Review Article
Volume 1 Issue 2 - 2017
The Role of Two-Dimensional Speckle Tracking Echocardiography in Cardio-Oncology
1University of KwaZulu-Natal, Durban, South Africa
2Mediclinic Heart Hospital, Pretoria, South Africa
3London School of Economics and Political Science, London, UK
2Mediclinic Heart Hospital, Pretoria, South Africa
3London School of Economics and Political Science, London, UK
*Corresponding Author: Mamotabo R Matshela, University of KwaZulu-Natal, Durban, South Africa.
Received: October 11, 2017; Published: October 17, 2017
Abstract
Echocardiography has recently evolved into an advanced non-invasive diagnostic modality in the field of cardiology and cardiovascular medicine; and has undergone innovations due to the availability of deformation parameters including strain, strain rate, twist and torsion that allow for accurate assessment of myocardial mechanical function. The myocardial deformation parameters have been highlighted, and have important clinical and research applications for daily clinical practice in managing diverse etiologies of systemic and other diseases where myocardial function could be compromised.
The deformation parameters are largely implicated for the early detection of myocardial dysfunction or subclinical myocardial dysfunction, particularly in asymptomatic patients. Speckle tracking strain analysis is extensively applied to cardiomyopathies, coronary artery disease, valvular heart diseases or simply for prognostic purposes in condition where myocardial function has been compromised. More recently, several other clinical situations including cardio-oncology and metabolic conditions have benefited from special evaluation by speckle tracking strain analysis methods. In the modern era, speckle tracking strain evaluation, particularly Global Longitudinal Strain (GLS) and Global Circumferential Strain have been shown to provide useful information to our daily clinical practice, however GLS in particular has recently been implicated as an important strain parameter in cardiovascular or cardio-oncology patients with regard to management and long-term follow-up. Lately, some specific aspects of strain evaluation, particularly GLS have been shown to provide useful information of clinical relevance in the case of cancer patients.
Keywords: Cancer; Cardio-oncology; Echocardiography; Radiation; Speckle tracking
Abbreviations: GLS: Global Longitudinal Systolic strain; LV: Left Ventricle, Left Ventricular LVEF: Left Ventricular Ejection Fraction; STE: Speckle Tracking Echocardiography;
Introduction
It is crucial to have a constant and regular follow-up; and standardized imaging protocol to monitor myocardial function which has been repeatedly highlighted in clinical cardiology practice. The literature has extensively implicated the utility of left ventricular ejection fraction (LVEF) with deformation parameters, particularly strain which represents a more accurate method for assessing subclinical myocardial mechanical dysfunction. [33-34, 36-37] This premise is scientifically true and feasible, especially in patients with sub-clinically ventricular myocardial dysfunction and also those with borderline or reduced LVEF. [36] From the physiological stand-point, deformation imaging in the form of strain, particularly global longitudinal systolic strain (GLS) may provide useful information in cardio-oncology which is supported by the knowledge inert effect on LVEF, Figure 1. [34,36-37] As a result we opted to take this opportunity to review cardiotoxicity in patients who receive chemotherapeutic treatment, radiotherapy or both; and the role of advanced echocardiographic imaging modalities, particularly speckle tracking strain parameters to detect subclinical myocardial dysfunction in cancer patients.
