Research Article
Volume 2 Issue 2 - 2017
The Anti-Inflammatory Actions of an Intra-Oral Adhesive Patch Containing Botanical Extracts Exert Inhibitory Effects on the Development of Dental Biofilm and Improved Clinical Outcomes in the Management of Peri-Implant Mucosal Inflammation and Peri-Implantitis
1Professor of Periodontology, Faculty of Dentistry, Professor, Department of Laboratory Medicine and Pathophysiology, Faculty of Medicine, Dentist-in-Chief, Sinai Health System, University of Toronto
2Jerusalem Perio Center, Jerusalem Israel
2Jerusalem Perio Center, Jerusalem Israel
*Corresponding Author: Dani E Weichholz, Jerusalem Perio Center, Jerusalem Israel.
Received: November 29, 2017; Published: December 04, 2017
Abstract
Peri-implant mucosal inflammation (PIMI) and the more aggressive form of the disease, often referred to as peri-implantitis (PI), are characterized typically by both infection and inflammation. Yet it is unclear as to what the initiating factors for these two related conditions are; infection and/or inflammation. Despite the fact that both aforementioned pathophysiological factors are present concurrently in both PIMI and PI, treatment approaches for such conditions most often focus on the microbial element. Hence these disorders are typically treated using an antimicrobial therapeutic paradigm while generally ignoring the inflammatory portion of the equation. This narrow focus regarding treatment could explain, at least in part, why successful management of PIMI and PI has remained an elusive goal. To address the need for more effective ways to manage the inflammatory elements of PIMI/PI, we describe the use of an anti-inflammatory intra-oral patch that can be applied to tissues affected by PIMI/PI. Notably, this patch treatment was performed as a sole therapy, without any anti-microbial intervention. Clinical parameters, including gingival index (GI), bleeding on probing (BOP) and pocket depth (PD) were measured prior to treatment and 1 week later. In addition, microbial sampling was performed to ascertain the relative numbers of five known periodontal pathogenic bacterial species prior to treatment and, again, one week later.
Following this treatment significant improvement in all three clinical measures, as well as a clear reduction in microbial load (even though antimicrobial therapy was not used), were demonstrated. These findings suggest that PIMI/PI are conditions that are exacerbated by microbial infection but that pathologically unregulated inflammation could be a critically important factor. Treatment of microbial infection alone, then, might be insufficient for management of PIMI/PI. We suggest that a combined treatment approach that utilizes both anti-inflammatory, as well as antimicrobial, therapy could lead to more reliable and predictable outcomes in relation to treatment of PIMI/PI.
Introduction
PIMI and PI present as inflammation in the tissue surrounding an implant, with destruction of hard tissue (bone) being associated with the latter only. It is generally assumed that PIMI must be present in most cases before an affected implant can then develop PI. Insofar as PI is concerned it must be recognized that even around healthy implants there is some degree of bone loss related to normal remodeling but, in the presence of PI, the bone loss is exaggerated significantly to the point where, if left untreated, it could lead to loss of the implant [1] As regards the prevalence of either condition it has been suggested that, for PI in particular, the findings vary widely and range from 6% to 36%. [2] This disparity in statistics about PI is due largely to the lack of clear defining terms and factors, including “differing thresholds of bone loss used in various studies to define its existence.” [3] This is unfortunate, because early diagnosis and prompt treatment require a highly sensitive index, particularly when it comes to its earliest signs and symptoms as will be addressed below. Insofar as PIMI is concerned the prevalence also varies widely, with incidence rates of 18-56% with respect to individual implants, while the condition itself is seen in about 60-80% of patients. [4,5]
There are several studies focused on prevention of PIMI and/or PI, including one report by Froum., et al. [6] These authors have focused on what are currently believed to be ‘best practices’ for prevention of PIMI/PI, as well as treatment of these conditions when they occur. In agreement with what was pointed out above, it is thought that intervention in the treatment of PIMI should be carried out at the earliest possible opportunity to prevent progression to PI and, similarly, the earlier one treats PI the better insofar as prevention or at least delaying the loss of an implant is concerned.
