neurons, in the brain die or become impaired. Although many brain areas are affected, the most common symptoms result from the loss of neurons in an area near the base of the brain called the substantia nigra. Normally, the neurons in this area produce the important brain chemical known as dopamine, a chemical messenger responsible for transmitting signals between the substantia nigra and the next "relay station" of the brain, the corpus striatum, to produce smooth, purposeful movement. Loss of dopamine results in abnormal nerve firing patterns within the brain that cause impaired movement. Studies have shown that most people with PD have lost 60 to 80 percent or more of the dopamine-producing cells in the substantia nigra by the time symptoms appear, and have also lost the nerve endings that produce the neurotransmitter norepinephrine. Norepinephrine, which is closely related to dopamine, is the main chemical messenger of the sympathetic nervous system (SNS), the part of the nervous system that controls many automatic functions of the body, such as pulse and blood pressure. The loss of norepinephrine might explain several of the non-motor features seen in PD, including fatigue and abnormalities of blood pressure regulation.
The affected brain cells of people with PD contain Lewy bodies-deposits of the protein alpha-synuclein. We do not yet know why Lewy bodies form or what role they play in the disease. Some research suggests that the cell’s protein disposal system may fail in people with PD, causing proteins to build up to harmful levels and trigger cell death. Additional studies have found evidence that clumps of protein that develop inside brain cells of people with PD may contribute to the death of neurons. Some researchers speculate that the protein buildup in Lewy bodies is part of an unsuccessful attempt to protect the cell from the toxicity of smaller aggregates, or collections, of synuclein (see Figure 3).
While the precise cause of PD is unknown, some cases are hereditary and can be traced to specific genetic mutations (see Table 3). Nonetheless, most cases are sporadic-i.e., the disease does not typically run in families. It is thought that PD likely results from a combination of genetic susceptibility and exposure to one or more unknown environmental factors that trigger the disease.
Environmental causes
Exposure to certain toxins has caused parkinsonian symptoms in rare circumstances (such as exposure to MPTP, an illicit drug, or in miners exposed to the metal manganese). Other still-unidentified environmental factors may also cause PD in genetically susceptible individuals. Main environmental causes are:
Exposure to certain toxins has caused parkinsonian symptoms in rare circumstances (such as exposure to MPTP, an illicit drug, or in miners exposed to the metal manganese). Other still-unidentified environmental factors may also cause PD in genetically susceptible individuals. Main environmental causes are:
- Postencephalitic parkinsonism: Just after the first World War, the viral disease encephalitis lethargica affected almost 5 million people throughout the world, and then suddenly disappeared in the 1920s. Known as sleeping sickness in the United States, this disease killed one-third of its victims and led to post-encephalitic parkinsonism in many others. This resulted in a movement disorder that appeared sometimes years after the initial illness. In rare cases, other viral infections, including equine encephalomyelitis (western, easter, and Japanese B encephalitis) have caused parkinsonian symptoms.
- Drug-induced parkinsonism: A reversible form of parkinsonism sometimes results from use of certain drugs, such as Chlorpromazine and Haloperidol, which are typically prescribed for patients with psychiatric disorders. Some drugs used for stomach disorders (Metoclopramide), high blood pressure (Reserpine), and others such as Valproate can cause tremor and bradykinesia. Stopping the medication or lowering the dosage of these medications usually causes the symptoms to go away.
- Toxin-induced parkinsonism. Some toxins can cause parkinsonism by various mechanisms. The chemical MPTP also causes a permanent form of parkinsonism that closely resembles PD. Investigators discovered this reaction in the 1980s when heroin addicts in California who had taken an illicit street drug contaminated with MPTP began to develop severe parkinsonism. This discovery, which showed that a toxic substance could damage the brain and produce parkinsonian symptoms, led to a dramatic breakthrough in Parkinson's research.
- Parkinsonism-dementia complex of Guam. This disease occurs among the Chamorro populations of Guam and the Mariana Islands and may be accompanied by a motor neuron disease resembling amyotrophic lateral sclerosis (ALS, Lou Gehrig's disease). The course of the disease is rapid, with death typically occurring within 5 years.
Diagnosis
There are currently no blood or laboratory tests that diagnose sporadic PD. Therefore, the diagnosis is based on medical history and a neurological examination. In some cases, PD can be difficult to diagnose accurately early on in the course of the disease. Early signs and symptoms of PD may sometimes be dismissed as the effects of normal aging. Doctors may sometimes request brain scans or laboratory tests in order to rule out other disorders. Computed Tomography (CT) scans of people with PD usually appear normal. Magnetic Resonance Imaging (MRI) has become more accurate in diagnosis of the disease over time, specifically through iron-sensitive T2* (a relaxation time) and Spin-Weighted Images (SWI) sequences at a magnetic field strength of at least 3 Tesla (T), both of which can demonstrate absence of the characteristic 'swallow tail' imaging pattern in the dorsolateral substantia nigra. In a meta-analysis, absence of this pattern was 98% sensitive and 95% specific for the disease. Diffusion MRI has shown potential in distinguishing between PD and Parkinson-plus syndromes, though its diagnostic value is still under investigation. CT and MRI are also used to rule out other diseases that can be secondary causes of parkinsonism, most commonly encephalitis and chronic ischemic insults, as well as less frequent entities such as basal ganglia tumors and hydrocephalus.
Dopamine-related activity in the basal ganglia can be directly measured with Positron Emission Tomography (PET) and Single Photon Emission Computed Tomograpjhy (SPECT) scans. A finding of reduced dopamine-related activity in the basal ganglia can rule out drug-induced parkinsonism, but reduced basal ganglia dopamine-related activity is seen in both PD and the Parkinson-plus disorders so these scans are not reliable in distinguishing PD from other neurodegenerative causes of parkinsonism. However, CT and MRI brain scans of people with PD usually appear normal. Since many other diseases have similar features but require different treatments, making a precise diagnosis is important so that people can receive the proper treatment.
There are four approaches to diagnosis:
- Initial clinico-medical: It is based on a careful medical history, neurological examination and a Levodopa test which, if resulting in any improvement in motor impairment helps confirm the diagnosis. This is followed by periodical review to confirm accuracy of the diagnosis. However, anti-PD medications are less effective at controlling Parkinson-plus syndrome. Note that a history of stroke and drugs, faster progression rates, early cognitive dysfunction or postural instability, minimal tremor or symmetry at onset may rather indicate a Parkinson plus disease. Genetic forms with an autosomal dominant or recessive pattern of inheritance are sometimes referred to as familial Parkinson's disease or familial parkinsonism.
- The U.K. Queen Square Brain Bank for Neurological Disorders (QSBBND) at the UCL Institute of Neurology: It holds a unique archive of brains donated by individuals with neurodegenerative disease and neurologically normal controls. It specializes in parkinsonian movement disorders, including PD and multiple system atrophy and holds the national collection of brains donated by individuals with progressive supranuclear palsy (PSP). Recently, the collection has been developed to include donated brains from prospectively studied people with familial dementia, in collaboration with the Dementia Research Group (DRG). The QSBBND also banks brains donated by people with dystonia and Gilles de la Tourette syndrome. The diagnosis requires slowness of movement (bradykinesia) plus either rigidity, resting tremor, or postural instability. Other possible causes of these symptoms need to be ruled out. Finally, three or more of the following supportive features are required during onset or evolution: unilateral onset, tremor at rest, progression in time, asymmetry of motor symptoms, response to Levodopa for at least five years, clinical course of at least ten years and appearance of dyskinesia induced by the intake of excessive Levodopa.