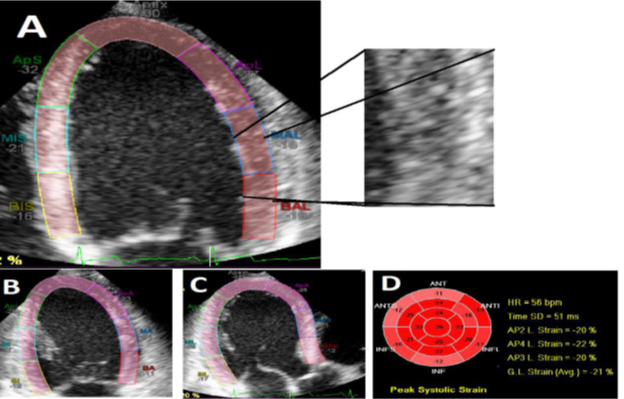
Figure 1: [47] Showing the three different apical chamber views in a normal person, where speckles
from frame to frame were used to calculate myocardial deformation in different directions. Images A, B
and C represent the left ventricle in four-chamber, two-chamber and three-chamber views which were
analyzed with speckle tracking software; respectively. The regional and global longitudinal strain values
are displayed in image D. Note that the averaged GLS is -21%. Abbreviation: GLS, global longitudinal
systolic strain, RT, radiotherapy. (Images, courtesy of SUVI TUOHINEN). [47]
Clinical applications of 2D-Speckle tracking echocardiography in cardiology and cardio-oncology
Although, circumferential strain has previously been used to evaluate and differentiate constriction from restrictive cardiomyopathies; and contributed immensely to evaluate suspected myocardial damage and dysfunction in patients with metabolic syndrome, infections and other systemic diseases; data are still limited with its potential applications in cancer patients and also during the current era of cardio-oncology. [39-41,45] Currently, studies are essentially limited to the use of GLS in cancer, cardio-oncology and chemotherapeutic induced cardiotoxicity.
Although, circumferential strain has previously been used to evaluate and differentiate constriction from restrictive cardiomyopathies; and contributed immensely to evaluate suspected myocardial damage and dysfunction in patients with metabolic syndrome, infections and other systemic diseases; data are still limited with its potential applications in cancer patients and also during the current era of cardio-oncology. [39-41,45] Currently, studies are essentially limited to the use of GLS in cancer, cardio-oncology and chemotherapeutic induced cardiotoxicity.
The recent literatures suggest that a highly reproducible, sensitive and easily attainable deformation parameter with potential broader sense of clinical utility in a diverse pathology of myocardial disease is GLS, Figure 1. [33,38] The GLS is clinically applicable and globally proposed in a multiple clinical context including several cardiac and non-cardiac diseases. The GLS can be utilized, among other things, in ischemic heart diseases, systemic and pulmonary arterial hypertension, valvular heart diseases and in the cases of post-valvular repair’s or replacement’s follow-ups, pericardial diseases including both pre- and post-pericardiectomy in the setting of constriction, heart failure managements and follow-up, cardiomyopathies including hypertrophic cardiomyopathies, renal patients including post- renal transplant, and cardiotoxicity in patients on or after chemotherapy, to aid differentiate whether changes observed are either the pathological or simply physiological. [39-41]
The clinical application of strain indices, apart from cardio-oncology, and particularly GLS measurement, can be extended to other medical conditions and appeared therefore to be a sensitive indicator for early detection of sub-clinical diseases, Figure 1. Multiple actual clinical applications can be proposed in the case of coexistence of many non-communicable diseases. [33, 35, 38-41]
Definition of cardio-toxicity
Chemotherapy induced cardiotoxicity
Definitions: Cardiotoxicity is currently defined as an expression of LV systolic dysfunction, which could be divided into two criteria based on recent reports: 1) as a 5% reduction in symptomatic patients, or 2) a 10% reduction in asymptomatic patients, in the LVEF from baseline to a value less than 55%. [22-24, 46]
Definitions: Cardiotoxicity is currently defined as an expression of LV systolic dysfunction, which could be divided into two criteria based on recent reports: 1) as a 5% reduction in symptomatic patients, or 2) a 10% reduction in asymptomatic patients, in the LVEF from baseline to a value less than 55%. [22-24, 46]
Mechanisms and risk factors for chemotherapy induced cardio-toxicity
In the recent past, the reports have proposed two main mechanisms which are widely publicized for myocardial injury in cancer patients on chemotherapy for any form of cancer: 1) the first mechanism which is irreversible, is the dose dependent mechanism reported in era of cytostatic drugs. The common agents associated with this irreversible myocardial toxicity include the antracycline. The main pathognomic mechanism for myocardial toxicity is related to the over production of free radicals from the oxidative stress pathways and 2) secondly, the dose independent mechanism also referred to as the “no dose cumulative dependence”, which is a reversible mechanism related to receptor inhibitors. [25-28]
