Research Article
Volume 1 Issue 1 - 2016
Role of Podoplanin in Analyzing Neoplastic Potential of Keratocystic Odontogenic Tumor- A Retrospective Immunohistochemical Study.
1Dr.Chaitanya C.Varangaonkar, MDS, Dr.Uhlas Patil Medical College, Jalagon
2Dr.Pratima A.Dongre MDS, (Department of Oral Pathology and Microbiology). Government Dental College and Hospital, Nagpur
3Dr.Sindhu M.Ganvir MDS, Professor, Head of the Department (Department of Oral Pathology and Microbiology), Government Dental College and Hospital, Nagpur
4Dr.Shailesh D.Kumbhare MDS, Consultant, (Asha Hospital), Nagpur
5Dr.Sharvari Tawale MDS, (Residence), Shri.Yashwantrao Chavan Dental College, Ahmadnagar
6Dr.Manasi Deshpande MDS (Private Practioner), Nagpur
2Dr.Pratima A.Dongre MDS, (Department of Oral Pathology and Microbiology). Government Dental College and Hospital, Nagpur
3Dr.Sindhu M.Ganvir MDS, Professor, Head of the Department (Department of Oral Pathology and Microbiology), Government Dental College and Hospital, Nagpur
4Dr.Shailesh D.Kumbhare MDS, Consultant, (Asha Hospital), Nagpur
5Dr.Sharvari Tawale MDS, (Residence), Shri.Yashwantrao Chavan Dental College, Ahmadnagar
6Dr.Manasi Deshpande MDS (Private Practioner), Nagpur
*Corresponding Author: Dr. Pratima A.Dongre MDS, Department of Oral Pathology and Microbiology, Government Dental College and Hospital, Nagpur.
Received: December 10, 2016; Published: December 16, 2016
Abstract
Background: The aim of this study was to investigate and compare the podoplanin expression in ameloblastoma, keratocystic odontogenic tumor, orthokeratinised odontogenic cyst and dentigerous cyst and to examine its role in deciding neoplastic potential of KCOT by comparing its expression in ameloblastoma.
Methods: Fifteen paraffin embedded specimens of ameloblastoma, 15 paraffin embedded specimens of KCOT, 15 orthokeratinised odontogenic cysts and 15 dentigerous cysts were obtained and were analyzed by immunohistochemistry using anti-human podoplanin antibody. Podoplanin expression in odontogenic epithelial cells was evaluated using a scoring method and by calculating the percentage of positive odontogenic cells.
Results: Podoplanin was expressed strongly (at the invasive front) in the peripheral odontogenic epithelial cells in Ameloblastoma. In KCOT, most of basal and suprabasal cells, areas of budding basal cell proliferation, epithelial nests and peripheral cells of daughter cyst in stromal connective tissue showed positivity for Podoplanin. Whereas in case of OOC and DC, only cases associated with inflammation were positive for podoplanin.
Conclusion: Expression of podoplanin (at the invasive front) in peripheral cells of ameloblastoma is considered to be associated with neoplastic odontogenic tissues, this molecule is strongly expressed in KCOT in comparison with OOCs. The pattern of staining for podoplanin in KCOT could be related to its neoplastic nature and suggests that this protein might play a role in progression and local invasion of odontogenic tumors.
Keywords: Immunohistochemistry; Keratocystic odontogenic tumor; Orthokeratinized odontogenic cyst; Podoplanin
Introduction
Ameloblastoma (AM) is one of the most frequent odontogenic tumors of the jaw and is characterized by benign but locally aggressive behavior with a high rate of recurrence. [1,2] Histologically, AMs occur in six main patterns, follicular and plexiform,acanthomatous, granular cell and basal cell variants. [1,2] Recent studies have identified genetic and molecular alterations in epithelial odontogenic tumors. [2,3] However, details of the mechanism of oncogenesis, cytodifferentiation and tumor progression remain unknown. [2]
Keratocystic odontogenic tumor (KCOT) defined by the World Health Organization (WHO), is a benign, intraosseous neoplasm of dental origin, with a characteristic lining of parakeratinized stratified squamous epithelium. It was previously known as Odontogenic keratocyst (OKC) and was first described by Phillipsen in 1956. [4] The term Keratocystic odontogenic tumor was recommended by WHO which describe its neoplastic nature. However, evidence based clinical parameters cannot predict the potential of KCOT for neoplastic behavior or aggressive and localized infiltration.
