Review Article
Volume 2 Issue 6 - 2018
Health Aspects of Nutritional Fats and Oils. A Review of Recent Findings
University of Basel, and Endocrine Practice, Missionsstr. 24 CH-4055 Basel, Switzerland
*Corresponding Author: Ulrich Keller, University of Basel, and Endocrine Practice, Missionsstr. 24, Switzerland.
Received: April 05, 2018; Published: April 17, 2018
Abstract
Current guidelines of professional organizations and governmental health authorities recommend to limit the consumption of saturated fats for the prevention of chronic diseases such as cardiovascular disease, and to propose upper limits for the intake of mono- and polyunsaturated fatty acids.
The present review of meta-analyses of prospective cohort studies and of randomized controlled trials published during the past 5 years indicates that these recommendations may need certain revisions. Regarding cardiovascular disease, current data suggest that intake of total and saturated fat (in % of energy intake) is not clearly associated with cardiovascular morbidity and mortality. A small benefit regarding cardiovascular risk results from reduction of saturated fat when it is replaced by polyunsaturated fat. This benefit was not observed in patients with established CVD.
According to recent data consumption of n-6 PUFA acid has been associated with diminished (and not with increased) cardiovascular morbidity and mortality, and there is insufficient evidence to prioritize a specific type of unsaturated fats replacing other macronutrients such as saturated fats or starchy or sugary foods.
Concerning risk of diabetes type 2, recent data show that total fat or saturated fat intake are not clearly associated with increased risk. In contrast, increased consumption of MUFA, olive oil and in some instances of PUFA have been associated with diminished diabetes risk and with improved metabolic control in patients with established diabetes when carbohydrates were replaced by MUFA.
Regarding overweight and obesity, lowering the proportion of fat in the diet resulted in a small decrease of body weight. Therefore, these new epidemiological trial data suggest that there is insufficient evidence to recommend limited consumption of the various types of fats and oils to improve health outcomes.
Keywords: Dietary fats and oils; Cardiovascular disease; Diabetes type 2; Cancer risk; Public health recommendations
Abbreviations: CHD: Coronary Heart Disease; CVD: Cardiovascular Disease; EPA: Eisosapentaenoic Acid; DHA: Docosahexaenoic Acid; DM: diabetes mellitus; PC: Prospective cohorts; RCTs: Randomised Controlled Trials; RR: Relative Risk; MUFA: Monounsaturated Fatty Acids; PUFA: Polyunsaturated Fatty Acids; SFA: Saturated Fatty Acids
Introduction
Dietary fats and oils cover approximately one third of total energy requirements and are the most energy-dense nutrients. As nutrition-related diseases such as obesity, cardiovascular diseases and diabetes type 2 are becoming more and more prevalent; the role of fats and oils in their development and prevention is of particular interest. Not only the quantity but particularly the quality of fats and their fatty acid content is of great interest, specifically in relation to atherosclerotic diseases.
Guidelines such as the AHA/American College of Cardiology Guideline on Lifestyle Management to Reduce Cardiovascular Risk (2013) [1], the WHO and the 2010 Dietary Guidelines for Americans [2] recommend to limit consumption of saturated fats to less than 10% of energy consumption.
In addition, several governmental health recommendations such as the WHO/FAO Expert Consultation stated that a maximum of 10% of energy should be consumed as n–6 PUFA for CHD risk reduction. The Swiss Federal Commission on Nutrition has issued recommendations on the consumption of fats and oils for the public in 1992, 2006 [3] and 2012 [4]. These recommendations proposed a limitation of fat intake, both of the saturated and the unsaturated varieties.
In the meantime, results of several large epidemiological studies reporting associations between fat consumption and health outcomes have been published. The present article reviews and summarizes meta-analyses of epidemiological studies published in international journals related to the subject during the last decade, with the goal to propose possible revisions of the current recommendations.
Definition and scope
Fats are present in visible form or they are contained in food products with a mixture of nutrients (“hidden fat”). Solid fats are mostly of animal origin, and oils are usually of plant origin. Dietary fats and oils contain fatty acids of various chain lengths and degrees of saturation which exert different effects on the risk of non-communicable diseases. Since most fats and oils contain mixtures of fatty acids, and certain fatty acids are often present in both animal and plant-based sources, they are discussed as a whole group. The main focus of this review is to summarize associations between consumption of certain fats and oils (defined by their fatty acid composition) and health outcomes according to epidemiological studies. Specific foodstuff with mixtures of nutrients including fat (e.g. dairy, processed meat) is not discussed.
Fats are present in visible form or they are contained in food products with a mixture of nutrients (“hidden fat”). Solid fats are mostly of animal origin, and oils are usually of plant origin. Dietary fats and oils contain fatty acids of various chain lengths and degrees of saturation which exert different effects on the risk of non-communicable diseases. Since most fats and oils contain mixtures of fatty acids, and certain fatty acids are often present in both animal and plant-based sources, they are discussed as a whole group. The main focus of this review is to summarize associations between consumption of certain fats and oils (defined by their fatty acid composition) and health outcomes according to epidemiological studies. Specific foodstuff with mixtures of nutrients including fat (e.g. dairy, processed meat) is not discussed.