- The U.S. National Institute of Neurological Disorders and Stroke (NINDS): It is an Institute within the National Institutes of Health (NIH) that aims to seek fundamental knowledge about the brain and nervous system and to use that knowledge to reduce the burden of neurological disease.
- The International Parkinson and Movements Disorder Society (PMDS)' s task force: Has proposed diagnostic criteria for PD as well as research criteria for the diagnosis of prodromal disease, but these will require validation against the more established criteria.
Staging of the Disease
The average life expectancy of a person with PD is generally the same as for people who do not have the disease. Fortunately, there are many treatment options available for people with PD. However, in the late stages, PD may no longer respond to medications and can become associated with serious complications such as choking, pneumonia, and falls.
PD is a slowly progressive disorder. It is not possible to predict what course the disease will take for an individual person. One commonly used scale neurologists use for describing how the symptoms of PD have progressed in a patient is the “Hoehn and Yahr Scale” (HYS) for the staging of PD (Table 4), published in 1967, and its modified version:
| Stage | Symptoms |
| 1 | Symptoms on one side of the body only |
| 2 | Symptoms on both sides of the body. No impairment of balance |
| 3 | Balance impairment. Mild to moderate disease. Physically independent |
| 4 | Severe disability, but still able to walk or stand unassisted |
| 5 | Wheelchair-bound or bedridden unless assisted |
Table 4: The Hoehn and Yahr scale for staging Parkinson's disease.
Another commonly used scale is the “Movement Disorders Society-Unified Parkinson's Disease Rating Scale” (MDS-UPDRS), which measures motor movement in PD (Table 5):
| Stage | Symptoms |
| 1 | Non-motor experiences of daily living |
| 2 | Motor experiences of daily living |
| 3 | Motor examination |
| 4 | Motor complications |
Table 5: The Hoehn and Yahr scale for staging Parkinson's disease.
Both the HYS and the MDS-UPDRS scales are used to describe how individuals are faring and to help assess treatment response.
Prognosis
As stated earlier, PD is both chronic, meaning it persists over a long period of time, and progressive, meaning its symptoms grow worse over time. Although some people become severely disabled, others experience only minor motor disruptions. Tremor is the major symptom for some individuals, while for others tremor is only a minor complaint and other symptoms are more troublesome. It is currently not possible to predict which symptoms will affect an individual, and the intensity of the symptoms also varies from person to person.
PD invariably progresses with time. A severity rating method known as the “Unified Parkinson's Disease Rating Scale” (UPDRS) is the most commonly used metric for clinical study. A modified version known as the MDS-UPDRS is also sometimes used. Left untreated, motor symptoms advance aggressively in the early stages of the disease and more slowly later. Untreated, individuals are expected to lose independent ambulation after an average of eight years and be bedridden after ten years. However, it is uncommon to find untreated people nowadays. Medication has improved the prognosis of motor symptoms, while at the same time it is a new source of disability, because of the undesired effects of Levodopa after years of use. In people taking Levodopa, the progression time of symptoms to a stage of high dependency from caregivers may be over 15 years. However, it is hard to predict what course the disease will take for a given individual. Age is the best predictor of disease progression. The rate of motor decline is greater in those with less impairment at the time of diagnosis, while cognitive impairment is more frequent in those who are over 70 years of age at symptom onset.
Since current therapies improve motor symptoms, disability at present is mainly related to non-motor features of the disease. Nevertheless, the relationship between disease progression and disability is not linear. Disability is initially related to motor symptoms. As the disease advances, disability is more related to motor symptoms that do not respond adequately to medication, such as swallowing/speech difficulties, and gait/balance problems; and also to Levodopa-induced complications, which appear in up to 50% of individuals after 5 years of Levodopa usage. Finally, after ten years, most people with the disease have autonomic disturbances, sleep problems, mood alterations and cognitive decline. All of these symptoms, especially cognitive decline, greatly increase disability.
The life expectancy of people with PD is reduced. Mortality ratios are around twice those of unaffected people. Cognitive decline and dementia, old age at onset, a more advanced disease state and presence of swallowing problems are all mortality risk factors. On the other hand, a disease pattern mainly characterized by tremor as opposed to rigidity predicts an improved survival. Death from aspiration pneumonia is twice as common in individuals with PD as in the healthy population.
Treatment
At present, there is no cure for PD, but medications or surgery can often provide improvement in the motor symptoms.
Drug therapy
A variety of medications provide dramatic relief from the symptoms. Anticholinergics (category 5 in Table 6 below) and surgery (lesioning of the corticospinal pathway or some of the basal ganglia structures) were the only treatments until the arrival of Levodopa, which reduced their use dramatically. The motor symptoms of PD are the result of reduced dopamine production in the brain's basal ganglia. Dopamine does not cross the BBB, so it cannot be taken as a medicine to boost the brain's depleted levels of dopamine. However a precursor of dopamine, Levodopa, can pass through the BBB to the brain where it is readily converted to dopamine, and administration of Levodopa temporarily diminishes the motor symptoms of PD. Levodopa has been the most widely used PD treatment for over 40 years. However, only 5–10% of Levodopa crosses the BBB. Much of the remainder is metabolized to dopamine elsewhere in the body, causing a variety of side effects including nausea, vomiting and orthostatic hypotension.
Carbidopa and Benserazide are dopa decarboxylase inhibitors, which do not cross the BBB and inhibit the conversion of Levodopa to dopamine outside the brain, reducing side effects and improving the availability of Levodopa for passage into the brain. One of these drugs is usually taken along with Levodopa, often combined with Levodopa in the same pill. Levodopa use leads in the long term to the development of complications: involuntary movements called dyskinesias, and fluctuations in the effectiveness of the medication. When fluctuations occur, a person can cycle through phases with good response to medication and reduced PD symptoms ("on" state), and phases with poor response to medication and significant PD symptoms ("off" state). Using lower doses of Levodopa may reduce the risk and severity of these Levodopa-induced complications. A former strategy to reduce Levodopa-related dyskinesia and fluctuations was to withdraw Levodopa medication for some time. This is now discouraged since it can bring on dangerous side effects such as neuroleptic malignant syndrome (NMS). Most people with PD will eventually need Levodopa and will later develop Levodopa-induced fluctuations and dyskinesias.
There are controlled-release versions of Levodopa. Older controlled-release Levodopa preparations have poor and unreliable absorption and bioavailabilty and have not demonstrated improved control of PD motor symptoms or a reduction in Levodopa-related complications when compared to immediate release preparations. A newer extended-release Levodopa preparation does seem to be more effective in reducing fluctuations but in many patients problems persist. Intestinal infusions of Levodopa (Duodopa) can result in striking improvements in fluctuations compared to oral Levodopa when the fluctuations are due to insufficient uptake caused by gastroparesis. Other oral, longer acting formulations are under study and other modes of delivery (inhaled, transdermal) are being developed.