Podoplanin is a transmembrane glycoprotein that has been largely used as an identifying marker of the lymphatic endothelium. [5] Published findings indicate that this protein has a relatively broad spectrum of reactivity including its expression in a wide variety of benign and malignant oral tumors. [6-15] In oral squamous cell carcinoma, the strong expression of podoplanin by malignant cells was associated with lymph node metastasis and poor clinical outcome. [6,7,10, 12,13,16]
An investigation to verify if podoplanin expression could be an useful parameter for reclassification of the keratocystic odontogenic tumour from cyst to tumour status was recently published. [11] Despite the numerous studies about the presence of podoplanin expression in various oral tissues and tumours, little is known about its physiologic or pathologic function. Sawa., et al. suggested an association of podoplanin in cellular proliferative activity due to its expression in tooth germ, which is present in cells with high mitotic activity, i.e. in dental lamina, terminal portion of Hertwig’s root sheath and preameloblasts. [17]
In view of the above considerations, the present study was designed to investigate the distribution of cells expressing podoplanin in human Ameloblastoma (AM), Keratocystic Odontogenic Tumors (KCOT), Orthokeratinized Odontogenic Cyst (OOC) and Dentigerous cysts and the role of podoplanin in deciding neoplastic potential and invasive behavior of KCOT by comparing its expression with ameloblastoma.
To the best of our knowledge this study is one of the few studies comparing intensity of podoplanin expression between cystic and neoplastic tissue and thus deciding the role of podoplanin in tumor progression. None of the study has investigated and compared the differential expression of Podoplanin between cystic and tumor component.
Subjects and Methods
All paraffin-embedded tissue specimens from15 ameloblastomas, 15 KCOT, 15 Orthokeratinized Odontogenic Cyst and 15 Dentigerous cyst were retrived from the department of oral Pathology and microbiology, Government dental college and hospital, Nagpur. The inclusion criteria were as follows: (i) patients with microscopically confirmed diagnoses of solid⁄multicystic ameloblastoma or unicystic ameloblastoma, KCOT, OOC, Dentigerous cysts determined by the sum of the clinical, radiographic, and microscopic data; (ii) the availability of the paraffin block with a sufficient amount of tissue for microscopic analysis. These samples were analyzed by immunohistochemistry for podoplanin. The diagnosis of Ameloblastoma, KCOT, OOC, dentigerous cysts was reviewed according to the World Health Organization histological classification of odontogenic tumors. [1] This study was approved by the Ethics Committee of the Government dental college and hospital, Nagpur.
Immunohistochemistry
Formalin-fixed 4 um sections of Ameloblastomas, KCOT, OOC and Dentigerous cysts were obtained from the pathology archive and subjected to immunohistochemical analysis using anti-podoplanin antibodies to determine their expression in odontogenic cells. After antigen retrieval using 10 mm citrate buffer, pH 6.0, in EZ antigen retriver for 10 min of 2 cycles, endogenous peroxidase activity was blocked by incubating in 3% H2O2 for 20 min. Each ameloblastoma, KCOT, OOC, Dentigerous cyst section was incubated for one hour in a 1:200 dilution of the primary monoclonal anti-podoplanin antibody (D2-40 clone, code#3619-1; Dako North America, Inc., Carpinteria, CA, USA). After the primary antibody incubation, each section was incubated using the Advance HRP Link System (code #K4067; Dako North America, Inc.) for 30 min at 37°C. The antibodies podoplanin was detected using 3.3 diaminobenzidine tetrahydrochloride (cod# D-5637; Sigma). Sections were counterstained with Mayer’s hematoxylin before being dehydrated and cover slipped. Lymph node was used as the positive control. The primary antibody was omitted during immunohistochemical staining for the negative control.