Research of the literature and grading of evidence
The literature search focused on meta-analyses quoted in PubMed during the past 5 years (2012-2017). Key words used were dietary fats, fatty acids, oils, and health outcomes, cardiovascular disease, coronary heart disease, obesity, diabetes type 2, mortality, blood lipids, cancer, depression, cognitive impairment. A total of 122 meta-analyses were retrieved. Only original publications dealing with adults, healthy individuals were considered; studies with focus on biomarkers were excluded. Tables of individual meta-analyses of cohort studies and randomized controlled studies including their main features and conclusions was prepared.
The literature search focused on meta-analyses quoted in PubMed during the past 5 years (2012-2017). Key words used were dietary fats, fatty acids, oils, and health outcomes, cardiovascular disease, coronary heart disease, obesity, diabetes type 2, mortality, blood lipids, cancer, depression, cognitive impairment. A total of 122 meta-analyses were retrieved. Only original publications dealing with adults, healthy individuals were considered; studies with focus on biomarkers were excluded. Tables of individual meta-analyses of cohort studies and randomized controlled studies including their main features and conclusions was prepared.
Classification of levels of evidence (LOE, according to WHO [5] was the following:
LOE I: Ia Meta-analyses of randomised controlled intervention studies;
Ib randomised controlled intervention studies
LOE II: IIa Meta-analyses of cohort studies;
IIb cohort studies
LOE I: Ia Meta-analyses of randomised controlled intervention studies;
Ib randomised controlled intervention studies
LOE II: IIa Meta-analyses of cohort studies;
IIb cohort studies
A few selected epidemiological studies which appeared to be of importance in relation to the subject of this publication were quoted.
Mechanisms
Dietary fats are more energy-dense than other energy providing foods. The different fatty acids in fats and oils determine their physical (melting point or fluidity of cell membranes) and chemical (e.g. process of chemical reactions) behaviour and their biological functions. They exert different effects on plasma lipoprotein concentrations and are precursors of eicosanoids as metabolites of n-3 and n-6 fatty acids. Dietary fats are also sources of fat-soluble vitamins and of flavouring agents. Vegetable oils such as extra virgin olive oil contain also phenolic compounds which may exert anti-inflammatory properties [6].
Dietary fats are more energy-dense than other energy providing foods. The different fatty acids in fats and oils determine their physical (melting point or fluidity of cell membranes) and chemical (e.g. process of chemical reactions) behaviour and their biological functions. They exert different effects on plasma lipoprotein concentrations and are precursors of eicosanoids as metabolites of n-3 and n-6 fatty acids. Dietary fats are also sources of fat-soluble vitamins and of flavouring agents. Vegetable oils such as extra virgin olive oil contain also phenolic compounds which may exert anti-inflammatory properties [6].
Survey of publications
Basic considerations
There has been a tendency during the past years in international nutritional recommendations [7,8] to recommend consumption of certain foods or food groups rather than quantities of specific nutrients such as fats or carbohydrates. Reason for this is that health effects of certain nutrients depend on the type of food in which they are consumed, due to the texture of the food and to other food components [9-11]. Examples: Identical quantities of SFA in the form of butter or cheese may have slightly different effects on serum lipids [12]. In addition, fermented dairy products have different health effects compared to unfermented products [13] even with identical content of macronutrients.
Basic considerations
There has been a tendency during the past years in international nutritional recommendations [7,8] to recommend consumption of certain foods or food groups rather than quantities of specific nutrients such as fats or carbohydrates. Reason for this is that health effects of certain nutrients depend on the type of food in which they are consumed, due to the texture of the food and to other food components [9-11]. Examples: Identical quantities of SFA in the form of butter or cheese may have slightly different effects on serum lipids [12]. In addition, fermented dairy products have different health effects compared to unfermented products [13] even with identical content of macronutrients.
Nevertheless, the present survey focusses on fats and oils as they are defined by their biochemical composition. The reason for this is the fact that the currently available epidemiological literature was largely based on this aspect.
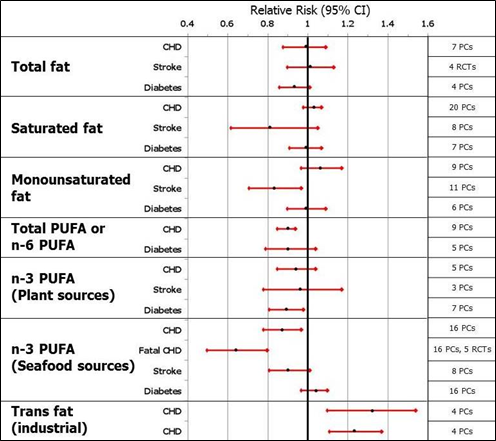
Summary of a publication of meta-analyses of the relationship between dietary fats and oils and coronary heart disease, stroke, and diabetes mellitus published between 2006 and 2014 [14]
Figure 1: Shows the relative risks with 95% confidence intervals for major health outcomes
during high versus low consumption of specific fats (redrawn from Figure 7 in [14].