Nerve cells can use Levodopa to make dopamine and replenish the brain's reduced supply. People cannot simply take dopamine pills because, as stated above, dopamine does not easily pass through the BBB, a protective lining of cells inside blood vessels that regulate the transport of oxygen, glucose, and other substances in the brain. Usually, people are given Levodopa combined with another substance called Carbidopa. When added to Levodopa, Carbidopa prevents the conversion of Levodopa into dopamine except for in the brain; this stops or diminishes the side effects due to dopamine in the bloodstream. Levodopa/Carbidopa is often very successful at reducing or eliminating the tremors and other motor symptoms of PD during the early stages of the disease. It allows the majority of people with PD to extend the period of time in which they can lead active, productive lives. People often see noticeable improvement in their symptoms after starting Levodopa/Carbidopa therapy.
However, they may need to increase the dose gradually for maximum benefit. Levodopa is often so effective that some people may not show symptoms during the early stages of the disease as long as they take the medicine. But Levodopa is not a cure. Although it can reduce the symptoms of PD, it does not replace lost nerve cells and it does not stop the progression of the disease. Levodopa/Carbidopa can have a variety of side effects. The most common initial side effects include nausea, low blood pressure, and restlessness. The nausea and vomiting caused by Levodopa are greatly reduced by the right combination of Levodopa and Carbidopa. The drug also can cause drowsiness or sudden sleep onset, which can make driving and other activities dangerous. Long-term use of Levodopa sometimes causes hallucinations and psychosis. Safinamide tablets can be used as an add-on treatment for individuals with PD who are currently taking Levodopa/Carbidopa and experiencing "off" episodes (when the person's medications are not working well, causing an increase in PD symptoms).
Although Levodopa/Carbidopa helps most people with PD, not all symptoms respond equally to the drug. Levodopa usually helps most with bradykinesia and rigidity. Problems with balance may not respond. Medications for PD fall into six categories (see Table 6). The cornerstone of therapy for PD remains the drug Levodopa (also called L-dopa).
- Drugs that increase the level of dopamine in the brain: The most common drugs for PD are dopamine precursors-substances such as Levodopa that cross the BBB and are then changed into dopamine. Other drugs mimic dopamine or prevent or slow its breakdown. Usually, affected individuals are given Levodopa combined with Carbidopa. Carbidopa delays the conversion of Levodopa into dopamine until it reaches the brain. Nerve cells can use Levodopa to make dopamine and replenish the brain's dwindling supply. Although Levodopa helps at least three-quarters of parkinsonian cases, not all symptoms respond equally to the drug. Bradykinesia and rigidity respond best, while tremor may be only marginally reduced. Problems with balance and other symptoms may not be alleviated at all.
- Drugs that mimic dopamine (dopamine agonists): These drugs, which include Apomorphine, Pramipexole, Ropinirole, and Rotigotine, mimic the role of dopamine in the brain. They can be given alone or with Levodopa. They are somewhat less effective than Levodopa in treating PD symptoms, but work for longer periods of time. Many of the potential side effects are similar to those associated with the use of Levodopa, including drowsiness, sudden sleep onset, hallucinations, confusion, dyskinesias, edema (swelling due to excess fluid in body tissues), nightmares, and vomiting. In rare cases, they can cause an uncontrollable desire to gamble, hypersexuality, or compulsive shopping. Several dopamine agonists that bind to dopamine receptors in the brain have similar effects to Levodopa. These were initially used as a complementary therapy to Levodopa for individuals experiencing Levodopa complications (on-off fluctuations and dyskinesias); they are now mainly used on their own as first therapy for the motor symptoms of PD with the aim of delaying the initiation of Levodopa therapy and so delaying the onset of Levodopa's complications. Dopamine agonists include Bromocriptine, Pergolide, Pramipexole, Ropinirole, Piribedil, Cabergoline, Apomorphine and Lisuride. Though dopamine agonists are less effective than Levodopa at controlling PD motor symptoms, they are usually effective enough to manage these symptoms in the first years of treatment. Dyskinesias due to dopamine agonists are rare in younger people who have PD but, along with other complications, become more common with older age at onset. Thus dopamine agonists are the preferred initial treatment for younger onset PD, and Levodopa is preferred for older onset PD. Dopamine agonists produce significant, although usually mild, side effects including drowsiness, hallucinations, insomnia, nausea, and constipation. Sometimes side effects appear even at a minimal clinically effective dose, leading the physician to search for a different drug. Agonists have been related to impulse control disorders (such as compulsive sexual activity, eating, gambling and shopping) even more strongly than Levodopa. They tend to be more expensive than Levodopa. Apomorphine, a non-orally administered dopamine agonist, may be used to reduce off periods and dyskinesia in late PD. It is administered by intermittent injections or continuous subcutaneous infusions. Since secondary effects such as confusion and hallucinations are common, individuals receiving Apomorphine treatment should be closely monitored. Two dopamine agonists that are administered through skin patches (Lisuride and Rotigotine) are useful for people in the initial stages and possibly to control off states in those in the advanced state.
- Drugs that inhibit dopamine breakdown (MAO-B inhibitors): These drugs inhibit the enzyme monoamine oxidase B, or MAO->B, which breaks down dopamine in the brain. MAO-B inhibitors cause dopamine to accumulate in surviving nerve cells and reduce the symptoms of PD. Studies supported by the (U.S.) National Institute of Neurological Disorders and Stroke (NINDS) have shown that Selegiline (also called Deprenyl) can delay the need for Levodopa therapy by up to a year or more. When Selegiline is given with Levodopa, it appears to enhance and prolong the response to Levodopa and thus may reduce wearing-off. Selegiline is usually well-tolerated, although side effects may include nausea, orthostatic hypotension, or insomnia. It should not be taken with the antidepressant Fluoxetine or the sedative Meperidine, because combining Selegiline with these drugs can be harmful. The drug Rasagiline is used in treating the motor symptoms of PD with or without Levodopa. Whether Rasagiline slows progression of PD is still controversial. MAO-B inhibitors (Safinamide, Selegiline and Rasagiline) increase the amount of dopamine in the basal ganglia by inhibiting the activity of MAO-B, an enzyme which breaks down dopamine. Like dopamine agonists, their use may delay the commencement of Levodopa therapy in early disease, but MAO-B inhibitors produce more adverse effects and are less effective than Levodopa at controlling PD motor symptoms. There are few studies of their effectiveness in the advanced stage, although results suggest that they are useful to reduce fluctuations between on and off periods. An initial study indicated that Selegiline in combination with Levodopa increased the risk of death, but this was later disproven.
- Drugs that inhibit dopamine breakdown (COMT inhibitors): Catechol-O-methyltransferase (COMT) is another enzyme that breaks down dopamine. The drugs Entacapone and Tolcapone prolong the effects of Levodopa by preventing the breakdown of dopamine. COMT inhibitors can decrease the duration of "off periods” of one's dose of Levodopa. The most common side effect is diarrhea. The drugs cause nausea, sleep disturbances, dizziness, urine discoloration, abdominal pain, low blood pressure, or hallucinations in a few rare cases. The usefulness of Tolcapone is limited by possible complications as it has caused severe liver disease, and people taking Tolcapone need regular monitoring of their liver function. A similarly effective drug, Entacapone, has not been shown to cause significant alterations of liver function. Licensed preparations of Entacapone contain Entacapone alone or in combination with Carbidopa and Levodopa.