Given the potentially dire consequences of PIMI and PI with respect to loss of implants, the American Academy of Periodontology wrote a report focused on prevention, diagnosis, and early treatment. [7] The report stresses the importance of preventing the progression of PIMI and also recommended the use of methods that “monitor implant health and determine inflammatory complications as part of an ongoing implant maintenance program.” [7]
The study described herein is based on the concept that an increase in inflammatory exudate alters the local environment (around a tooth or implant) thereby actually ‘nurturing’ microbial growth in general and possibly even providing, ultimately, an environment that is even more supportive for the establishment of the more well-known pathogenic or ‘red complex’ bacteria. [8] Either way, it can be postulated that these sometimes invasive groups of bacteria not only have the capacity to cause direct tissue-damaging effects, but likely also activate the host immune response that results in localized tissue destruction. Therefore, it is hypothesized that the reduction in the concentration of inflammatory products contained within the exudate alone could decrease the localized tissue destruction and simultaneously decrease the bacterial load.
This study is an open-label trial and, therefore, has to be considered purely as a pilot investigation in support of a concept. The goals are to focus treatment solely on treatment/reduction of local inflammation about implants affected with PIMI or PI as a means towards reduction in overall disease activity and reestablishment of health.
Materials and Methods
Outcome Measures
It was decided to focus on particular outcome measures of treatment for PIMI or PI. Therefore, the following parameters were assessed and included clinical measures, microbial assessment, and inflammatory response of patients with either PIMI or PI. The treatment consisted strictly of the application of a novel, botanically-based anti-inflammatory adhesive patch (PerioPatch®; IZUN Oral Care) over affected sites. This patch has anti-inflammatory effects only and has no direct antimicrobial activity.
It was decided to focus on particular outcome measures of treatment for PIMI or PI. Therefore, the following parameters were assessed and included clinical measures, microbial assessment, and inflammatory response of patients with either PIMI or PI. The treatment consisted strictly of the application of a novel, botanically-based anti-inflammatory adhesive patch (PerioPatch®; IZUN Oral Care) over affected sites. This patch has anti-inflammatory effects only and has no direct antimicrobial activity.
The study included 18 patients of at least 18 years of age, with a total of 23 dental implant sites with either PI or PIMI. With respect to the diagnosis of PIMI, involved sites demonstrated only inflamed mucosal tissue about the implant with no evidence (radiographic or clinical) for loss of bone beyond what might be expected normally as a consequence of bone remodeling, as described by others. [9] In cases where PI was diagnosed, the site had to demonstrate inflamed mucosal tissue as noted above for PIMI, in addition to excessive bone loss demonstrated clinically and/or radio graphically. Typically, sites with PI had evidence for radiographic bone loss of ≥ 2 mm from the time of placement. Implants had been loaded for at least 1 year, with a range of 1 to 10 years.
Excluded from the study were pregnant women, smokers, and patients who had undergone debridement in the implant area within the previous 3 months, were taking anti-inflammatory drugs on a regular basis, or who had been on antibiotics within the previous 6 months.
The treatment protocol involved a single course of treatment with the anti-inflammatory botanical intra-oral patch (PerioPatch®, IZUN Oral Care Corp. http://izunoralcare.com). The treatment regimen involved applying the patches twice a day for 3 days, for a total of six patches over the treatment period. Patients were evaluated at baseline, and returned for post-treatment evaluation 1 week after commencement of the therapy-i.e., 4 days after completion of the treatment.
At baseline, patients were evaluated according to methods described below:
Pocket depth (PD)-PD was assessed and recorded in millimeters at six sites per implant: mesiobuccal (MB), buccal (B), distobuccal (DB), mesiolingual (MP/L), lingual (L), and distolingual (DP/L).
Pocket depth (PD)-PD was assessed and recorded in millimeters at six sites per implant: mesiobuccal (MB), buccal (B), distobuccal (DB), mesiolingual (MP/L), lingual (L), and distolingual (DP/L).
Bleeding on probing (BOP)-Using Y or N, Y indicated any evidence of bleeding around an implant site.
Gingival inflammation (GI)-Gingival inflammation was assessed using the 0-3 gingival index developed by Löe and Silness [10] and Löe [11] (0= normal gingiva; 1 = mild inflammation; 2 = moderate inflammation; 3 = severe inflammation).
Gingival inflammation (GI)-Gingival inflammation was assessed using the 0-3 gingival index developed by Löe and Silness [10] and Löe [11] (0= normal gingiva; 1 = mild inflammation; 2 = moderate inflammation; 3 = severe inflammation).