Conclusions from this review of meta-analyses: Total fat and saturated fat is not clearly associated with CHD, stroke and diabetes, Monounsaturated fats appear to be protective for stroke. Total or n-6 PUFA are associated with diminished CHD events. Plant-based n-3 PUFA are shown to be protective for diabetes type 2, however, inspection of reference Wu J., et al. (2012) [15] shows that the decrease of diabetes risk was not statistically significant. Seafood-derived n-3 PUFA are associated with diminished CHD risk, and they diminish CHD death. The most striking association is the association of industrial trans fats with CHD risk.
| Source | Study category | Disease | End point | Main nutritional theme | No. of included studies | No. of subjects | Subject group | Duration | RR (95%CI) | Limitations | Conclusions | LOE |
| Harcombe Z, 2017 [20] | Meta-analysis of PCs | CHD | Mortality | Total fat and SFA intake | 6 PCs | 89'801 | Adults without CHD | 6-20 yrs. | The RR 1.04 (0.98-1.1) for total fat, and 1.08 (0.94-1.25) for SFA | Lack of generalisability, dietary recalls are unreliable | Epidemiological evidence to date found no significant association between CHD mortality and total fat or saturated fat intake | II a |
| Micha R 2017 (PLoS1) [21] | Meta-analysis and systematic review of meta-analyses of PCs & RCTs | CVD & diabetes | Disease risk | 10 foods & 7 nutrients (including PUFA & trans) | 23 meta-analyses | 140'000-820'000 | Adults | Not stated | Refers to individual meta-analyses | Possible bias by clustering of dietary patterns which could still cause unmeasured confounding, e.g., from clustering of healthful factors. | There was evidence for protective cardiometabolic effects of seafood omega-3s, polyunsaturated fats, and adverse effects of trans-fats. Optimal mean population intake of PUFA replacing SAFA or CHO: 11% E [of 2000 kcal] | I a & II a |
| Micha R 2017 (JAMA) [22] | Data from NHANES & meta-analyses of PCs & RCTs | CVD & diabetes | Mortality | 10 dietary factors (including PUFA & seafood omega-3 fats) | not stated | not stated | Adults | years | CHD: PUFAs,% energy replacing carbohydrates or saturated fats per 5% energy/d (age 50): RR 0.88 (0.83-0.94); Seafood omega 3 per 100 mg/d: RR0.82 (0.075-0.90) | Dietary habits were based on self-reported 24-hour recalls, which have known measure-ment errors for individual people | Most cardiometabolic deaths in USA were estimated to be related to excess sodium intake, insufficient intake of nuts/seeds, high intake of processed meats, and low intake of seafood omega-3 fats | I a & II a |
| Alexander D. et al. 2017 [23] |
Meta-analysis of PCs & RCTs | CHD | Risk & mortality | EPA &DHA from foods or supplements | 18 RCTs & 16 PCs | 93,000 (RCT trials) & 732,000 in PC studies | Adults with and without CHD | 5-40 yrs. | Among RCTs, risk reduction (CHD) with EPA&DHA (SRRE=0.94; 95% CI, 0.85-1.05) was n.s. Subgroup analyses indicated a significant CHD risk reduction with EPA&DHA in higher-risk populations (e.g. with elevated TG levels (SRRE=0.84; 95% CI, 0.72-0.98) and elevated LDL-c (SRRE=0.86; 95% CI, 0.76-0.98). Meta-analysis of PCs resulted in a significant SRRE of 0.82 (95% CI, 0.74-0.92) for higher intakes of EPA&DHA | Large heterogeneity of studies | EPA&DHA may be associated with reducing CHD risk, with a greater benefit observed among higher-risk populations in RCTs | I a & II a |
| Pimpin 2016 [24] | Meta-analysis of PCs | CVD, Mortality | Risk & Mortality | Butter | 15 PCs | 636'151 | Adults | 10-22 yrs. | Butter consumption (14 g/d) was weakly associated with mortality; RR = 1.01, 95%CI = 1.00, 1.03, P =0.045) but not with any CVD( RR = 1.00,95%CI = 0.98, 1.02; P = 0.704), CHD (RR = 0.99, 95%CI = 0.96,1.03; P = 0.537), or stroke (N = 3; RR = 1.01, 95%CI = 0.98, 1.03; P = 0.737) | No evidence for heterogeneity nor publication bias | There were relatively small or neutral overall associations of butter with mortality & CVD | II a |
| de Souza RJ, 2015 [25] | Meta-analysis of PCs | CVD, stroke, diabetes | Risk & mortality | SFA & trans fats (industrial & ruminant) | 12 PCs | 90'500-339'000 | Adults | Not stated | RR SFA 0.99 (0.91-1.09) for total mortality, 0.95 (0.88-1.03) for CVD mortality, 1.02 (0.9-1.15) for stroke, 0.95 (0.88-1.03) for DM. Industrial, but not ruminant, trans fats were associated with CHD mortality (1.18 (1.04 to 1.33) v 1.01 (0.71 to 1.43)) and CHD (1.42 (1.05 to 1.92) v 0.93 (0.73 to 1.18)) | Evidence is heterogeneous; methodological limitations | SFA are not associated with all-cause mortality, CVD, CHD, ischemic stroke, or type 2 diabetes, but the evidence is heterogeneous with metho-dological limitations. Trans fats are associated with all-cause mortality, total CHD, and CHD mortality, probably because of higher levels of intake of industrial than ruminant trans fat | II a |