- Drugs that decrease the action of acetylcholine (anticholinergics): These drugs, which include Trihexyphenidyl, Benztropine, and Ethopropazine, decrease the activity of the neurotransmitter acetylcholine (production or uptake) and can be particularly effective in reducing tremors. Side effects may include dry mouth, constipation, urinary retention, hallucinations, memory loss, blurred vision, and confusion. They affect other neurotransmitters in the body in order to ease some of the symptoms of the disease. Anticholinergics may help control tremor and rigidity. Other drugs, such as Bromocriptine, Pramipexole, and Ropinirole, mimic the role of dopamine in the brain, causing the neurons to react as they would to dopamine. An antiviral drug, Amantadine, also appears to reduce symptoms. In May 2006, the FDA approved Rasagiline to be used along with Levodopa for patients with advanced PD or as a single-drug treatment for early PD. In March 2017, the FDA approved Safinamide tablets as an add-on treatment for individuals with PD who are currently taking Levodopa/Carbidopa and experiencing "off" episodes (when the person's medications are not working well, causing an increase in PD symptoms). The drug Amantadine may help control dyskinesias but if dyskinesias are severe, surgical treatment such as deep brain stimulation (DBS) may be considered. Other difficulties may be encountered later in the course of the disease. People with PD may begin to notice more pronounced symptoms before their first dose of medication in the morning and between doses as the period of effectiveness after each dose begins to shorten (this is the so-called wearing-off effect). People experience sudden, unpredictable “off periods,” where the medications do not seem to be working. One approach to alleviating these side effects is to take Levodopa more often and in smaller amounts. People with PD should never stop taking Levodopa without their physician's input, because rapidly withdrawing the drug can have potentially serious side effects. Amantadine, an antiviral drug, can help reduce symptoms of PD and Levodopa-induced dyskinesia. It is often used alone in the early stages of the disease. It may also be used with an anticholinergic drug or Levodopa. After several months, Amantadine's effectiveness wears off in up to half of the people taking it. Amantadine's side effects may include insomnia, mottled skin, edema, agitation, or hallucinations. Researchers are not certain how Amantadine works in PD, but it may increase the effects of dopamine.
- Drugs with an unknown mechanism of action for PD: Dyskinesias, or involuntary movements such as twisting and writhing, commonly develop in people who take Levodopa over an extended period of time. These movements may be either mild or severe. Some doctors start younger individuals with PD on drugs that act directly like dopamine itself and add Levodopa later in the course of the disease. The dosage of Levodopa is sometimes reduced in order to lessen these drug-induced movements.
- Drugs that help control the non-motor symptoms of the disease: The non-motor symptoms are the symptoms that do not affect movement. For example, people with PD-related depression may be prescribed antidepressants.
When recommending a course of treatment, a doctor will assess how much the symptoms disrupt the person’s life and then tailor therapy to the person's particular condition. Since no two people will react the same way to a given drug, it may take time and patience to get the dose just right. Even then, symptoms may not be completely alleviated.
| Category | Generic | Brand name |
| 1. Drugs that increase brain levels of dopamine | Levodopa/Carbidopa | Parcopa, Sinemet |
| 2. Drugs that mimic dopamine (dopamine agonists) | Apomorphine Pramipexole Ropinirole Rotigotine |
Apokyn Mirapex Requip Neupro |
| 3. Drugs that inhibit dopamine breakdown (MAO-B inhibitors) | Rasagiline Selegiline (deprenyl) |
Azilect Eldepryl, Zelapar |
| 4. Drugs that inhibit dopamine breakdown (COMT inhibitors) | Entacapone Tolcapone |
Comtan Tasmar |
| 5. Drugs that decrease the action of acetylcholine (anticholinergics) | Benztropine Ethopropazine Trihexyphenidyl |
Cogentin Parsidol Artane |
| 6. Drugs with an unknown mechanism of action for PD | Amantadine | Symmetrek |
| 7. Drugs that help control the non-motor symptoms of PD | Antidepressants | |
| 8. Other drugs | Quetiapine (for psychosis) Cholinisterase inhibitors for dementia Modafinil for daytime sleepiness |
Table 6: Medications to Treat the Motor Symptoms of Parkinson’s Disease.
Surgery
Treating motor symptoms with surgery was once a common practice, but since the discovery of Levodopa, the number of operations has declined. Studies in the past few decades have led to great improvements in surgical techniques, so that surgery is again being used in people with advanced PD for whom drug therapy is no longer sufficient (17).
Treating motor symptoms with surgery was once a common practice, but since the discovery of Levodopa, the number of operations has declined. Studies in the past few decades have led to great improvements in surgical techniques, so that surgery is again being used in people with advanced PD for whom drug therapy is no longer sufficient (17).
Surgery for PD can be divided into three main groups:
- Lesional;
- Deep brain stimulation(DBS): Target areas for DBS or lesions include the thalamus, the globus pallidus or, and the subthalamic nucleus. DBS is the most commonly used surgical treatment. Developed in the 1980s by Alim Louis Benabid and others, it involves the implantation of electrodes into the brain. Th electrodes are connected to a medical electrical device called a neurostimulator or pulse generator that can be externally programmed to send electrical impulses to specific parts of the brain. DBS can reduce the need for Levodopa and related drugs. It is recommended for people who have PD with motor fluctuations and tremor inadequately controlled by medication, or to those who are intolerant to medication, as long as they do not have severe neuropsychiatric problems (18, 19).
- Intentional formation of lesions: These are less common surgical therapies that involve suppressing overactivity of specific subcortical areas. For example, pallidotomy involves surgical destruction of the globus pallidus to control dyskinesia.
Pallidotomy and Thalamotomy: The earliest types of surgery for PD involved selectively destroying specific parts of the brain that contribute to PD symptoms. Surgical techniques have been refined and can be very effective for the motor symptoms of PD. The most common lesion surgery is called pallidotomy. In this procedure, a surgeon selectively destroys a portion of the brain called the globus pallidus. Pallidotomy can improve symptoms of tremor, rigidity, and bradykinesia, possibly by interrupting the connections between the globus pallidus and the striatum or thalamus. Some studies have also found that pallidotomy can improve gait and balance and reduce the amount of Levodopa people require, thus reducing drug-induced dyskinesias. Another procedure, called thalamotomy, involves surgically destroying part of the thalamus; this approach is useful primarily to reduce tremors. Because these procedures cause permanent destruction of small amounts of brain tissue, they have largely been replaced by deep brain stimulation for treatment of PD.
Deep Brain Stimulation: DBS uses an electrode surgically implanted into part of the brain, typically the subthalamic nucleus or the globus pallidus. Similar to a cardiac pacemaker, a pulse generator (battery pack) implanted in the chest area under the collarbone sends finely controlled electrical signals to the electrode(s) via a wire placed under the skin. When turned on using an external wand, the pulse generator and electrodes painlessly stimulate the brain in a way that helps to block signals that cause many of the motor symptoms of PD. DBS is FDA-approved and is widely used as a treatment for PD. It can be used on one or both sides of the brain. If it is used on just one side, it will affect symptoms on the opposite side of the body. DBS is primarily used to stimulate one of three brain regions: the subthalamic nucleus, the globus pallidus interna, or the thalamus. Stimulation of either the globus pallidus or the subthalamic nucleus can reduce tremor, bradykinesia, and rigidity. Stimulation of the thalamus is useful primarily for reducing tremors.
People who initially responded well to treatment with Levodopa tend to respond well to DBS. While the motor function benefits of DBS can be substantial, it usually does not help with speech problems, "freezing", posture, balance, anxiety, depression, or dementia. One advantage of DBS compared to pallidotomy and thalamotomy is that the electrical current can be turned off using a handheld device. The pulse generator also can be externally programmed.
