Review Article
Volume 1 Issue 2 - 2017
A Neglected advance in Cardiac Therapeutics
University of Aberdeen, Aberdeen AB24 3FX, Scotland, UK
*Corresponding Author: Mark I M Noble, University of Aberdeen, Aberdeen AB24 3FX, Scotland, UK.
Received: July 25, 2017; Published: August 28, 2017
Abstract
From 1986 up to the present, there has been a stream of published papers indicating the great efficacy of 5HT2 serotonin receptor antagonism in inhibition of arterial thrombus growth. The particular receptor sub-type of importance in this regard is the 5HT2A subtype located on the platelet membrane. The reasons for the failure to make such drugs available for the treatment of patients with arterial disease are uncertain. One may be the fact that serotonin is a weak agonist for platelet aggregation in standard testing, much of which is performed in citrated platelet rich plasma which has an unphysiologically low extracellular calcium ion concentration. Thus it is possible that haematological opinion leaders assume that antagonising the platelet serotonin receptor will be ineffective. The discrepancy between such views and the consistently opposite evidence derived from in vivo animal experiments can be explained on the basis of a misconception of the mechanism of action. In arterial stenoses, platelets are activated by increased platelet shear stress caused by the narrowing of the lumen and the consequent increase in blood velocity. There is also a well-accepted positive feedback between serotonin released from such activated platelets and activation of more platelets via the 5HT2A receptor. A potential advantage of developing such a drug for clinical use is that, in the absence of serotonin in wounds, bleeding during surgical trauma would not be increased.
Keywords: Arterial thrombosis; Shear stress activation of platelets; Platelet to platelet serotonin mediated positive feedback
Abbreviations: 5HT: 5-hydroxytryptamine; QT: Time interval between the QRS complex and the T wave of the electrocardiogram; CNS: Central Nervous System; CVS: Cardiovascular System; LFTs: Liver Functions Tests
Introduction
Although there have been major improvements in the treatment of arterial disease in recent years, and a decline in disease due to changes in life style such as smoking cessation, there remains a need for further improvements. In the developing countries, there is a worrying increase in obesity, type 2 diabetes and pre-diabetes (occult insulin resistance). These patients are particularly prone to arterial disease, and the burden on treatment provision remains high. In this article, published evidence suggesting that an old approach which has been neglected be resurrected, is reviewed
History of Serotonin 5HT2 receptor antagonism applied to the cardiovascular system.
In 1986, Ashton., et al. [1] measured coronary blood flow in the circumflex coronary artery of the anaesthetised dog upon which they had imposed a stenosis using an arterial constrictor. They observed that the blood flow was not constant, but kept falling and then jumping up again. They called these “cyclic blood flow variations”, and correctly attributed them to thrombus growth followed by embolism. The startling results, as replotted in Figure 1, showed complete abolition of these phenomena after treatment with a 5HT2 blocking drug. The drug used, ketanserin, created questions owing to its hypotensive action which proved to be due contamination with alpha-1 adrenergic antagonistic properties, and also with QT prolongation properties. Subsequently, the use of more selective 5HT antagonists proved that the anti-thrombotic activity action was mediated by the platelet 5HT2A receptor antagonism and the consistency of anti-thrombotic activitiy across many drugs of this class was impressive [1 - 11]. Pure 5HT2A antagonists have no effect on blood pressure or QT interval.
In 1986, Ashton., et al. [1] measured coronary blood flow in the circumflex coronary artery of the anaesthetised dog upon which they had imposed a stenosis using an arterial constrictor. They observed that the blood flow was not constant, but kept falling and then jumping up again. They called these “cyclic blood flow variations”, and correctly attributed them to thrombus growth followed by embolism. The startling results, as replotted in Figure 1, showed complete abolition of these phenomena after treatment with a 5HT2 blocking drug. The drug used, ketanserin, created questions owing to its hypotensive action which proved to be due contamination with alpha-1 adrenergic antagonistic properties, and also with QT prolongation properties. Subsequently, the use of more selective 5HT antagonists proved that the anti-thrombotic activity action was mediated by the platelet 5HT2A receptor antagonism and the consistency of anti-thrombotic activitiy across many drugs of this class was impressive [1 - 11]. Pure 5HT2A antagonists have no effect on blood pressure or QT interval.