| Hooper L. 2015 (Cochrane) [26] | Meta-analysis of RCTs | CVD | Morbidity, mortality | Replacing SFA with CHO, PUFA or other nutrients | 15 RCTs | 59'000 | Adults | >2 yrs. | Reducing dietary saturated fat reduced the risk of cardiovascular events by 17% (risk ratio (RR) 0.83; 95% confidence interval (CI) 0.72 to 0.96, mainly when saturated fat calories replaced polyunsaturated fat | The studies provide mode-rate-quality evi-dence that redu-cing SFA and replacing it with PUFA reduces our risk of CVD | A small but potentially important reduction in cardiovascular risk on reduction of saturated fat intake is observed when replacing SFA with PUFA | I a |
| Farvid M.S. 2014 [27] | Meta-analysis of PCs | CHD | Risk & death | Dietary linoleic acid | 13 PCs | 310'602 | Adults | 5.3-30 yrs. | Highest vs lowest category of LA intake resulted in a 15% lower risk of CHD events (pooled RR, 0.85; 95% CI 0.78–0.92; I2=35.5%), and a 21% lower risk of CHD deaths (pooled RR, 0.79; 95% CI 0.71–0.89; I2=0.0%). A 5% of energy increment in LA intake replacing SFA was associated with a 9% lower risk of CHD events (RR, 0.91; 95% CI, 0.87–0.96) and a 13% lower risk of CHD deaths (RR, 0.87; 95% CI, 0.82–0.94) | No evidence of publication bias for either CHD events or death. | In prospective observational studies, dietary LA intake is inversely associated with CHD risk in a dose– response manner. These data provide support for current recommendations to replace saturated fat with polyunsaturated fat for primary prevention of CHD | II a |
| Wen YT, 2014 [28] | Meta-analysis of RCTs | CV events & mortality | CV events & mortality | Omega 3 PUFA supplements | 14 RCTs | 16'338 | Patients with CHD | 3 mo.- 4.6 yrs. | Omega-3 PUFAs did not demonstrate satisfactory improvements of major cardiovascular events (OR, 0.93; 95% CI, 0.86 to 1.01; P Z 0.08; I2 Z 46%). By contrast, omega3 PUFAs reduced risks of death from cardiac causes and death from all causes (OR, 0.88; 95% CI, 0.80 to 0.96; P= 0.003; OR, 0.86; 95% CI, 0.76 to 0.98; P= 0.03; and OR, 0.92; 95% CI, 0.85 to 0.99; P= 0.02) | No evidence of publication bias for either CHD events or death | Supplement of Omega-3 PUFAs in patients with CHD does not prevent major cardiovascular events, but reduces death from cardiac causes and death from all causes. Whether dietary supplementation with Omega-3 PUFAs should be still considered in patients with CHD is currently debated | I a |
| Schwings-hackl L, 2014 [BMJ open] [29] | Meta-analysis of RCTs | CHD | Risk & death | Fat reduction; replacing SFA with PUFA or other nutrients | 12 RCTs | 7'150 | Patients with CHD | 1-6 yrs. | When comparing modified fat diets versus control diets no significant risk reduction could be observed considering all-cause mortality (RR 0.92, p=0.60; I2=59%) and cardiovascular mortality (RR 0.96, p=0.84; I2=69%), combined cardiovascular events (RR 0.85, p=0.30; I2=75%) and myocardial infarction (RR 0.76, p=0.13; I2=55%). Sensitivity analyses did not reveal a significant risk reduction for any outcome parameter when polyunsaturated fat was increased in exchange for saturated fat | Some studies were >50 yrs. old. Substantial heterogeneity for several outcomes | Recommending higher intakes of PUFA in replacement of SFA was not associated with risk reduction in patients with CHD | I a |
| Chowdhury R, 2014 [30] | Systematic review & meta-analysis of observational studies & of RCTs | CHD | Risk | Dietary & circulating fatty acids | 32 observational studies, 27 RCTs | up to 512‘000 | Adults, with and without CHD | 5-23 yrs. in PCs, 1-8 yrs. in RCTs | In observational studies, relative risks for CHD were 1.03 (95% CI, 0.98 to 1.07) for SFA, 1.00 (CI, 0.91 to 1.10) for MUFA, 0.87 (CI, 0.78 to 0.97) for LC n-3 PUFA, 0.98 (CI, 0.90 to 1.06) for n-6 PUFA, and 1.16 (CI, 1.06 to 1.27) for trans fatty acids when the top and bottom thirds of baseline dietary fatty acid intake were compared. In RCTs, relative risks for CHD were 0.97 (CI, 0.69 to 1.36) for ALA, 0.94 (CI, 0.86 to 1.03) for LC n-3 PUFA, and 0.86 (CI, 0.69 to 1.07) for n-6 PUFA supplementations | Potential biases from preferential publication and selective reporting | Current evidence from RCTs does not clearly support cardiovascular guidelines that encourage high consumption of polyunsaturated fatty acids and low consumption of total saturated fats | I a & II a |