In the recent past, the reports have proposed two main mechanisms which are widely publicized for myocardial injury in cancer patients on chemotherapy for any form of cancer: 1) the first mechanism which is irreversible, is the dose dependent mechanism reported in era of cytostatic drugs. The common agents associated with this irreversible myocardial toxicity include the antracycline. The main pathognomic mechanism for myocardial toxicity is related to the over production of free radicals from the oxidative stress pathways and 2) secondly, the dose independent mechanism also referred to as the “no dose cumulative dependence”, which is a reversible mechanism related to receptor inhibitors. [25-28]
Apart from this postulated mechanisms, it is pivotal to note that some of the cardiotoxic effects from any of these chemotherapeutic agents can still occur at least two decades after the first dosing of the initial chemotherapeutic regimen, as a result it is crucial to implement all potential prophylactic strategies to reduce the risk of cardiotoxicity, in particular anthracycline-induced cardiotoxicity. [28-32, 43]
The role of deformation – speckle tracking train echocardiographic parameters in cardio-oncology, chemotherapeutics
Despite previously limited data on chemotherapy related cardiotoxicity, it is also important to consider that the incidences of cardiotoxicity related to chemotherapy agents are currently reported to be higher than previously anticipated. The strain measurement by speckle tracking echocardiography (STE), particularly GLS appears to be the most sensitive and reproducible for early detection of sub-clinical cardiac or cardiovascular diseases where many acute and chronic metabolic chronic or patho-physiological conditions are implicated. [33,38] Most importantly, STE may be useful in areas where standard echocardiographic parameters including LVEF or tissue Doppler indices cannot assist us to clarify or identify the possible primary clinical diagnosis. [36-37] The deformation imaging need be implicated for early diagnosis and risk stratification, including prognostication of cancer patients receiving cardio-toxic cancer therapeutic agents. [33-35] This approach should be emphasized and considered for routine daily clinical practice, where feasible. With current literature, roughly a third of cancer patients develop asymptomatic LV dysfunction and around 2%-6% may become symptomatic with features of overt heart failure syndrome. [34,42]
Despite previously limited data on chemotherapy related cardiotoxicity, it is also important to consider that the incidences of cardiotoxicity related to chemotherapy agents are currently reported to be higher than previously anticipated. The strain measurement by speckle tracking echocardiography (STE), particularly GLS appears to be the most sensitive and reproducible for early detection of sub-clinical cardiac or cardiovascular diseases where many acute and chronic metabolic chronic or patho-physiological conditions are implicated. [33,38] Most importantly, STE may be useful in areas where standard echocardiographic parameters including LVEF or tissue Doppler indices cannot assist us to clarify or identify the possible primary clinical diagnosis. [36-37] The deformation imaging need be implicated for early diagnosis and risk stratification, including prognostication of cancer patients receiving cardio-toxic cancer therapeutic agents. [33-35] This approach should be emphasized and considered for routine daily clinical practice, where feasible. With current literature, roughly a third of cancer patients develop asymptomatic LV dysfunction and around 2%-6% may become symptomatic with features of overt heart failure syndrome. [34,42]
Strain in cardio-oncology - global longitudinal systolic strain:In the current era, reported data indicates that myocardial deformation imaging, strain parameters and in particular the GLS is regarded as the most important tool which plays an incremental role for early detection of suppressed myocardial function in the field of cardiology and cardiovascular medicine; and most importantly in patients receiving chemotherapy. The GLS has been regarded as an appropriate echocardiographic strain parameter proposed in cardio-oncology and one should consider applying GSL on all patients on or post-chemotherapy for early detection of myocardial dysfunction. [33-34,44] Tracking of LV myocardial deformation using STE for early detection of LV myocardial mechanical dysfunction in cardio-oncology and follow-up of cancer patients on chemotherapeutic agents, GLS is considered the best strain parameter compared with circumferential strain. Although, GLS is regarded as an important parameter for early detection of sub-clinical myocardial dysfunction, recent reports suggest a combination of GLS with LVEF, and also the addition of troponin, for early detection of any potential cardiac damage. [43-44]