Formalin-fixed 4 um sections of Ameloblastomas, KCOT, OOC and Dentigerous cysts were obtained from the pathology archive and subjected to immunohistochemical analysis using anti-podoplanin antibodies to determine their expression in odontogenic cells. After antigen retrieval using 10 mm citrate buffer, pH 6.0, in EZ antigen retriver for 10 min of 2 cycles, endogenous peroxidase activity was blocked by incubating in 3% H2O2 for 20 min. Each ameloblastoma, KCOT, OOC, Dentigerous cyst section was incubated for one hour in a 1:200 dilution of the primary monoclonal anti-podoplanin antibody (D2-40 clone, code#3619-1; Dako North America, Inc., Carpinteria, CA, USA). After the primary antibody incubation, each section was incubated using the Advance HRP Link System (code #K4067; Dako North America, Inc.) for 30 min at 37°C. The antibodies podoplanin was detected using 3.3 diaminobenzidine tetrahydrochloride (cod# D-5637; Sigma). Sections were counterstained with Mayer’s hematoxylin before being dehydrated and cover slipped. Lymph node was used as the positive control. The primary antibody was omitted during immunohistochemical staining for the negative control.
Immunostaining evaluation
Cytoplasmic and/or membranous podoplanin expression by epithelial odontogenic cells in each ameloblastoma, KCOT, OOC and dentigerous cyst was assessed by the evaluation of the staining intensity and percentage of podoplanin positive cells according to the method used by Etemad –Moghadam., et al. [18]
Cytoplasmic and/or membranous podoplanin expression by epithelial odontogenic cells in each ameloblastoma, KCOT, OOC and dentigerous cyst was assessed by the evaluation of the staining intensity and percentage of podoplanin positive cells according to the method used by Etemad –Moghadam., et al. [18]
The tissue sections were evaluated for podoplanin positivity by scanning the section at 100x magnification. The counting of podoplanin positive cells was performed by counting in ten randomly selected, most representative fields showing podoplanin positivity at magnification of 400x, using the measurement tool on the computer assisted image analyser. Average of the values of these 10 selected fields were calculated to get the final value of percentage of positive cells per section. The result was presented as the mean number of podoplanin positive cells per section.
The percentage of podoplanin positive cells in the three group were counted as follows-
- 0% = no positive cells.
- 1% = 1-33 % positive cells.
- 2% = 34-66% positive cells.
- 3% = 67-100 % positive cells.
Staining intensity was calculated as
- 0% = when there was no staining.
- 1% = in parts where positivity was observed only at a magnification of 400x.
- 2% = in cases where the staining intensity was obvious at
100x, but not at 40x. and
- 3% = in fields where immunopositive cells were seen even at 40x.
Staining index was calculated of each specimen as follows- Percentage of immunopositive cells x intensity score.
This index was classified as:
- Zero = 0
- Low = 1 to 2.
- Moderate = 3 to 4.
- High = 6 to 9.
All the haematoxylin–eosin and immunohistochemical–stained sections were evaluated by two observers to eliminate interobserver bias, and any disagreement were resolved with a pentahead microscope.
Statistical analysis
Statistical analysis was done using statistical software SPSS, (Statistical Package of Social Software) version 16.0, to make the study statistically significant. After clinical observations, the data collected was tabulated and all observed results were then subjected to various statistical analyses as per requirement
Statistical analysis was done using statistical software SPSS, (Statistical Package of Social Software) version 16.0, to make the study statistically significant. After clinical observations, the data collected was tabulated and all observed results were then subjected to various statistical analyses as per requirement
Results
Clinical features
* - One-way ANOVA test
The age of patients with ameloblastoma ranged from 19 to 65 years (mean of 44 years) and KCOT of 13 to 70 years ( mean of 28.80) (Table 1).