| de Goede J, 2013 [31] | Meta-Analysis of 2 cohort studies | CHD | Mortality | Associations with plasma fatty acid cholesteryl esters | 2 observational cohorts | 558 | Dutch adults | 8-19 yrs. | After adjustment for confounders, the OR (95%CI) for fatal CHD per SD increase in plasma linoleic acid was 0.89 (0.74–1.06). The ORs (95%CI) for fatal CHD for an SD increase in n-3 PUFA were 0.92 (0.74–1.15) for alpha-linolenic acid and 1.06 (0.88–1.27) for EPA-DHA. In the meta-analysis, a 5% higher linoleic acid level was associated with a 9% lower risk (relative risk: 0.91; 95% CI: 0.84–0.98) of CHD | Blood samples were stored >10 yrs. Data of plasma n-3 FA esters were possibly unreliable | Linoleic acid in plasma cholesteryl is inversely associated with CHD. There was no such relation with n-3 PUFA cholesteryl esters | II a |
| Ramsden CE, 2013 [32] | RCT (Sydney Diet Heart Study) & meta-Analysis of RCTs | CHD | Mortality | Dietary linoleic acid (LA) | 1 (+2+4) RCTs | 458 | Men with recent CHD | 12 mo. | Replacement of dietary SFA with omega 6 LA (intervention) had higher rates of death than controls (all cause 17.6% v 11.8%, HR 1.62 (95% CI 1.00 to 2.64), P=0.05; CVD 17.2% v 11.0%, 1.70 (1.03 to 2.80), P=0.04; CHD 6.3% v 10.1%, 1.74 (1.04 to 2.92), P=0.04) | Results of borderline significance. Small trial | -Linoleic acid intervention trials showed no evidence of cardiovascular benefit | I a |
| Pan A, 2012 [33] | Meta-analysis of cohorts | CVD | Risk | Dietary -linolenic acid (ALA) | 27 cohorts (pro-& retrospective) | 251'049 | Adults | 5- 33.7 yrs. | The overall pooled RR was 0.86 (95% CI: 0.77, 0.97; I2 = 71.3%). The association was n.s. with biomarkers of ALA | High unexplained heterogeneity | Higher ALA exposure is associated with a moderately lower risk of CVD. The results were generally consistent for dietary studies but were not statistically significant for biomarker studies | II a |
| Kotwal S, 2012 [34] | Meta-analysis of RCTs | CVD | Risk & death | Omega 3 PUFA supplements (fish oil) or intervention | 20 RCTs | >60'000 | Mostly patients with CHD | 0.6-7 yrs. | There was no overall effect of ω-3 FA on composite cardiovascular events (RR=0.96; 95% CI, 0.90–1.03; P=0.24) or on total mortality (RR=0.95; 95% CI, 0.86–1.04; P=0.28). ω-3 FA did protect against vascular death (RR=0.86; 95% CI, 0.75–0.99; P=0.03) but not coronary events (RR=0.86; 95% CI, 0.67–1.11; P=0.24) | Significant heterogeneity between the trials | Omega 3 fatty acids did not protect against composite cardiovascular events but showed some protection against CV death. There is no clear effect on total mortality, sudden death, stroke, or arrhythmia. The beneficial effects of omega 3 fatty acids are not as large as previously implied | I a |
| Hooper L. 2012 (Cochrane) [35] | Meta-analysis of RCTs | CVD | Risk & death | Fat intake, replacement of fat with other macronutrients | 48 RCTs | >80'000 | Adults, with and without CHD | >6 mo. | Reducing SFA by reducing and/or modifying dietary fat reduced the risk of CV events by 14% (RR 0.86, 95%CI 0.77 to 0.96, 24 comparisons, 65'508 participants of whom 7% had a cardiovascular event).Subgrouping suggested that this reduction was observed only in studies of at least two years duration and in men (not of women). Dietary fat reduction/modification had no effect on total and on CV mortality | Uncertainty over allocation concealment, lack of blinding and presence of systematic differences- but scale and consistency of evidence makes findings relatively robust | Modifying fat in our food (replacing some SFA with plant oils and unsaturated spreads) may reduce risk of heart and vascular disease, but it is not clear whether MUFA or PUFA are more beneficial. There were no clear effects of dietary fat changes on total and cardiovascular mortality | I a |
| Schwings-hackl L, 2014 [Lipids Health Dis] [36] | Meta-analysis of PCs | CVD & stroke | CV events & mortality, stroke risk | Monounsaturated fatty acids, olive oil | 32 PCs | 841'211 | Adults, most of them without CVD at baseline | 4.6- 30 yrs. | The comparison of the top versus bottom third of the distribution of a combination of MUFA (of both plant and animal origin) showed reduced all-cause mortality (RR: 0.89, 95% CI 0.83, 0.96, p = 0.001; I2 = 64%), CV mortality (RR: 0.88, 95% CI 0.80,0.96, p = 0.004; I2 = 50%), CV events (RR: 0.91, 95% CI 0.86, 0.96, p = 0.001; I2 = 58%), and stroke (RR: 0.83,95% CI 0.71, 0.97, p = 0.02). | Potential public-cation bias for combined CV events (p = 0.018) & total mortality (p = 0.041). No evidence of publication bias for risk of CHD (p = 0.28) and stroke (p = 0.28) | There was an overall risk reduction of stroke (17%) when comparing the top versus bottom third of MUFA, olive oil, oleic acid, and MUFA: SFA ratio. Only olive oil seems to be associated with reduced risk | II a |