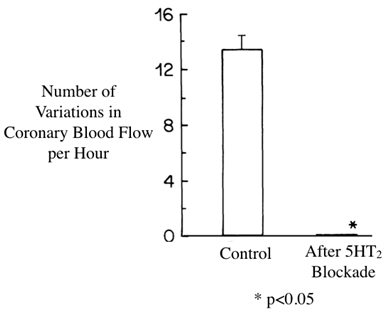
Figure 1: Variations in coronary blood flow through a stenosis due to thrombosis were abolished by the 5HT2 antagonist ketanserin. Adapted from Ashton., et al. [1].
A refinement in the method of recording the effects of substances upon thrombus growth rate is illustrated in Figure 2.
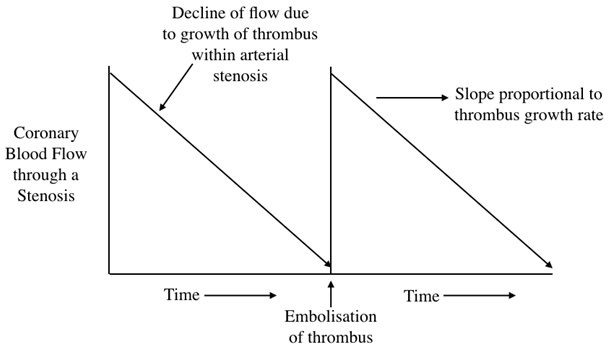
Figure 2: The decline of blood flow as a stenosis closes with thrombus is almost linear with time, so that the slopes can be used as an index of thrombus growth rate. A refinement in the method from [3].
With this refinement, it was possible to obtain dose/effect relationships for the 5HT2A antagonist used, resulting in a dose that caused complete dispersion of the thrombus [Figure 3].
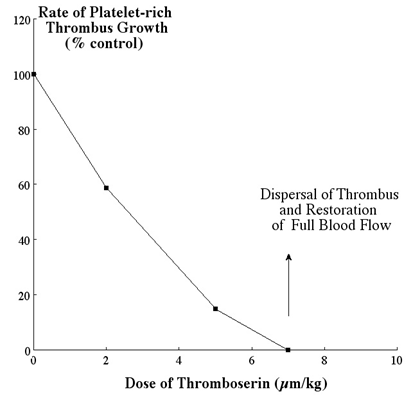
Figure 3: Effect of increasing doses of 5HT2A antagonist on thrombus growth rate with final complete dispersion. Plotted from data in [3] (this drug originated as ICI 170809, was then called thromboserin, and is now called Th001 (ArteclereTM).
It was also possible to show that thrombus growth rate was accelerated by adrenaline and that this acceleration was also blocked by the 5HT2A antagonist [3]. A subsequent study showed that when complete thrombotic occlusion was relieved by thrombolysis, but then re-occluded, full dispersion was achieved by addition of a 5HT2A antagonist [12].
Rejection by the Pharmaceutical Industry of proposals for developing 5HT2A antagonists for clinical use
The reasons for the failure to make such drugs available for treatment of patients with arterial disease are unclear. One may be the fact that serotonin is a weak agonist for platelet aggregation in standard testing, much of which is performed in citrated platelet rich plasma which has an unphysiologically low extracellular calcium ion concentration. Thus it is possible that haematological opinion leaders assume that antagonising the platelet serotonin receptor will be ineffective. Another possible reason is that the many 5HT2 antagonistic drugs have been synthesized, but have mostly been assigned for use in the Central Nervous System (CNS) departments. Another possible explanation is the success of purine P2Y12 receptor antagonists, such as clopidogrel.
The reasons for the failure to make such drugs available for treatment of patients with arterial disease are unclear. One may be the fact that serotonin is a weak agonist for platelet aggregation in standard testing, much of which is performed in citrated platelet rich plasma which has an unphysiologically low extracellular calcium ion concentration. Thus it is possible that haematological opinion leaders assume that antagonising the platelet serotonin receptor will be ineffective. Another possible reason is that the many 5HT2 antagonistic drugs have been synthesized, but have mostly been assigned for use in the Central Nervous System (CNS) departments. Another possible explanation is the success of purine P2Y12 receptor antagonists, such as clopidogrel.