The age of patients with ameloblastoma ranged from 19 to 65 years (mean of 44 years) and KCOT of 13 to 70 years ( mean of 28.80) (Table 1).
| Mean | SD | P* value | |
| KCOT | 28.8000 | 20.01856 | < 0.01 |
| OKC | 28.5333 | 10.92747 | |
| Ameloblastoma | 44.0000 | 13.36306 | |
| DC | 25.4667 | 7.67991 |
Table 1: Comparison of mean age of the participants between test groups.
* - Chi-square test
A predominance of men was observed in relation to women in both the groups (Table 2).
A predominance of men was observed in relation to women in both the groups (Table 2).
| Sex | P* value | |||
| Female | Male | |||
| Groups | KCOT | 6 | 9 | 0.81 |
| 33.3% | 21.4% | |||
| OKC | 4 | 11 | ||
| 22.2% | 26.2% | |||
| Ameloblastoma | 4 | 11 | ||
| 22.2% | 26.2% | |||
| DC | 4 | 11 | ||
| 22.2% | 26.2% | |||
| Total | 18 | 42 | ||
| 100.0% | 100.0% | |||
Table 2: Gender distribution of test groups.
* - Chi-square test
The distribution of tumors according to the staining intensity was listed in Table 3.
The distribution of tumors according to the staining intensity was listed in Table 3.
| Staining Intensity | P* value | ||||
| Low | Medium | High | |||
| Groups | KCOT | 1 | 7 | 7 | < 0.01 |
| 4.2% | 36.8% | 41.2% | |||
| OKC | 12 | 3 | 0 | ||
| 50.0% | 15.8% | .0% | |||
| Ameloblastoma | 1 | 4 | 10 | ||
| 4.2% | 21.1% | 58.8% | |||
| DC | 10 | 5 | 0 | ||
| 41.7% | 26.3% | .0% | |||
| Total | 24 | 19 | 17 | ||
| 100.0% | 100.0% | 100.0% | |||
Table 3: Distribution of groups with respect to staining intensity.
Podoplanin expression in odontogenic epithelial cells from ameloblastomas, KCOT, Orthokeratinized odontogenic cyst and Dentigerous cyst.
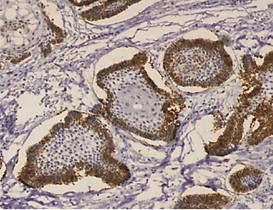
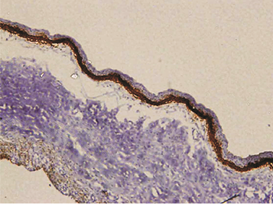
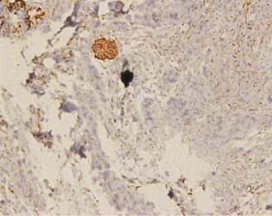
The expression of podoplanin in follicular ameloblastomas was predominantly observed in the peripheral columnar cells of tumoral islands, while the reticulum stellate-like cells exhibited virtually no immunostaining (Figure 1a).
Figure 1: a – Follicular ameloblastoma podoplanin expression was obseverd in peripheral cells while central cells were negative.
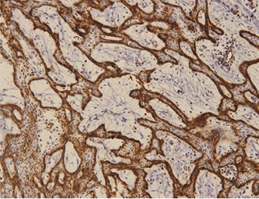
The outer cells of the plexiform AMs expressed podoplanin based on immunohistochemistry staining. In a few specimens, its expression reached the central cells (Figure 2a).
The expression of podoplanin was quite variable in the unicystic AMs. Its positivity was restricted to the basal and supra-basal layers of the cystic lining in some tumors and no reaction in the upper layer (Figure 3). The acanthomatous areas did not express podoplanin (Figure 4).