Left Ventricular Mechanical Dysfunction in Radiation-induced cardio-toxicity
Radiation-induced myocardial dysfunction
Radiation induced myocardial fibrosis which in turn leads to a decrease in myocardial elasticity and distensibility, can subsequently lead to decreased LVEF and later overt heart failure. [1,2,4-5] Radiation induced cardiac disease may affect all mediastinal or cardiovascular structures including pericardium, myocardium, valves and coronary arteries. [1] In addition; one needs to worry about other potential mechanisms of radiation induced cardiac disease, primarily vascular, where atherosclerosis is accelerated by radiotherapy as either direct or indirect mediastinal radiation induced coronary artery injury. [1]
Radiation-induced myocardial dysfunction
Radiation induced myocardial fibrosis which in turn leads to a decrease in myocardial elasticity and distensibility, can subsequently lead to decreased LVEF and later overt heart failure. [1,2,4-5] Radiation induced cardiac disease may affect all mediastinal or cardiovascular structures including pericardium, myocardium, valves and coronary arteries. [1] In addition; one needs to worry about other potential mechanisms of radiation induced cardiac disease, primarily vascular, where atherosclerosis is accelerated by radiotherapy as either direct or indirect mediastinal radiation induced coronary artery injury. [1]
Risk factors and mechanisms of radiation induced myocardial toxicity
A cluster of environmental and traditional risk factors for coronary and cardiovascular diseases may be present in cancer patients which in turn may subsequently affect the general risk profiling of cardiac diseases, where ionizing radiation become one very important factor in these patients. [5-13] Higher doses and longer duration of exposure during radiotherapy are also important factors for cardiac and vasculature injuries, which significantly increases the risk of radiation induced cardiac diseases. [5-14] Reports demonstrated that radiation-induced heart diseases are due to long-term outcomes after radiotherapy treatment of malignant disease, in particular breast cancer. [15-16] In addition, pathological structural changes in situations related to high-doses of radiotherapy on the heart are due to direct damage leading to coronary artery diseases, pericardial adhesions, fibrosis of the pericardium and myocardium, microvascular injuries, and valvular stenoses. [13,17-18] In addition, the presence of multiple risk factors may also increase the risk in those who receive smaller and moderate doses of radiation during their radiotherapy sessions. [5-13] Radiotherapy, particularly in patients receiving mediastinal radiation, leads to an increased collagen deposition after irradiation which may contribute to impaired myocardial contractility and dysfunction. [3,13,19] In addition, cardiomyocytes are known to react to stress signals directly by initiating an inflammatory response through activation of macrophages, which also applies to radiation patients. [13,20] All these may later lead to decreased myocardial contractility, resulting in decreased diastolic filling and subsequently impaired ventricular systolic function. [2,13,21]
A cluster of environmental and traditional risk factors for coronary and cardiovascular diseases may be present in cancer patients which in turn may subsequently affect the general risk profiling of cardiac diseases, where ionizing radiation become one very important factor in these patients. [5-13] Higher doses and longer duration of exposure during radiotherapy are also important factors for cardiac and vasculature injuries, which significantly increases the risk of radiation induced cardiac diseases. [5-14] Reports demonstrated that radiation-induced heart diseases are due to long-term outcomes after radiotherapy treatment of malignant disease, in particular breast cancer. [15-16] In addition, pathological structural changes in situations related to high-doses of radiotherapy on the heart are due to direct damage leading to coronary artery diseases, pericardial adhesions, fibrosis of the pericardium and myocardium, microvascular injuries, and valvular stenoses. [13,17-18] In addition, the presence of multiple risk factors may also increase the risk in those who receive smaller and moderate doses of radiation during their radiotherapy sessions. [5-13] Radiotherapy, particularly in patients receiving mediastinal radiation, leads to an increased collagen deposition after irradiation which may contribute to impaired myocardial contractility and dysfunction. [3,13,19] In addition, cardiomyocytes are known to react to stress signals directly by initiating an inflammatory response through activation of macrophages, which also applies to radiation patients. [13,20] All these may later lead to decreased myocardial contractility, resulting in decreased diastolic filling and subsequently impaired ventricular systolic function. [2,13,21]