Plaque index (PI)-The presence of bacterial plaque was rated using a scale ranging from 0-3 based on the plaque index described by Löe and Silness [10] and Löe [11] at four sites per implant (M, D, B, P/L) (0 = no plaque; 1 = plaque that can be detected using a probe or disclosing solution only; 2 = moderate; visible to human eye; 3 = abundance of soft matter within the gingival pocket and/or on the tooth and gingival margin).
Microbial assessment- Samples were collected at sites with the deepest probing depths using paper points and sent to an external laboratory to analyze for the following Gram-negative anaerobic bacteria: Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, Bacteroides forsythus and Treponema denticola. Laboratory measurement was carried out by the use of cDNA probes to measure the quantity of known periodontal pathological bacteria.
After baseline measurements were taken, the first of six PerioPatches was placed on the affected tissue. Patients received a total of six such treatments over a 3-day period-i.e., twice a day for 3 days. The initial patch was placed by the dentist and the remaining patches were applied by the patient.
One week after baseline, the parameters noted above were recorded again while additional microbial sampling was also performed.
Results
Baseline and follow-up data (i.e., one week after treatment) were compared. Statistical analysis was then performed on outcomes of change in PD, GI, and on the bacterial findings (Figure 1). Importantly, values obtained for PD, GI, and BOP were analyzed using frequency distributions since assessment of mean values for the outcomes derived from scales is potentially misleading.
Reduced inflammation was observed in all cases of PI where the PerioPatch was used. As well, there was a statistically significant reduction in microbial levels as measured by cDNA following treatment.
As shown in Figure 1, bacterial analysis showed a decrease in microbial load after PerioPatch treatment; it was reduced on average from 5.07 to 3.04 units, GI on average dropped from 1.45 to 0.7 GI units and probing depths on average were reduced from 5.19 mm to 3.27 mm.
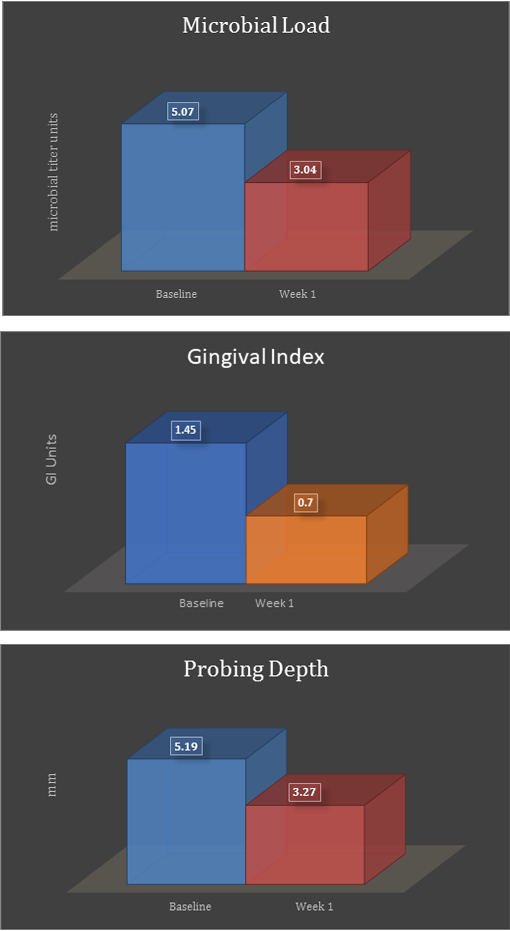
Figure 1: Data analysis for microbial load, gingival index, and probing depth reflects improvements in
all values compared to baseline values, just one week after commencement of the three-day treatment.
Values presented reflect the average of the data analyzed.
The frequency distribution data demonstrate a clear shift to the left, connoting improved health with regard to all three measured parameters (PD, GI and BoP) following treatment (Figures 2-4).
When probing depths were assessed by frequency distribution analysis as opposed to mean probing depths, an increase in the percentage of sites with probing depths of less than 5 mm, from 41% to 64%, was found after treatment, while there was a concomitant reduction in probing depths within the 7 mm range, from 33% to 23%. That is, prior to treatment at least 26% of sites were in the 7mm range while, after treatment, this was reduced to only 13% (Figure 2).