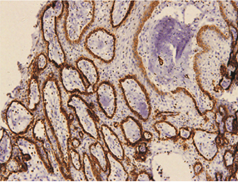
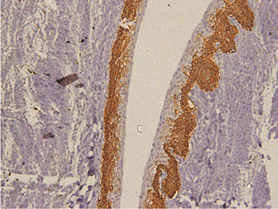
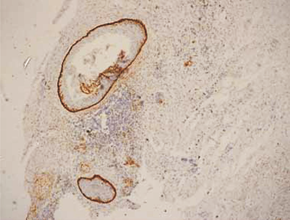
In keratocystic odontogenic tumours, basal and suprabasal layers from epithelial tumoral lining presented high membranous immunoreaction to anti-podoplanin antibody while the upper layers were negative for this protein, areas of budding cell proliferation, epithelial nests in connective tissue also shows positivity for this protein. Peripheral cells from daughter cysts also expressed the antibody (Figure 5 a,b,c,d,e,f). Three KCOT cases were negative for this protein.
Figure 5a: KCOT-Basal and suprabasal layer of cystic lining stongly express podoplanin while cells from the upermost layer were negative.
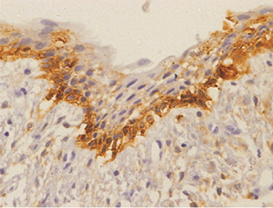
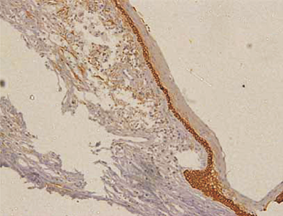
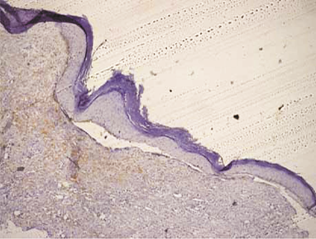
Orthokeratinized odontogenic cysts did not stain with podoplanin (Figure 6), except for few cells from basal layer, which presented slight immunoreaction for the protein associated to inflammatory infiltrate.
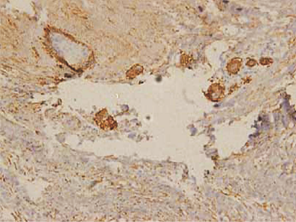
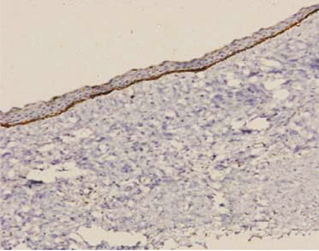
In case of dentigerous cysts weak positivity was observed when mild to moderate inflammatory reaction was present. Some of the DCs without an inflammatory reaction were entirely negative for podoplanin in the basal cells. (Figure 7).
Discussion
The distribution of podoplanin immunoreaction in the odontogenic tumours found in this study is corroborated by previous investigations. [8,9,11,14,19,20]. Its expression had been studied only in few exclusively epithelial odontogenic tumours and odontogenic cysts. [8,11], [12,14] The uniqueness of this study is in evaluation and comparison of neoplastic and cystic odontogenic lesions. [13] Thus, to contribute to this line of investigation, analysis of the immunostaining pattern of podoplanin in 15 ameloblastomas, 15 KCOT, 15 OOC, 15 dentigerous cysts was carried out.
The glycoprotein Podoplanin is well established as a lymphatic-specific marker that is widely used in histopathology, and has been reported to be associated with tumor-induced platelet aggregation and tumor metastasis [21]. In addition, enhanced podoplanin expression has been demonstrated in squamous cell carcinoma of the lung, uterine cervix, and head and neck, oral leukoplakia, and pleomorphic adenoma. [10,22,23,24]
The expression of podoplanin was basically restricted to the peripheral epithelial cells of the odontogenic neoplasias, indicating that this protein probably has a role in the process of tumoral invasion. As shown in Figure 1,2,3 the peripheral epithelial odontogenic cells of all studied tumours were positive for podoplanin while the central ones were negative. Interestingly, positive immunoreactions for podoplanin in OOCs and DCs was found in the basal cell layer only where inflammatory changes were present in the connective tissue. This phenomenon can also be seen in the inflamed gingival tissue.