Individuals must return to the medical center frequently for several months after DBS surgery in order to have the stimulation adjusted very carefully to give the best results. After a few months, the number of medical visits usually decreases significantly, though individuals may occasionally need to return to the center to have their stimulator checked. Currently, the battery for the pulse generator must be surgically replaced every three to five years. DBS does not stop PD from progressing, and some problems may gradually return. It is not a good option for everyone. It is generally appropriate for people with Levodopa-responsive PD who have developed dyskinesias or other disabling "off" symptoms despite drug therapy. It is not generally an option for people with memory problems, hallucinations, severe depression, poor health, or a poor response to Levodopa. DBS has not been demonstrated to be of benefit for "atypical" parkinsonian syndromes such as multiple system atrophy, progressive supranuclear palsy, or post-traumatic parkinsonism, which also do not improve with Parkinson’s medications. As with any brain surgery, DBS has potential complications, including stroke or brain hemorrhage. These complications are rare, however. There is also a risk of infection, which may require antibiotics or even replacement of parts of the DBS system.
Complementary and Supportive Therapies
A wide variety of complementary and supportive therapies may be used for PD. Among these therapies are:
- Therapeutic approach: It involves speech and swallowing evaluation and therapy. Certain techniques can help with the low voice volume that individuals with Parkinson’s often experience.
- Standard physical, occupational, and speech therapy techniques: Thesecan help with such problems as gait and voice disorders, tremors and rigidity, and cognitive decline.
- Diet: At this time, there are no specific vitamins, minerals, or other nutrients that have any proven therapeutic value in PD. An NINDS clinical study of the dietary supplement coenzyme Q10 was stopped in 2011 when results from an interim analysis showed active treatment with the supplement was unlikely to demonstrate a statistically significant difference than from a placebo. The NINDS and other components of the National Institutes of Health are funding research to determine if caffeine, antioxidants, and other dietary factors may be beneficial for preventing or treating PD. While there is currently no proof that any specific dietary factor is beneficial, a normal, healthy diet can promote overall well-being for people with PD just as it would for anyone else. Eating a fiber-rich diet and drinking plenty of fluids also can help alleviate constipation. A high protein diet, however, may limit Levodopa's absorption, highlighting the importance of the timing of medications.
- Exercise: Exercise can help people with PD improve their mobility and flexibility. Some doctors prescribe physical therapy or muscle-strengthening exercises to tone muscles and to put underused and rigid muscles through a full range of motion. The effects of exercise on disease progression are not known, but it may improve body strength so that the person is less disabled. Exercises also improve balance, helping people minimize gait problems, and can strengthen certain muscles so that people can speak and swallow better. Exercise can improve emotional well-being and general physical activity, such as walking, gardening, swimming, calisthenics, and using exercise machines, can have other benefits. An NINDS-funded clinical trial demonstrated the benefits of tai chi exercise compared to resistance or stretching exercises. People with PD should always check with their doctors before beginning a new exercise program.
- Others: Other complementary and supportive therapies that are used by some individuals with PD include massage therapy, yoga, hypnosis, acupuncture, and the Alexander technique, which optimizes posture and muscle activity.
Rehabilitation
Exercise programs are recommended in people with PD. There is some evidence that speech or mobility problems can improve with rehabilitation, although studies are scarce and of low quality. Regular physical exercise with or without physical therapy can be beneficial to maintain and improve mobility, flexibility, strength, gait speed, and quality of life. When an exercise program is performed under the supervision of a physiotherapist, there are more improvements in motor symptoms, mental and emotional functions, daily living activities, and quality of life compared to a self-supervised exercise program at home. In terms of improving flexibility and range of motion for people experiencing rigidity, generalized relaxation techniques such as gentle rocking have been found to decrease excessive muscle tension. Other effective techniques to promote relaxation include slow rotational movements of the extremities and trunk, rhythmic initiation, diaphragmatic breathing, and meditation techniques. As for gait and addressing the challenges associated with the disease such as hypokinesia (slowness of movement), shuffling and decreased arm swing, physiotherapists have a variety of strategies to improve functional mobility and safety. Areas of interest with respect to gait during rehabilitation programs focus on, but are not limited to improving gait speed, the base of support, stride length, trunk and arm swing movement. Strategies include utilizing assistive equipment (pole walking and treadmill walking), verbal cueing (manual, visual and auditory), exercises (marching and PNF patterns) and altering environments (surfaces, inputs, open vs. closed). Strengthening exercises have shown improvements in strength and motor function for people with primary muscular weakness and weakness related to inactivity with mild to moderate PD. However, reports show a significant interaction between strength and the time the medications was taken. Therefore, it is recommended that people with PD should perform exercises 45 minutes to one hour after medications when they are at their best. Also, due to the forward flexed posture, and respiratory dysfunctions in advanced PD, deep diaphragmatic breathing exercises are beneficial in improving chest wall mobility and vital capacity. Exercise may improve constipation.
One of the most widely practiced treatments for speech disorders associated with PD is the Lee Silverman Voice Treatment (LSVT). Speech therapy and specifically LSVT may improve speech. Occupational therapy (OT) aims to promote health and quality of life by helping people with the disease to participate in as many of their daily activities as possible. There have been few studies on the effectiveness of OT and their quality is poor, although there is some indication that it may improve motor skills and quality of life for the duration of the therapy.
Palliative Care
Palliative care (PC) is specialized medical care for people with serious illnesses, including PD. The goal of this speciality is to improve quality of life for both the person suffering from PD and the family by providing relief from the symptoms, pain, and stress of illnesses. As PD is not a curable disease, all treatments are focused on slowing decline and improving quality of life, and are therefore palliative in nature.
PC should be involved earlier, rather than later in the disease course. PC specialists can help with physical symptoms, emotional factors such as loss of function and jobs, depression, fear, and existential concerns. Along with offering emotional support to both the patient and family, PC serves an important role in addressing goals of care. People with PD may have many difficult decisions to make as the disease progresses such as wishes for feeding tube, non-invasive ventilator, and tracheostomy; wishes for or against cardiopulmonary resuscitation; and when to use hospice care. PC team members can help answer questions and guide people with PD on these complex and emotional topics to help them make the best decision based on their own values.
Muscles and nerves that control the digestive process may be affected by PD, resulting in constipation and gastroparesis (food remaining in the stomach for a longer period than normal). A balanced diet, based on periodical nutritional assessments, is recommended and should be designed to avoid weight loss or gain and minimize consequences of gastrointestinal dysfunction. As the disease advances, swallowing difficulties (dysphagia) may appear. In such cases it may be helpful to use thickening agents for liquid intake and an upright posture when eating, both measures reducing the risk of choking. Gastrostomy to deliver food directly into the stomach is possible in severe cases.
Levodopa and proteins use the same transportation system in the intestine and the BBB, thereby competing for access. When they are taken together, this results in a reduced effectiveness of the drug. Therefore, when Levodopa is introduced, excessive protein consumption is discouraged and well balanced Mediterranean diet is recommended. In advanced stages, additional intake of low-protein products such as bread or pasta is recommended for similar reasons. To minimize interaction with proteins, Levodopa should be taken 30 minutes before meals. At the same time, regimens for PD restrict proteins during breakfast and lunch, allowing protein intake in the evening.