| Cheng P, 2016 [37] | Meta-analysis of cohorts | Stroke | Risk & death | SFA | 15 PCs | 476'569 | Adults | 7.6- 18 yrs. | Higher SFA intake was associated with reduced stroke risks for East-Asians [RR = 0.79 (95 % CI 0.69–0.90)], for dose <25 g/day [RR = 0.81 (95 % CI 0.71–0.92)], for males [RR = 0.85 (95 % CI 0.75–0.96)], and for individuals with body mass index (BMI) <24 [RR = 0.75 (95 % CI 0.65–0.87)], but not for non-East- Asians, females, and individuals with dose >25 g/day and BMI >24 | Possible threshold effect of SFA consumption | Higher consumption of SFA was associated with decreased stroke risk (morbidity, mortality) in certain groups of subjects (not in Non-East-Asians) | II a |
| Cheng P 2015 [38] | Meta-analysis of cohorts | Stroke | Risk & death | Long-chain n-3 PUFA | 14 PCs | 514'483 | Adults | 4-21.2 yrs. | Higher long chain n-3 PUFA intake was associated with reduced overall stroke risk [relative risk (RR) = 0.87; 95% confidence interval (CI), 0.79–0.95 | Significant heterogeneity between the trials | Higher long chain n-3 PUFA intake is inversely associated with risk of stroke morbidity and mortality | II a |
| Martínez-González MA 2014 [39] | Meta-analysis of cohorts; 1 RCT | Stroke | Risk | Olive Oil consumption | 2 PCs, 1 RCT | Ca. 40’000 | Adults | years | The combined RR of stroke for an increment of 25 g olive oil consumed per d was 0·76 (95% CI 0·67, 0·86; P,0·001), with a negligible change after including the PREDIMED trial | Relatively few trials | Higher olive oil intake is inversely associated with risk of stroke incidence | I a & II b |
| Larssen SC 2012 [40] | Meta-analysis of PCs | Stroke | Risk | Long-chain n-3 PUFA | 8 PCs | 242'076 | Adults | 4-28 yrs. | The combined RR of total stroke was 0.90 (95 % CI, 0.81–1.01) for the highest versus lowest category of long-chain omega-3 PUFA intake, without heterogeneity among studies (P = 0.32) | No association between stroke risk & n-3 PUFA intake | II a | |
| Chowdhury R, 2012 [41] | Meta-analysis of PC & RCTs | Stroke (cerebro-vascular disease) | Risk & mortality | Long-chain n-3 PUFA | 26 PC2 & 12 RCTs | 794'000 | Adult with & without CVD | 3- 15.1 yrs. | The RR for cerebrovascular disease comparing the top thirds of baseline LC omega 3 fatty acids with the bottom thirds for circulating biomarkers was 1.04 (0.90 to 1.20) and for dietary exposures was 0.90 (0.80 to 1.01). In the RCTs the RR for cerebrovascular disease in the LC omega 3 supplement compared with the control group in primary prevention trials was 0.98 (0.89 to 1.08) and in secondary prevention trials 1.17 (0.99 to 1.38) | There were moderate, inverse associations of fish consump-tion and LC omega 3 fatty acids with cerebrovascular risk. LC omega 3 fatty acids in RCTs with supplements had no significant effect | I a & II a |
Table 1: Chemical composition (g/100 g) of starches from 8 dry legume seeds.
These studies demonstrate that consumption of total fat and saturated fat (in % of energy intake) is not clearly associated with cardiovascular morbidity and mortality.
A small but potentially important benefit regarding cardiovascular risk results from reduction of saturated fat when it is replaced by polyunsaturated fat. This benefit has not been observed in patients with established CVD. Consumption of the PUFA linoleic acid has been associated with diminished cardiovascular morbidity and mortality; however, there is insufficient evidence to prioritize a specific type of unsaturated fat replacing saturated fats. Seafood-derived PUFA (n-3) supplements have been shown to diminish cardiovascular and total mortality in cardiovascular high risk patients. Consumption of industrial trans fatty acids has been associated with increased cardiovascular morbidity and mortality and total mortality. Regarding stroke risk, higher consumption of MUFA (particularly olive oil) has been associated with diminished risk. There is evidence from cohort studies that consumption of long-chain n-3 PUFAs is associated with diminished stroke risk, however, RCTs with long-chain n-3 PUFAs are inconclusive.