Our present study demonstrated that the expression of podoplanin was evidently intense in KCOTs than in orthokeratinized odontogenic cysts (OOCs) and Dentigerous cysts (DCs), probably because KCOT has more of a neoplastic character, with progressive behaviour and local invasiveness. Similarly, we have investigated and compared podoplanin expression of KCOT with ameloblastoma and found that this protein was strongly expressed at invasive front area of ameloblastoma same as that of KCOT. Ameloblastoma is a benign odontogenic tumor showing local invasion and recurrence.
It was reported in the literature that podoplanin is expressed in limited myoepithelial elements of pleomorphic adenoma. [24] Although pleomorphic adenoma is a common benign epithelial tumor of the salivary glands, it has tendency to invade surrounding tissue. Similarly, AM is also a benign odontogenic tumor but shows local invasion and recurrence.
The tumoral invasion relies on intense active remodeling of the cellular actin cytoskeleton. Moreover, podoplanin is expressed in podocytes and myofibroblasts, both cell types with a high amount of cytoskeletal remodeling. [26,27] Adding to these findings with increased membranous podoplanin expression at the invasive edge (in the peripheral cells) of KCOT and ameloblastomas, it is possible to speculate that this protein might be associated with the remodeling of the cytoskeleton of neoplastic cells responsible of causing local invasion and recurrance.
Podoplanin might favor tumor invasion through its ability to remodel actin in the cytoskeleton of tumor cells, contributing to their increased motility; this association, the podoplanin–cytoskeleton, seems to be mediated by ezrin, which is markedly phosphorylated in the presence of Podoplanin overexpression. [27,28] In addition, human podoplanin expressed in immortal HaCaT keratinocytes fails to trigger a complete EMT but induces scattering associated with increased plasma membrane motility and reduced cell–cell cohesion. [23]
Conclusion
The podoplanin expression pattern was detected and compared between tumor (ameloblastoma) and developmental cysts by Immunohistochemistry, which showed that podoplanin is strongly expressed at the invasive edge of ameloblastoma and KCOT but it is negative in other developmental cysts like Orthokeratinized odontogenic cyst and Dentigerous cysts unless they are associated with inflammation. This pattern of expression suggest that podoplanin expression is required during processes demanding high cellular activities such as proliferation and differentiation and this protein seems to participate on the process of local invasion of neoplasias like ameloblastoma and KCOT probably by orchestrating the cytoskeleton movement. Thus, our results suggest the possible contribution of podoplanin in the local invasiveness and the neoplastic nature of KCOT.
References
- Philipsen HP., et al. “Odontogenic tumours. In: Barnes L, Evenson JW, Reichart PA, Sidransky D, eds. WHO classification of tumours. Pathology and genetics. Head and neck tumours. Lyon: IARC Press, 2005; 283–327.
- Kumamoto H. “Molecular pathology of odontogenic tumors”. Journal of Oral Pathology & Medicine 35.2 (2006): 65-74.
- Heikinheimo K., et al. “Gene expression profiling of ameloblastoma and human tooth germ by means of a cDNA microarray”. Journal of Dental Research 81.8 (2002): 525-530.
- Philipsen HP. “Om keratocystedr (Kolesteratomer) and kaeberne”. Tandlaegebladet 60 (1956): 963-971.
- Schacht V., et al. “T1alpha podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema”. The EMBO Journal 22.14 (2003): 3546-3556.
- Schacht V., et al. “Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors”. American Journal of Pathology 166.3 (2005): 913-921.
- Yuan P., et al. “Overexpression of podoplanin in oral cancer and its association with poor clinical outcome”. Cancer 107.3 (2006): 563-569.
- Gonza´ lez-Alva P., et al. “Enhanced expression of podoplanin in ameloblastomas”. Journal of Oral Pathology & Medicine 39.1 (2009): 103–109.
- Gonza´ lez-Alva P., et al. “Podoplanin expression in odontomas: clinicopathological study and immunohistochemical analysis of 86 cases”. Journal of Oral Science 53.1 (2011): 67-75.