Other imaging modalities
Echocardiography plays an integral part as the first non-invasive and easily accessible imaging approach to be considered; the most cheap and effective in cardiovascular diseases and cardio-oncology, Figure 1. Other imaging modalities which should be in co-operated during the initial visits an d for follow-up of patients in cardiovascular medicine and chemotherapy induced cardiotoxicity, are chosen based on the attending clinician’s judgment depending on the patient’s clinical presentation and. Imaging modalities, including the Magnetic Resonance Imaging, Single Photon Emission Computed Tomography, positron emission tomography and computer tomography are some of the most important imaging options which should form part of our daily clinical practice, however their choices always depend on the attending clinical judgment, cost and availability of these modalities at the clinical center. The implementation of cardiac imaging forms an integral part of the imaging protocol in cardiovascular practice. In addition, most recently research has summarized and reported the role of nuclear imaging modalities for early detection of myocardial damage related to chemotherapeutic agents and cardio-chemo-radiotherapy at the reversible stages, which need be implemented on our daily clinical practice.
Echocardiography plays an integral part as the first non-invasive and easily accessible imaging approach to be considered; the most cheap and effective in cardiovascular diseases and cardio-oncology, Figure 1. Other imaging modalities which should be in co-operated during the initial visits an d for follow-up of patients in cardiovascular medicine and chemotherapy induced cardiotoxicity, are chosen based on the attending clinician’s judgment depending on the patient’s clinical presentation and. Imaging modalities, including the Magnetic Resonance Imaging, Single Photon Emission Computed Tomography, positron emission tomography and computer tomography are some of the most important imaging options which should form part of our daily clinical practice, however their choices always depend on the attending clinical judgment, cost and availability of these modalities at the clinical center. The implementation of cardiac imaging forms an integral part of the imaging protocol in cardiovascular practice. In addition, most recently research has summarized and reported the role of nuclear imaging modalities for early detection of myocardial damage related to chemotherapeutic agents and cardio-chemo-radiotherapy at the reversible stages, which need be implemented on our daily clinical practice.
Summary and Conclusion
Strain analysis should form an important part of our daily clinical practice in the field of cardiovascular and cardio-oncology. Strain parameters should be implemented in the management of cancer patients, and need be highlighted to improve primary and secondary prevention of cardiomyopathies, particularly in patients receiving chemotherapy with or without radiotherapy. The GLS should form an integral part in cardio-oncology management protocol to exclude any potential myocardial mechanical dysfunction and further guide management in patients with or those at risk of potential cardiotoxicity. The strain analysis, particularly GLS principally forms an important aspect in patients who are completely asymptomatic or those with a history of a transient heart failure, and where regular and continuous follow-up is fundamental.
References
- Claus P., et al. “Tissue tracking technology for assessing cardiac mechanics: Principles, normal values, and clinical applications”. JACC: Cardiovascular Imaging 8.12 (2015): 1444-1460.
- Laura Stefani., et al.“Clinical Application of 2D Speckle Tracking Strain for Assessing Cardio-Toxicity in Oncology”. Journal of Functional Morphology and Kinesiology 1.4 (2016): 343-354.
- Aurigemma GP., et al.“Quantitative evaluation of left ventricular structure, wall stress and systolic function. In The Practice of Clinical Echocardiography”. WB Saunders Company: Philadelphia(2002): 65-87.
- Ewer MS., et al.“A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving Adriamycin”.Journal of Clinical Oncology2.2 (1984): 112-117.