| Source | Study category | Disease | End point | Main nutritional theme | No. of included studies |
No. of subjects | Subject group | Duration | RR (95%CI) | Limitations | Conclusions | LOE |
| Jovanovski E 2017 [42] | Systematic review & meta-analysis of RCTs | Diabetes T2 | Glycemic control, insulin sensitivity | -linolenic acid | 8 RCTs | 212 | Adults with DM T2 | 3 months | n.s. for: HbA1c, IR (HOMA), FBG | Considerable unexplained heterogeneity | -linolenic acid-enriched diets did not affect HbA1c, FBG, or FBI. | I a |
| Wu J.H.Y 2017 [43] | Systematic review & meta-analysis of PCs | Diabetes T2 | New diabetes risk | Omega-6 fatty acid biomarkers | 20 PCs | 39'740 | Adults | mean 8 yrs. | Higher proportions of linoleic acid biomarkers as % of total fatty acid were associated with a lower risk of type 2 diabetes [RR per interquintile range 0∙65, 95% CI 0∙60–0∙72, p<0.0001). Levels of arachidonic acid were n.s. | Linoleic acid biomarkers reflect dietary intake but are not identical to dietary intake | Linoleic acid has long-term benefits for the prevention of type 2 DM and that arachidonic acid is not harmful | II a |
| Schwingshackl L 2017 [44] | Systematic review & meta-analysis of PCs | Diabetes T2 | Diabetes T2 risk & glycemic control | Olive oil | 4 PCs, 29 RCTs | 15’784 DM T2 | Adults with and without DM T2 | 5- 22 yrs. for PCs, 2 wks.- 4 yrs. for RCTs | The highest olive oil intake category showed a 16% reduced risk of T2D (RR: 0.84; 95% CI: 0.77, 0.92) compared with the lowest. In T2D patients olive oil supplementation resulted in a significantly more pronounced reduction in HbA1c (MD: − 0.27%; 95% CI: − 0.37, − 0.17) and fasting plasma glucose (MD: − 0.44 mmol/; 95% CI − 0.66, − 0.22) as compared with the control groups | There was evidence for a nonlinear relationship | Olive oil could be beneficial for the prevention and management of T2D | II a |
| Lin N 2016 [45] | Systematic review & meta-analysis of RCTs | Diabetes T2 | CRP, other markers of inflammation | n-3 PUFA, mostly fish oil | 8 RCTs | 955 | Adults with DM T2 | 6- 12 weeks | N-3 PUFAs significantly reduced CRP concentration compared with control [SMD 95 % CI, 1.90 (0.64, 3.16), Z = 2.96, P = 0.003, random effect model | Small trials, short duration | N-3 PUFAs decrease CRP concentration in type-2 DM mellitus | I a |
| Pimpin 2016 [24] | Meta-analysis of PCs | Diabetes | Risk | Butter | 11 PCs | 23‘954 incident DM | Adults | 10- 22 yrs. | Butter consumption (14 g/d) was inversely associated with incidence of diabetes (N = 11; RR = 0.96, 95%CI = 0.93, 0.99;P = 0.021) | No evidence for heterogeneity nor publication bias | There was a relatively small association of butter with diminished risk of DM | II a |
| Qian F 2016 [46] | Systematic review & meta-analysis of RCTs | Diabetes T2 (T2D) |
Glycemic control, blood pressure lipids | MUFA compared to CHO & PUFA | 24 RCTs comparing with CHO, 4 RCTs with PUFA | 1'504 | Adults with DM T2 | 2- 48 weeks | High-MUFA compared to high-CHO diets reduced fasting plasma glucose (WMD -0.57mmol/L [95%CI -0.76,-0.39]), triglycerides (-0.31 mmol/L [-0.44, -0.18]), body weight (-1.56 kg [-2.89,-0.23]), and systolic blood pressure (-2.31 mm Hg), &-increased HDL cholesterol (0.06 mmol/L [0.02, 0.10]). High-MUFA diets compared with high-PUFA diets reduced fasting plasma glucose (-0.87 mmol/L [-1.67, -0.07]) | Low to medium levels of heterogeneity | Evidence that consuming diets high in MUFA can improve metabolic risk factors among patients with T2D | I a |
| Imamura F 2016 [47] | Systematic review & meta-analysis of RCTs | Diabetes T2, metabolic syndrome | Glucose-insulin homeostasis (HOMA model) |
SFA, PUFA, MUFA, and carbohydrate | 102 RCTs | 4'220 | Adults with and without DM T2 | 3- 168 days | Replacing 5% energy from carbohydrate with SFA had no significant effect on fasting glucose; replacing carbohydrate with MUFA lowered HbA1c (-0.09%; -0.12, -0.05; n = 23), 2 h post-challenge insulin (-20.3 pmol/L; -32.2, -8.4; n = 11), and HOMA-IR (-2.4%; -4.6, -0.3; n = 30). Replacing carbohydrate with PUFA significantly lowered HbA1c (-0.11%; -0.17, -0.05) and fasting insulin (-1.6 pmol/L; -2.8, -0.4). Replacing SFA with PUFA significantly lowered glucose, HbA1c, C-peptide, and HOMA | Small number of trials for some out-comes and potential issues of blinding, compliance, generalisability, heterogeneity due to unmea-sured factors, and public-cation bias | In comparison to carbohydrate, SFA, or MUFA, most consistent favourable effects were seen with PUFA, which were linked to improved glycaemia, diminished insulin resistance, and improved insulin secretion capacity | I a |