- Ito T., et al. “Low podoplanin expression of tumor cells predicts poor prognosis in pathological stage IB squamous cell carcinoma of the lung, tissue microarray analysis of 136 patients using 24 antibodies”.Lung cancer 63.3 (2009): 418–424.
- Okamoto E., et al. “Significance of podoplanin expression in keratocystic odontogenic tumor”. Journal of Oral Pathology & Medicine 39.1 (2009): 110-114.
- Vormittag L., et al. “Co-expression of Bmi-1 and podoplanin predicts overall survival in patients with squamous cell carcinoma of the head and neck treated with radio (chemo) therapy”. International Journal of Radiation Oncology Biology Physics 73.3 (2009): 913-918.
- Kreppel M., et al. “Impact of podoplanin expression in oral squamous cell carcinoma: clinical and histopathologic correlations”. Virchows Archiv 456.5 (2010): 473-482.
- Zustin J., et al. “Podoplanin expression in human tooth germ tissues and cystic odontogenic lesions: an immunohistochemical study”. Journal of Oral Pathology & Medicine 39.1 (2010): 115-120.
- Friedrich RE and Zustin J. “Calcifying epithelial odontogenic tumour of the maxilla: a case report with respect to immunohistochemical findings”. In Vivo 25.2 (2011): 259-264.
- Wicki A and Christofori G. “The potential role of podoplanin in tumour invasion”. British Journal of Cancer 96.1 (2007): 1-5.
- Sawa Y., et al. “Expression of podoplanin in the mouse tooth germ and apical bud cells”. Acta histochemica et cytochemica 4.5 (2008): 121-126.
- Etemad-Moghadam S., et al. “Evaluation of myofibroblasts in oral epithelial dysplasia and squamous cell carcinoma”. Journal of Oral Pathology & Medicine 38.8 (2009): 625-643.
- Tsuneki M., et al. “Podoplanin expression profiles characteristic of odontogenic tumor-specific tissue architectures”. Pathology Research and Practice 208.3 (2012): 140-146.
- Tjioe KC., et al. “Is podoplanin expression associated with the proliferative activity of ameloblastomas?” Oral Diseases 18.7 (2012): 673-679.
- Kono T., et al. “Immunohistochemical detection of the lymphatic marker podoplanin in diverse types of human cancer cells using a novel antibody”. International Journal of Oncology 31.3 (2007): 501-518.
- Kawaguchi H., et al. “Podoplanin: a novel marker for oral cancer risk in patients with oral premalignancy”. Journal of Clinical Oncology 26.3 (2008): 354–360.
- Wicki A., et al. “Tumor invasion in the absence of epithelial–mesenchymal transition: podoplanin- mediated remodeling of the actin cytoskeleton”. Cancer Cell 9.4 (2006): 261-272.
- Oku Y., et al. Podoplanin expression in human pleomorphic adenomas. In: Varma AK, Qiu WL, Zhang CP, Zhang ZY, eds. Oral oncology, Vol. 12. Shanghai: Ocean Papers & Printers, 2008; 251–3.
- Hata M., et al. “Expression of podoplanin in the mouse salivary glands”. Archives of Oral Biology 53.9 (2008): 835-841.
- Wetterwald A., et al. “Characterization and cloning of the E11 antigen, a marker expressed by rat osteoblasts and osteocytes”. Bone 18.2 (1996): 125-132.
- Martı´n-Villar E., et al. “Characterization of human PA2.26 antigen (T1alpha-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas”. International Journal of Cancer 113.6 (2005): 899-910.
- Martı´n-Villar E., et al. “Podoplanin binds ERM proteins to activate RhoA and promote epithelial–mesenchymal transition”. Journal of Cell Science 119.21 (2006): 4541-4553.
Citation:
Pratima A.Dongre., et al. “Role of Podoplanin in Analyzing Neoplastic Potential of Keratocystic Odontogenic Tumor- A Retrospective Immunohistochemical Study”. Oral Health and Dentistry 1.1 (2016): 56-66.
Copyright: © 2016 Pratima A.Dongre., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.