- Tadic M., et al. “Does masked hypertension impact left ventricular deformation?” American Society of Hypertension 10.9 (2016): 694-701.
- Kafa R., et al. “Association of abnormal postoperative left ventricular global longitudinal strain with outcomes in severe aortic stenosis following aortic valve replacement”. JAMA Cardiology 1.4 (2016): 494-496.
- Stefani L., et al.“Cardiovascular outcomes in renal transplant 2 recipients: Feasibility and clinical role of 2D speckle 3 tracking to assess myocardial function”.Journal of Functional Morphology and Kinesiology 1.1 (2016): 109-117.
- Sengupta PP., et al. “Disparate patterns of left ventricular mechanics differentiate constrictive pericarditis from restrictive cardiomyopathy”. JACC: Cardiovascular Imaging 1.1 (2008): 29-38.
- Shiino K., et al. “Intervendor consistency and reproducibility of left ventricular 2D global and regional strain with two different high-end ultrasound systems”. European Heart Journal - Cardiovascular Imaging18.6 (2016): 707-716.
- Bowles EJ., et al.“Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: A retrospective cohort study”. Journal of the National Cancer Institute 104.17 (2012): 1293-1305.
- Jose A Banchs. “The Role of Strain Imaging in Oncology and Vendor-to-Vendor Variability”. Expert Analysis (2016):
- Sawaya H., et al.“Early detection and prediction of cardiotoxicity in chemotherapy-treated patients”. American Journal of Cardiology 107.9 (2011): 1375-1380.
- Hare JL., et al.“Use of myocardial deformation imaging to detect preclinical myocardial dysfunction before conventional measures in patients undergoing breast cancer treatment with trastuzumab”. American Heart Journal 158.2 (2009): 294-301.
- Seidman A., et al.“Cardiac dysfunction in the trastuzumab clinical trials experience”. Journal of Clinical Oncology20.5 (2002): 1215-1221.
- Neilan TG., et al. “Tissue Doppler imaging predicts left ventricular dysfunction and mortality in a murine model of cardiac injury”. European Heart Journal 27.15 (2006): 1868-1875.
- Plana JC., et al. “Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging”. Journal of the American Society of Echocardiography 27.9 (2014): 911-939.
- Di Cosimo S. “Heart to heart with trastuzumab: a review on cardiac toxicity”. Targeted Oncology 6.4 (2011): 189-195.
- Marijana Tadic and Cesare Cuspidi. “The Role of Echocardiography in Detection of Chemotherapy-Induced Cardiotoxicity in Breast Cancer Patients”. International Journal of Cancer Management 10.5 (2017): e8109.
- Lal H., et al. “Cancer genetics and the cardiotoxicity of the therapeutics”. Journal of the American College of Cardiology 61.3 (2013): 267-274.
- Gulati G., et al. “Cancer and cardiovascular disease: the use of novel echocardiography measures to predict subsequent cardiotoxicity in breast cancer treated with anthracyclines and trastuzumab”. Current Heart Failure Reports 11.4 (2014): 366-373.
- Du XL., et al. “Cardiac toxicity associated with anthracycline-containing chemotherapy in older women with breast cancer”. Cancer115.22 (2009): 5296-5308.
- Bowles EJ., et al.“Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study”. Journal of the National Cancer Institute 104.17 (2012): 1293-1305.
- Chung WB and Youn HJ. “Pathophysiology and preventive strategies of anthracycline-induced cardiotoxicity”. The Korean Journal of Internal Medicine 31.4 (2016): 625-633.
- Fei HW., et al. “Left Ventricular Global Longitudinal Strain in HER-2 + Breast Cancer Patients Treated with Anthracyclines and Trastuzumab Who Develop Cardiotoxicity Is Associated with Subsequent Recovery of Left Ventricular Ejection Fraction”. Echocardiography33.4 (2016): 519-526.
- Potier A., et al. “Early detection of cancer therapeutics-related cardiac dysfunction”. Bulletin du Cancer 103.7-8 (2016): 667-673.