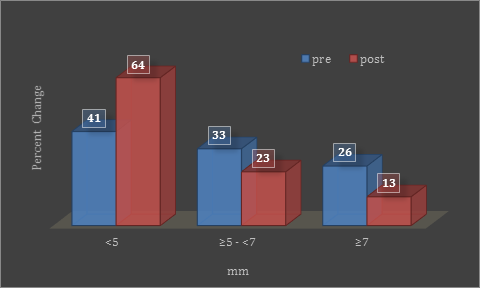
Figure 2: Frequency distribution graph shows percentage change in
probing depths before and after one week of treatment.
Based on the frequency distribution for GI, there was an almost 90% reduction in the severity of GI in patients who started treatment with initial GI in the 2-3 range (Figure 3). Correspondingly, there was an increase in the population of the GI category of 0-1 (i.e., more health associated). In addition, there was an overall reduction of BoP of just over 20% following treatment (Figure 4).
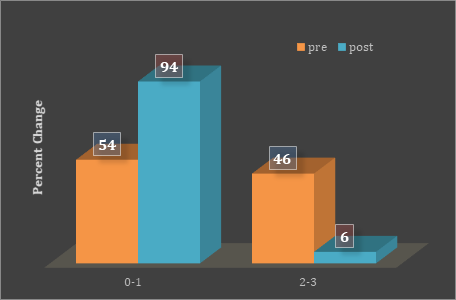
Figure 3: Frequency distribution graph shows percentage change in GI
before and after one week of treatment.
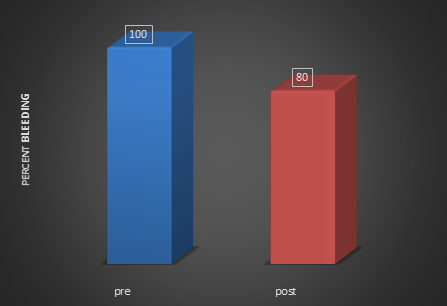
Figure 4: Frequency distribution graph shows percentage change in
BoP scores pre-treatment and post (after one week of treatment).
According to these findings, PerioPatch had a significant effect on pathogenic bacteria found in the soft tissue pockets of implants with PI, as well as on reduction in inflammation around implants with PI.
Discussion
While there are differences of opinion as to whether PIMI or PI are induced principally by microbial infection, the findings shown here suggest that inflammation itself could be playing a very important role not only in disease progression and tissue damage but even with respect to recruitment and maintenance of disease-associated bacteria in affected sites. Nevertheless, the overall question remains as to whether PIMI or PI are classic bacterial infections or are driven by changes in the inflammatory process. Once the inflammatory process is activated, it becomes the primary cause and the most important mitigating factor insofar as ongoing disease and destruction of tissue are concerned.
Most clinicians utilize a combination of therapies to properly address both the bacterial and inflammatory aspects of either PIMI or PI. Proposed treatment modalities range from debridement, surgical intervention, localized anti-microbial therapy, systemic antibiotics, laser ablation, and others - some of which carry their own risks. For example, low-dose doxycycline requires systemic administration and long-term therapy (> 3 months) to reduce matrix metalloproteinase (MMP) levels and to effect changes in the periodontal tissue. [12] Topical anti-inflammatory agents, such as COX-inhibitors, have been shown to irritate mucosal tissue, [13] and repeated application of topical steroids raises the risk of infection14 and reduces the capacity for normal tissue repair.
In contrast, a new topical option that can effectively reduce inflammation and, thereby, microbial load as this study suggests, may be a safer and more effective alternative. Botanical extracts contained in this intraoral patch (PerioPatch, Izun Oral Care corp., http://izunoralcare.com) are a known source of bioactive compounds that can modulate the inflammatory response. [15] They have also been shown to aid in absorption of pro-inflammatory mediators and, as such, support the natural resolution of the inflammation. This action has been observed clinically, as well as in in vitro assays not yet published. The primary effect of PerioPatch is therefore achieved by physical means, i.e., removal of toxic exudates and protection of healing tissue.
According to the findings in this study, PerioPatch significantly reduced the pathogenic bacterial load found in the pockets of implants with PI, as well as reduced the inflammation around the implants. Furthermore the frequency distribution analysis showed a clear shift to the left, i.e., towards health in all clinical parameters analyzed.