Coping with Parkinson's Disease
While PD usually progresses slowly, eventually daily routines may be affected-from socializing with friends to earning a living and taking care of a home. These changes can be difficult to accept. Support groups can help people cope with the disease’s emotional impact. These groups also can provide valuable information, advice, and experience to help people with PD, their families, and their caregivers deal with a wide range of issues, including locating doctors familiar with the disease and coping with physical limitations. Individual or family counseling may also help people find ways to cope with PD. People with PD may also benefit from being proactive and finding out as much as possible about the disease in order to alleviate fear of the unknown and to take a positive role in maintaining their health. Many people with PD continue to work either full- or part-time, although they may need to adjust their schedule and working environment to accommodate their symptoms.
Prediction or Prevention of Parkinson's Disease
- Prediction: In most cases, there is no way to predict or prevent sporadic PD. However, researchers are looking for a biomarker-a biological abnormality that all people with PD might share-that could be detected by screening techniques or by a simple chemical test given to people who do not yet have any parkinsonian symptoms. This could help doctors identify people at risk of the disease. It also might allow them to find treatments that will stop the disease process in the early stages. Studies demonstrated that synuclein builds up in nerve cells years before symptoms occur. Loss of a sense of smell, constipation, restless legs, and REM sleep disorder are potentially caused by these early changes. One important area of research in this domain involves imaging techniques, such as special MRI techniques or nuclear imaging techniques currently under study at the NIH and elsewhere. In rare cases, where people have a clearly inherited form of PD, researchers can test for known gene mutations as a way of determining an individual's risk of developing the disease. However, this genetic testing can have far-reaching implications and people should carefully consider whether they want to know the results of such tests.
- Prevention: Exercise in middle age may reduce the risk of PD later in life. Caffeine also appears protective with a greater decrease in risk occurring with a larger intake of caffeinated beverages such as coffee. People who smoke cigarettes or use smokeless tobacco are less likely than non-smokers to develop PD, and the more they have used tobacco, the less likely they are to develop PD. It is not known what underlies this effect. Tobacco use may actually protect against PD, or it may be that an unknown factor both increases the risk of PD and causes an aversion to tobacco or makes it easier to quit using tobacco. Antioxidants such as vitamins C and E have been proposed to protect against the disease, but results of studies have been contradictory and no positive effect has been proven. The results regarding fat and fatty acids have been contradictory, with various studies reporting protective effects, risk-increasing effects or no effects. There have been preliminary indications that the use of anti-inflammatory drugs and calcium channel blockers may be protective. A 2010 meta-analysis found that nonsteroidal anti-inflammatory drugs (apart from aspirin) have been associated with a reduction of incidence of the development of PD (at least 15% higher in long-term and regular users).
Management
There is no cure for PD but medications, surgery, and physical treatment can provide relief and are much more effective than treatments available for other neurological disorders like AD, motor neuron disease, and Parkinson plus syndromes. The main families of drugs useful for treating motor symptoms are Levodopa (always combined with a dopa decarboxylase inhibitor and sometimes also with a COMT inhibitor), dopamine agonists and MAO-B inhibitors. The stage of the disease and the age at disease onset determine which group is most useful.
Three stages may be distinguished:
- Initial stage: Here the individual with PD has already developed some disability requiring pharmacological treatment, a second stage associated with the development of complications related to Levodopa usage, and a third stage when symptoms unrelated to dopamine deficiency or Levodopa treatment may predominate. Treatment aims for an optimal trade-off between symptom control and treatment side-effects. The start of Levodopa treatment may be postponed by initially using other medications such as MAO-B inhibitors and dopamine agonists instead, in the hope of delaying the onset of complications due to Levodopa use. However, Levodopa is still the most effective treatment for the motor symptoms of PD and should not be delayed in patients whose quality of life is impaired by those symptoms. Levodopa-related dyskinesias correlate more strongly with duration and severity of the disease than duration of Levodopa treatment, so delaying this therapy may not really provide much longer dyskinesia-free time than early use.
- Second stage: Here, the aim is to reduce PD symptoms while controlling fluctuations in the effect of the medication. Sudden withdrawals from medication or overuse have to be managed. When oral medications are not enough to control symptoms, surgery, DBS, subcutaneous waking day apomorphine infusion and enteral dopa pumps can be of use.
- Third stage: This stage presents many challenging problems requiring a variety of treatments for psychiatric symptoms, orthostatic hypotension, bladder dysfunction, etc. In the final stages of the disease, palliative care is provided to improve quality of life.
Parkinsonism Resulting from Neurological Disorders
There are several instances where parkinsonism results from neurological disorders. These include:
There are several instances where parkinsonism results from neurological disorders. These include:
- Arteriosclerotic parkinsonism: Sometimes known as pseudo-parkinsonism, vascular parkinsonism, atherosclerotic parkinsonism, or arteriosclerotic parkinsonism (ASP). It involves damage to the brain due to multiple strokes. Tremor is rare in this type of parkinsonism, while dementia and difficulties with gait are common. Antiparkinsonian drugs are of little help to people with this form of parkinsonism.
- Post-traumatic parkinsonism: Also known as post-traumatic encephalopathy (PTE) or "punch-drunk syndrome," parkinsonian symptoms can develop after a severe head injury or frequent head trauma associated with boxing or other activities. This type of trauma can also cause a form of dementia called chronic traumatic encephalopathy (CTE).
- Essential tremor: Sometimes called benign essential tremor or familial tremor, this common condition tends to run in families and progresses slowly over time. The tremor is usually equal in both hands and increases when the hands are moving. It may involve the head but usually spares the legs. Essential tremor is not the same as PD and does not usually lead to it, although in some cases the two conditions may overlap in one person. People with essential tremor have no other parkinsonian features. Essential tremor does not respond to Levodopa or to most other PD drugs, but there are medications to treat it.
- Normal pressure hydrocephalus: Normal pressure hydrocephalus (NPH) is an abnormal increase of CSF in the brain's ventricles, or cavities. This causes the ventricles to enlarge, putting pressure on the brain. Symptoms include problems with walking, impaired bladder control leading to increased urinary frequency or incontinence, and progressive mental impairment and dementia. The person may also have a general slowing of movements or may complain that his or her feet feel "stuck". These symptoms may sometimes be mistaken for PD. They do not respond to Parkinson’s medications. Brain scans, intracranial pressure monitoring, and other tests can help to diagnose NPH. NPH can sometimes be treated by surgically implanting a CSF shunt that drains excess cerebrospinal fluid into the abdomen, where it is absorbed.
- Parkinsonism accompanying other conditions: Parkinsonian symptoms appear in individuals with other, clearly distinct neurological disorders such as Wilson's disease (WD), Huntington's disease (HD), AD, spinocerebellar ataxias, and Creutzfeldt-Jakob disease (CJD). Each of these disorders has specific features that help to distinguish it from PD.