- Syed Wamique Yusuf., et al. “Research Cardiology Research and Practice”. (2011): 317659.
- MJ Adams., et al. “Radiation-associated cardiovascular disease”. Critical Reviews in Oncology/Hematology 45.1 (2003): 55-75.
- JP Veinot and WD Edwards. “Pathology of radiation induced heart disease: a surgical and autopsy study of 27 cases”. Human Pathology 27.8 (1996): 766-773.
- Jaworski C., et al.“Cardiac complications of thoracic irradiation”. Journal of the American College of Cardiology 61.23 (2013): 2319-2328.
- Zamorano J. “An ESC position paper on cardio-oncology”. European Heart Journal 37 (2016): 2739-2748.
- Lancellotti P., et al. “Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography”. European Heart Journal - Cardiovascular Imaging 14.8 (2013): 721-740.
- Sardaro A., et al.“Radiationinduced cardiac damage in early left breast cancer patients: Risk factors, biological mechanisms, radiobiology, and dosimetric constraints”. Radiotherapy and Oncology 103.2 (2012): 133-142.
- Darby SC., et al. “Risk of ischemic heart disease in women after radiotherapy for breast cancer”. The New England Journal of Medicine 368 (2013): 987-998.
- Shimizu Y., et al. “Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950-2003”. BMJ 340 (2010): b5349.
- Hooning MJ., et al. “Longterm risk of cardiovascular disease in 10-year survivors of breast cancer”. Journal of the National Cancer Institute 99.5 (2007): 365-375.
- Gagliardi G., et al. “Radiation dose-volume effects in the heart”. International Journal of Radiation Oncology * Biology * Physics 76.3 (2010): S77-S85.
- Tapio S. “Pathology and biology of radiation-induced cardiac disease”. Journal of Radiation Research 57.5 (2016): 439-448.
- Adams J., et al. “Proteasome inhibition: a new strategy in cancer treatment”. Investigational New Drugs 18.2 (2000): 109-121.
- Darby S., et al. “Mortality from cardiovascular disease more than 10 years after radiotherapy for breast cancer: nationwide cohort study of 90 000 Swedish women”. BMJ 326.7383 (2003): 256-257.
- Hancock SL., et al.“Cardiac disease following treatment of Hodgkin’s disease in children and adolescents”. Journal of Clinical Oncology 11.7 (1993): 1208-1215.
- Demirci S., et al. “Radiation-induced cardiac toxicity after therapy for breast cancer: interaction between treatment era and follow-up duration”. International Journal of Radiation Oncology * Biology * Physics 73.4 (2009): 980-987.
- Adams MJ., et al. “Radiationassociated cardiovascular disease”. Critical Reviews in Oncology/Hematology 45.1 (2003): 55-75.
- M Chello P., et al. “Changes in the proportion of types I and III collagen in the left ventricular wall of patients with post-irradiative pericarditis”. Cardiovascular Surgery 4.2 (1996): 222-226.
- Kruse JJ., et al.“Structural changes in the auricles of the rat heart after local ionizing irradiation”. Radiotherapy and Oncology 58.3 (2001): 303-311.
- Boyd JH., et al.“S100A8 and S100A9 mediate endotoxin-induced cardiomyocyte dysfunction via the receptor for advanced glycation end products”. Circulation Research 102.10 (2008): 1239-1246.
- Simms MG and Walley KR. “Activated macrophages decrease rat cardiac myocyte contractility: importance of ICAM-1-dependent adhesion”. American Journal of Physiology 277.1 (1999): H253-260.
- Suvi Tuohinen. “Detection of the Early Radiotherapy-induced Cardiac Changes in Breast Cancer Patients Impact of advanced echocardiography techniques”. The International Journal of Cardiovascular Imaging (2017):
Citation:
Mamotabo R Matshela. “The Role of Two-Dimensional Speckle Tracking Echocardiography in Cardio-Oncology”. Therapeutic
Advances in Cardiology 1.2 (2017): 109-115.
Copyright: © 2017 Mamotabo R Matshela. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.