An attempt to break the resistance to development of 5HT2A antagonism for clinical use
Some of the Imperial Chemical Industries (ICI) pharmaceutical Division scientific staff considered the possibility of switching ICI 170809 from CNS to Cardiovascular applications [3,13]. It turned out that this drug had no cerebral effects because it does not pass the blood/brain barrier. ICI then abandoned the drug, even though it had proved safe in humans as well as in all the usual pre-clinical tests. This drug then became an "orphan".
Some of the Imperial Chemical Industries (ICI) pharmaceutical Division scientific staff considered the possibility of switching ICI 170809 from CNS to Cardiovascular applications [3,13]. It turned out that this drug had no cerebral effects because it does not pass the blood/brain barrier. ICI then abandoned the drug, even though it had proved safe in humans as well as in all the usual pre-clinical tests. This drug then became an "orphan".
Eventually a group in the Department of Medicine and Therapeutics of Aberdeen University obtained a supply of tablets of ICI 170809, and academic grants enabled them to perform a safety trial in patients with arterial disease [14]. These were stable patients, with no active thrombosis, so that anti-thrombotic efficacy could not be tested. One of the reasons for taking this study seriously is the lack of pass through the blood/brain barrier, so that concerns regarding interference with brain serotonin functions are negligible. Another was the theoretical absence of any effect on haemostasis, which was confirmed by lack of prolongation of bleeding time and lack of effect on a number of relevant laboratory tests. The only side effect was that, at doses higher than those considered efficacious, liver function tests (LFTs) increased, yet remaining within the normal range; this is similar to drugs sold over the counter (e.g., paracetamol, statins) that are metabolised in the liver. Similar tests are required for other potential 5HT2A antagonists, since all those tests show anti-thrombotic efficacy in animals [1-11].
Clinical trials that could test efficacy as well as safety of 5HT2A antagonists in patients
The most obvious target group is that of patients with acute coronary syndromes (ACS), a difficult group to recruit for a study from a logistic point of view. The correct treatment for these patients is angioplasty and stenting. However, before this could be broadly applied, the treatment was to attempt clearance of thrombotic obstruction with thrombolytic drugs such as streptokinase and rt-PA. Ambulance crews called to chest pain patients were trained to radio ECGs to the centre and, if the diagnosis was ACS, they would set up an intravenous infusion and administer the thrombolytic drug. The same set-up might be used for intravenous injection of a 5HT2A antagonist instead of a thrombolytic substance. Experimentally this is more effective in clearing arterial thrombi [12]. Resolution of ECG changes between ambulance pick up and arrival at the hospital or during hospitalization, could be taken into consideration in association with other diagnostic markers such as troponin [15]. The other advantage of such a procedure is that the safety of some 5HT2A antagonists means that its administration, if the diagnosis is uncertain will not be harmful.
The most obvious target group is that of patients with acute coronary syndromes (ACS), a difficult group to recruit for a study from a logistic point of view. The correct treatment for these patients is angioplasty and stenting. However, before this could be broadly applied, the treatment was to attempt clearance of thrombotic obstruction with thrombolytic drugs such as streptokinase and rt-PA. Ambulance crews called to chest pain patients were trained to radio ECGs to the centre and, if the diagnosis was ACS, they would set up an intravenous infusion and administer the thrombolytic drug. The same set-up might be used for intravenous injection of a 5HT2A antagonist instead of a thrombolytic substance. Experimentally this is more effective in clearing arterial thrombi [12]. Resolution of ECG changes between ambulance pick up and arrival at the hospital or during hospitalization, could be taken into consideration in association with other diagnostic markers such as troponin [15]. The other advantage of such a procedure is that the safety of some 5HT2A antagonists means that its administration, if the diagnosis is uncertain will not be harmful.
Another target group proposed for a clinical trial would be those receiving dual anti-platelet therapy (usually aspirin plus clopidogrel) who require surgery. At present there are various regimes to allow the surgeon to operate without the patient receiving anti-thrombotic drugs during the operation. This leads to potential problems in view of the activation of thrombosis by trauma [16]. Not stopping the dual anti-platelet therapy can lead to excessive operative bleeding. Substitution of dual anti-platelet therapy by 5HT2A antagonism might provide protection from arterial thrombosis without affecting operative bleeding. Measures of outcome would be clinical indices plus numbers of circulating macro aggregates, and indices of thrombosis such a prothrombin fragments.