Other Diseases and Conditions Resembling Parkinson's Disease
A number of disorders can cause symptoms similar to those of PD. People with symptoms that resemble PD but that result from other causes are considered to have parkinsonism. Some of these disorders include:
- Multiple system atrophy: Multiple system atrophy (MSA) refers to a set of slowly progressive disorders that affect the central and autonomic nervous systems. In MSA, the protein alpha-synuclein forms harmful filament-like aggregates in the supporting cells in the brain called oligodenreoglia. MSA may have symptoms that resemble PD. It may also take a form that primarily produces poor coordination and slurred speech, or it may involve a combination of these symptoms. Other symptoms may include swallowing difficulties, male impotence, constipation, and urinary difficulties. The disorder previously called Shy-Drager syndrome refers to MSA with prominent orthostatic hypotension—a fall in blood pressure every time the person stands up. MSA with parkinsonian symptoms is sometimes referred to as MSA-P (or striatonigral degeneration), while MSA with poor coordination and slurred speech is sometimes called MSA-C (or olivopontocerebellar atrophy). Unfortunately, many of the symptoms of MSA either do not respond to PD medications or the response is minimal or short-lived.
- Dementia with Lewy bodies: Dementia with Lewy bodies (DLB) is a neurodegenerative disorder associated with the same abnormal protein deposits (Lewy bodies) found in Parkinson’s disease but in widespread areas throughout the brain. Symptoms may range from primary parkinsonian symptoms such as bradykinesia, rigidity, tremor, and shuffling walk, to symptoms similar to those of AD (memory loss, poor judgment, and confusion). These symptoms may fluctuate, or wax and wane dramatically. Visual hallucinations are often one of the first symptoms, and individuals may suffer from other psychiatric disturbances such as delusions and depression. Cognitive problems also occur early in the course of the disease. Levodopa and other antiparkinsonian medications can help with the motor symptoms of dementia with Lewy bodies, but they may make hallucinations and delusions worse, and affected individuals may require treatment with atypical antipsychotic medications.
- Progressive supranuclear palsy. Progressive supranuclear palsy (PSP) is a rare, progressive brain disorder that causes problems with control of gait and balance. The symptoms of PSP are caused by a gradual deterioration of cells in the brain stem. People often tend to fall early in the course of PSP. One of the characteristic features of the disease is an inability to move the eyes properly, which some people describe as having blurred vision. People with PSP often show alterations of mood and behavior, including depression and apathy as well as mild dementia. PSP is often misdiagnosed because some of its symptoms are much like those of PD, AD, and other brain disorders. PSP symptoms usually do not respond to medication, or the response is minimal and short-lasting. PSP is characterized by aggregation of a protein called tau.
- Corticobasal degeneration: Corticobasal degeneration (CBD) results from atrophy of multiple areas of the brain, including the cerebral cortex and the basal ganglia. Initial symptoms may first appear on one side of the body, but eventually affect both sides. Symptoms are similar to some of the features found in PD, including rigidity, impaired balance, and problems with coordination. Often there is dystonia affecting one side of the body. Other symptoms may include cognitive and visual-spatial impairments, apraxia (loss of the ability to make familiar, purposeful movements), hesitant and halting speech, myoclonus (muscular jerks), and dysphagia (difficulty swallowing). Unlike PD, CBD usually does not respond to medication. Like PSP, it is characterized by deposits of the tau protein.
- Parkinson-plus diseases: Several diseases, including MSA, CBD, and PSP, are sometimes referred to as"Parkinson's plus" diseases because they have the symptoms of PD plus additional features.
Society and Culture
The costs of PD to society are high, but precise calculations are difficult due to methodological issues in research and differences between countries. The annual cost in the UK is estimated to be between 449 million and 3.3 billion pounds, while the cost per patient per year in the U.S. is probably around $10,000 and the total burden around 23 billion dollars.The largest share of direct cost comes from inpatient care and nursing homes, while the share coming from medication is substantially lower. Indirect costs are high, due to reduced productivity and the burden on caregivers. In addition to economic costs, PD reduces the quality f life of those with the disease and their caregivers.
Advocacy
World Parkinson's Day (11 April) is that of the birthday of James Parkinson. A red tulip was chosen by international organizations as the symbol of the disease in 2005: it represents the James Parkinson Tulip cultivar, registered in 1981 by a Dutch horticulturalist. Advocacy organizations include the National Parkinson Foundation (NPF) that since its founding by William Black in 1957 has provided more than $115 million for research and nearly $50 million for education and advocacy programs, the American Parkinson Disease Association, founded in 1961, and the European Parkinson's Disease Association founded in 1992.
Conclusions
The extent of our body of knowledge of Parkinson and other movement disorders is vast as gauged by the number of disorders cited. Likewise the number of treatment drugs is vast as equally gauged by the number of drugs listed. Nonetheless, there are currently no blood or laboratory tests and the diagnosis remains difficult and at times uncertain. There is also at present no cure. Yet, the disease is chronic and relentlessly progressive. While we know that the disorders are the result of the loss of dopamine-producing brain cells, no cure today, medication or/and surgery can provide substantial improvement of the motor systems. The symptoms are numerous and varied on all fronts (motor and neuropsychiatric). We are able to classify them and stage them, but the cure remains elusive.
The motor symptoms of PD are known to result from reduced dopamine production in the brain's basal ganglia. However, Dopamine does not cross the blood brain barrier so it cannot be taken as a medicine to boost the brain's depleted levels of dopamine. On the other hand a precursor of dopamine, Levodopa, can pass through this barrier to the brain where it is readily converted to dopamine. Administration of this drug temporarily diminishes the motor symptoms of PD. Unfortunately, only 5–10% of the drug crosses the barrier with much of the remainder being metabolized to dopamine elsewhere in the body, causing a variety of side effects including nausea, vomiting and orthostatic hypotension. More research is needed and the several lines of investigation currently pursued as well as new research vistas will be treated in a companion article.
Appendix
The Parkinson's Foundation's Ten Early Signs of Parkinson's Disease
The Parkinson's Foundation has provided the 10 signs below to alert to the presence of the disease. No single one of these signs is significant, but more than one sign should encourage considering a medical appointment:
The Parkinson's Foundation has provided the 10 signs below to alert to the presence of the disease. No single one of these signs is significant, but more than one sign should encourage considering a medical appointment:
1. Tremor
A slight shaking or tremor in the finger, thumb, hand or chin. At rest, this is a common early sign.
What is normal? Shaking after lots of exercise, or under stress or after an injury. Shaking could also be caused by medication.
A slight shaking or tremor in the finger, thumb, hand or chin. At rest, this is a common early sign.
What is normal? Shaking after lots of exercise, or under stress or after an injury. Shaking could also be caused by medication.
2. Small Handwriting
Handwriting smaller than it was in the past; writings of words on a page have changed (smaller letter sizes, words crowded together). This is called “micrographia”.
What is normal? Writing changes with advancing age, stiff hands or fingers or poor vision.
Handwriting smaller than it was in the past; writings of words on a page have changed (smaller letter sizes, words crowded together). This is called “micrographia”.
What is normal? Writing changes with advancing age, stiff hands or fingers or poor vision.
3. Loss of Smell
No longer smelling certain foods (bananas, dill pickles or licorice).
What is normal? Sense of smell can be changed by a cold, flu or a stuffy nose.
No longer smelling certain foods (bananas, dill pickles or licorice).
What is normal? Sense of smell can be changed by a cold, flu or a stuffy nose.
4. Trouble Sleeping
Thrashing around in bed or acting out dreams when deeply asleep or sudden movements during sleep.
What is normal? Occasional night(s) when 'tossing and turning' instead of sleeping; quick jerks of the body when initiating sleep or when in lighter sleep.
Thrashing around in bed or acting out dreams when deeply asleep or sudden movements during sleep.