While inflammation is necessary to initiate repair of injured tissue, when dysregulated it can damage tissue and prevent normal healing. Thus, the effective management of inflammation such as that demonstrated in chronic inflammatory disease-including gingivitis and periodontitis-is critical to promote healing. This is particularly the case after surgical procedures such as implant placement, periodontal surgery, ridge augmentation, and extractions.
The available topical approaches to managing inflammation in the oral environment have been associated with adverse events, including mucosal tissue irritation, increased risk of infection, and reduction in normal tissue repair. In contrast, the topical botanical patch described (PerioPatch) has demonstrated reduction of inflammatory cell activity in the crevicular fluid and significant reduction of the MMP activity in the gingiva in this and other studies-all without serious adverse events.
Conclusion
This latest study demonstrates the overall positive effect the PerioPatch treatment has on both clinical and microbial parameters as a stand-alone treatment. It shows that a decrease in inflammation can affect both the clinical parameters and the bacterial component of PI and of PIMI. Based on these findings, it would appear that such reduction in inflammation can impact on the severity of the localized inflammation and alter microbial load in a positive manner.
References
- Sanz M and Chapple IL. “Clinical research on peri-implant diseases: Consensus report of Working Group 4”. Journal of Clinical Periodontology39.S12(2012): 202-206.
- Koldsland OC., et al. “Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss”. Journal of Periodontology81.2 (2010): 231-238.
- Froum SJ.,et al. “A Regenerative Approach to the Successful Treatment of Peri-implantitis: A Consecutive Series of 170 Implants in 100 Patients with 2- to 10-Year Follow-up”. The International Journal of Periodontics and Restorative Dentistry 35.6 (2015): 857-863.
- Atieh MA., et al. “The frequency of peri-implant diseases: a systematic review and meta-analysis”. Journal of Periodontology 84.11 (2013): 1586-1598.
- Zitzmann NU and T Berglundh. “Definition and prevalence of peri-implant diseases”. Journal of Periodontology35. S8 (2008): 286-291.
- Froum S J., et al. “Successful management of peri-implantitis with a regenerative approach: a consecutive series of 51 treated implants with 3-to 7.5-year follow-up”. International Journal of Periodontics and Restorative Dentistry 32.1 (2012): 11-2.0
- American Academy of Periodontology. “Peri-Implant Mucositis and Peri-Implantitis: A Current Understanding of Their Diagnoses and Clinical Implications”. Journal of Periodontology84.4 (2013): 436-443.
- Socransky SS., et al. "Microbial complexes in subgingival plaque." Journal of Clinical Periodontology 25.2 (1998): 134-144.
- Oh TJ., et al. “The causes of early implant bone loss: myth or science?” Journal of Periodontology 73.3 (2002): 322-33.
- Löe H and Silness J. “Periodontal disease in pregnancy. I. Prevalence and severity”. Acta Odontologica Scandinavica21 (1963): 533-551.
- Löe H. “The Gingival Index, the Plaque Index and the Retention Index Systems”. Journal of Periodontology38.6 (1967): 610-616.
- Fisher DA. “Adverse effects of topical corticosteroid use”. The Western Journal of Medicine 162.2 (1995): 123-126.
- Reddy MS., et al. “Periodontal host modulation with antiproteinase, anti-inflammatory, and bone-sparing agents. A systematic review.” Annals of Periodontology 8.1 (2003): 12-37.
- Brewer AR., et al. “Update on the use of topical NSAIDs for the treatment of soft tissue and musculoskeletal pain: a review of recent data and current treatment options”. The Physician and Sportsmedicine 38.2 (2010): 62-70.
- Bisset NG. “Herbal Drugs and Phytopharmaceuticals”. Boca Raton: CRC Press; (1994).
Citation:
Dani E Weichholz., et al. “The Anti-Inflammatory Actions of an Intra-Oral Adhesive Patch Containing Botanical Extracts Exert
Inhibitory Effects on the Development of Dental Biofilm and Improved Clinical Outcomes in the Management of Peri-Implant Mucosal
Inflammation and Peri-Implantitis”. Oral Health and Dentistry 2.2 (2017): 333-340.
Copyright: © 2017 Dani E Weichholz., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.