Conflict of Interest
Since the performance of reference 14, an academic study, the author has become a sharehold in the company Thromboserin Ltd, which hold the patents for Th001 (ArteclereTM).
Since the performance of reference 14, an academic study, the author has become a sharehold in the company Thromboserin Ltd, which hold the patents for Th001 (ArteclereTM).
References
- Ashton JH., et al. “Serotonin as a mediator of cyclic flow variations in stenosed canine coronary arteries”. Circulation 73.3 (1986): 572-578.
- Torr S., et al.“Inhibition of acute platelet thrombosis formation in stenosed canine coronary arteries by the specific serotonin 5HT2 receptor antagonist ritanserin”. Cardiovascular Research 24.6 (1990): 465-470.
- McAuliffe SJG., et al. “Interaction between the effect of 5-hydroxytryptamine and adrenaline on the growth of platelet thrombi in the coronary artery of the anaesthetised dog”. British Journal of Pharmacology 109.2 (1993): 405-410.
- Belcher PR., et al. “Dispersion of coronary artery thrombi by antagonism of platelet serotonin receptor in the dog”. Cardiovascular Research 26.3 (1992): 292-296.
- Kirchengast M., et al. “Inhibition by the combined Ca2+ and 5-HT2 receptor antagonist nexopamil (LU 49938) of intracoronary thrombus formation in a canine model of arterial stenosis and intimal damage”. Journal of Cardiovascular Pharmacology 22.5 (1993): 687-694.
- Hsieh CP., et al. “Effects of MDL 28,133A, a 5-HT2 receptor antagonist, on platelet aggregation and coronary thrombosis in dogs. Journal of Cardiovascular Pharmacology 24.5 (1994): 761-772.
- Pawlak D., et al. “A potent 5HT receptor (5-HT2A) antagonist, DV-7028, delays arterial thrombosis development in rats”. Thrombosis Research 90.6 (1998): 259-270.
- Berry CN., et al. “Antiplatelet and antithrombotic activity of SL65.0472, a mixed 5HT1B/5-HT2A receptor antagonist”. Thrombosis and Haemostasis 85.3 (2001): 521- 528.
- Kihara H., et al. “Antithrombotic activity of AT-1015, a potent 5-HT2A receptor antagonist, in rat arterial thrombosis model and its effect on bleeding time”. European Journal of Pharmacology433.2 (2001): 157-162.
- Adams JW., et al. “Anti- thrombotic and vascular effects of AR246686, a novel 5-HT2A receptor antagonist”. European Journal of Pharmacology 586.1 (2008): 234-243.
- Przyklenk K., et al. “Targeted inhibition of the serotonin 5HT2A receptor improves coronary patency in an in vivo model of recurrent thrombosis”. Journal of Thrombosis and Haemostasis 8.2 (2010): 331-340.
- Belcher PR., et al. “Antagonism of the platelet 5HT2 receptor in the presence of thrombolysis”. International Journal of Cardiology 43.1 (1994):11-20.
- Millson DS., et al. “The effect of a selective 5HT2 receptor antagonist (ICI 170,809) on platelet aggregation and pupillary responses in healthy volunteers”. British Journal of Clinical Pharmacology 33.3 (1992): 281-288.
- Noble MIM., et al. “The Novel Anti-Thrombotic Drug with No-Bleeding Excess”. Journal of Cardiology & Cardiovascular Therapy 6.2 (2017): 1-6.
- Stubbs P., et al. “Prognostic significance of admission Troponin T concentrations in patients with myocardial infarction”. Circulation 94.6 (1996): 1291-1297.
- Gando S. “Hemostasis and thrombosis in trauma patients”. Seminars in Thrombosis and Hemostasis 41.1 (2015): 26-34.
Citation:
Mark I M Noble. “A Neglected advance in Cardiac Therapeutics”. Therapeutic Advances in Cardiology 1.2 (2017): 92-96.
Copyright: © 2017 Mark I M Noble. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.