What is normal? Occasional night(s) when 'tossing and turning' instead of sleeping; quick jerks of the body when initiating sleep or when in lighter sleep.
5. Trouble Moving or Walking
Feeling stiff in the body, stiff or in pain in the shoulders or hips, arms or legs, or arms don’t swing like they used to when walking. The feet seem “stuck to the floor.”
What is normal? Trouble moving or walking with arm or shoulder injured, or another illness like arthritis.
Feeling stiff in the body, stiff or in pain in the shoulders or hips, arms or legs, or arms don’t swing like they used to when walking. The feet seem “stuck to the floor.”
What is normal? Trouble moving or walking with arm or shoulder injured, or another illness like arthritis.
6. Constipation
Straining to move bowels.
What is normal? Not having enough water or fiber in the diet and some medicines can be causes.
Straining to move bowels.
What is normal? Not having enough water or fiber in the diet and some medicines can be causes.
7. A Soft or Low Voice
Very soft or sounds hoarse, or a change in voice.
What is normal? A chest cold or other virus can be the cause.
Very soft or sounds hoarse, or a change in voice.
What is normal? A chest cold or other virus can be the cause.
8. Masked Face
Having a serious, depressed or mad look on the face, even when not in a bad mood (“facial masking”).
What is normal? Some medicines can be the cause.
Having a serious, depressed or mad look on the face, even when not in a bad mood (“facial masking”).
What is normal? Some medicines can be the cause.
9. Dizziness or Fainting
Regularly feeling dizzy when standing up out of a chair or fainting.
What is normal? Not on a regular basis.
Regularly feeling dizzy when standing up out of a chair or fainting.
What is normal? Not on a regular basis.
10. Stooping or Hunching Over
Not standing up as straight as before, stooping, leaning or slouching when standing.
What is normal? If caused by pain from an injury or sickness or a problem with bones.
Not standing up as straight as before, stooping, leaning or slouching when standing.
What is normal? If caused by pain from an injury or sickness or a problem with bones.
References
- Mosley AD (2010). The encyclopedia of Parkinson's disease. (2nd ed.). New York: Facts on File. p. 89. ISBN9781438127491.
- Kalia, LV and Lang AE. “Parkinson's disease”. Lancet 386.9996 (2015): 896–912.
- Samii A., et al. “Parkinson's disease”. Lancet 363.9423 (2004): 1183-1193.
- Barranco Quintana JL., et al. “Parkinson's disease and tea: a quantitative review". Journal of American College of Nutrition 28.1 (2009): 1-6.
- Barichella M., et al. “Major nutritional issues in the management of Parkinson's disease”. Movement Disord 24.13 (2009):1881-1892.
- Ahlskog JE. “Does vigorous exercise have a neuroprotective effect in Parkinson disease?”. Neurology 77.3 (2011):288-294.
- National Parkinson Foundation (2017). “Parkinson's Disease vs. Parkinsonism”.
- Jankovic J. “Parkinson's disease: Clinical features and diagnosis”. Journal of Neurology, Neurosurgery, and Psychiatry 79.4 (2008): 36876.
- Cooper G., et al. “Parkinson's disease”. In Conn PM. Neuroscience in medicine. Totowa, NJ: Humana Press. pp. (2008): 508-512. ISBN 978-1-60327-454-8.
- Caballol N., et al. “Cognitive dysfunction and dementia in Parkinson disease”. Movement Disorders 22.Suppl 17 (2007): S358-66.
- Parker KL., et al. “Executive dysfunction in Parkinson;s disease and timing deficits”. Frontiers in Integrative Neuroscience 7(2013): 75.
- Garcia-Ptacek S., et al. “Parkinson Disease and Dementia”. Journal of Geriatric Psychiatry and Neurology 29.5 (2016): 261-270.
- Shergill SS., et al. “A preliminary investigation of laterality in Parkinson's disease ans susceptibility to psychosis”. Journal of Neurology, Neurosurgery, and Psychiatry 65.4 (1989): 610–11.
- Friedman JH. “Parkinson's disease psychosis 2010: A review article”. Parkinsonism Related Disorder 16.9 (2010): 553-560.
- Lesage S and Brice A. "Parkinson's disease: from monogenic forms to genetic susceptibility factors". Human Molecular Genetics 18.R1 (2009): R48-59.
- Shulman JM., et al. “Parkinson's disease: genetics and pathogenesis”. Annual Review of Pathology 6 (2011): 193-222.
- The National Collaborating Centre for Chronic Conditions, ed. (2006). "Surgery for Parkinson's disease". Parkinson's Disease. London: Royal College of Physicians. pp. 101–11. ISBN 1-86016-283-5.
- Bronstein M., et al. “Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues”. Archives of Neurology 68.2 (2011): 165.
- Coffey RJ. “Deep brain stimulation devices: a brief technical history and review”. Artifical Organs 33.3 (2009): 208-220.
Citation:
Alain L Fymat. “Parkinson’s Disease and other Movement Disorders: A Review”. Current Opinions in Neurological Science 2.1
(2017): 316-343.
Copyright: © 2017 Alain L Fymat. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























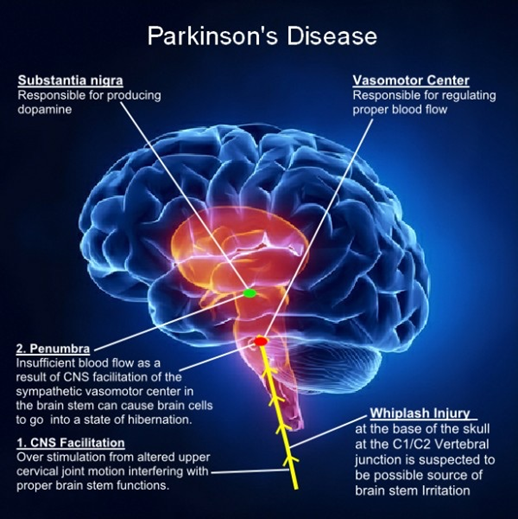
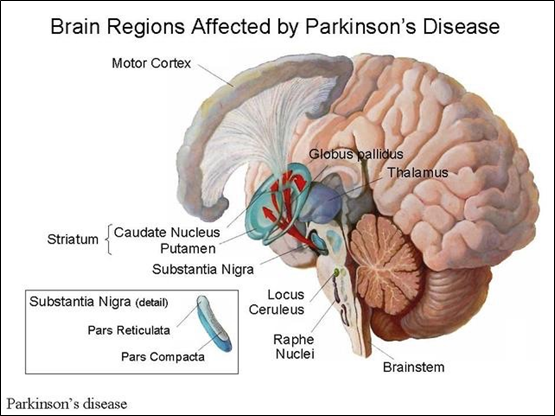
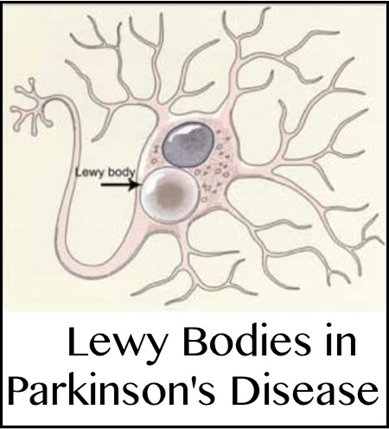
 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.