| Abbott KA 2016 [48] | Systematic review & meta-analysis of RCTs | Diabetes T2, metabolic syndrome | Insulin resistance (IR), in men and women | n-3 PUFA, mostly fish oil | 26 RCTs | 1'848 | Adults with and without DM T2 | 1-6 months | With all studies pooled, there was no effect of n–3 PUFA on IR at the group level (SMD: 0.089; 95% CI: 20.105, 0.283; P = 0.367). In trials of >6 wks., a significant improvement in IR was seen in women (SMD: 20.266; 95% CI: 20.524, 20.007; P =0.045) but not in men (SMD: 0.619; 95% CI: 20.583, 1.820; P = 0.313 | There was significant heterogeneity between groups and a limited number of trials in men and women separately | Improvement of insulin resistance with LC-n-3-PUFA in women but not in men | I a |
| Chen C 2015 [49] | Meta-analysis of RCTs | Diabetes T2 | Glucose control, lipids, BMI | n-3 PUFA, mostly fish oil | 20 RCTs | 1'209 | Adults with DM T2 | mostly <12 weeks | Triglyceride (TG) levels were significantly decreased by 0.24 mmol/L by n-3 PUFAs. No significant change of total cholesterol (TC), HbA1c, fasting plasma glucose, postprandial plasma glucose, BMI or body weight was observed. High ratio of EPA/DHA contributed to a greater decreasing tendency in plasma insulin, HbAc1, TC, TG, and BMI measures, although no statistical significance was identified (except TG). | Relatively small studies | Suggestion that a high EPA/DHA ratio affects glucose control favourably | I a |
| Souza RJ 2015 [25] | Systematic review & meta-analysis of PCs & RCTs | Diabetes T2 | Diabetes T2 risk | SFA & trans fats (industrial & ruminant) | 12 PCs | 90000-339000 | Adults | 1- 32 yrs. | SFA intake was not associated with type 2 diabetes (0.95, 0.88 to 1.03). Ruminant trans-palmitoleic acid was inversely associated with type 2 diabetes (0.58, 0.46 to 0.74) | The evidence is heterogeneous with method-logical limitations | SFA are not asso-ciated with risk of type 2 DM; ruminant trans fats appear to be associated with protection | I a & II a |
| Aronis KN 2012 [50] | Meta-analysis of RCTs | Diabetes T2 | Glucose, insulin & lipids | Trans fats (TFA) | 7 RCTs | 208 | Adults, non-diabetic | 4-16 wks. | Increased TFA intake did not result in significant changes in glucose or insulin concentrations. Increased TFA intake led to a significant increase in total and LDL-cholesterol [ES (95% CI): 0.28 (0.04, 0.51) and 0.36 (0.13, 0.60), respectively] and a significant decrease in HDL-cholesterol concentrations [ES (95% CI): 20.25 (20.48, 20.01) | No publication bias | TFA affect LDL-C & HDL-C but not glucose-insulin homeostasis | I a |
| Zheng J-S, 2012 [51] | Systematic review & meta-analysis of PCs | Diabetes T2 | Relative Risk of diabetes T2 | n-3 PUFA, mostly fish oil, and fish | 24 PCs | >500'000 | Adults | 4-18 yrs. | The RR of T2D for the highest vs lowest categories of total fish, marine n-3 PUFA and alpha-linolenic acid intake was 1.07 (95% CI: 0.91, 1.25), 1.07 (95% CI: 0.95, 1.20) and 0.93 (95% CI: 0.81, 1.07), respectively. For Asian populations the RR (highest vs lowest category) of T2D for fish and marine n-3 PUFA intake was 0.89 (95% CI: 0.81, 0.98) and 0.87 (95% CI: 0.79, 0.96) ; for Western populations the RR was 1.20 (95% CI: 1.01, 1.44) and 1.16 (95% CI: 1.04, 1.28) | Classifications of fish and n-3 PUFA intake amounts were inconsistent;; observational studies could not avoid residual confounders | Marine n-3 PUFA have beneficial effects on the prevention of T2DM in Asian populations | II a |
| Zhou Y, 2012 [52] | Systematic review & meta-analysis of PCs | Diabetes T2 | Relative Risk of diabetes T2 | n-3 PUFA, mostly fish oil, and fish | 13 PCs (mostly Western) | >100'000 | Adults | 6- 15 yrs. | Comparing the highest v. lowest categories, the pooled RR of T2DM for intake of fish and n-3 fatty acid was 1·146 (95% CI 0·975, 1·346) and 1·076 (95% CI 0·955, 1·213), respectively. In the linear dose–response relationship, the pooled RR for an increment of one time (about 105 g)/week of fish intake (four times/month) and of 0·1 g/d of n-3 fatty acid intake was 1·042 (95% CI 1·026, 1·058) and 1·057 (95% CI 1·042, 1·073), respectively | Potential biases and confounders could not be ruled out completely | Both fish oil and other n-3 fatty acids might be weakly positively associated with the T2DM risk (mostly Western populations) | II a |
| Wu J.H.Y 2012 [15] | Systematic review & meta-analysis of PCs | Diabetes T2 | Diabetes T2 incidence | n-3 PUFA, ALA & mostly fish oil | 18 PCs | 540'184 | Adults | 4-17 yrs. | Consumption of fish and/or seafood was not significantly associated with DM (n=13 studies; RR per 100 g/d = 1·12, 95% CI = 0·94, 1·34); nor were consumption of EPA &DHA (n= 16 cohorts; RR per 250 mg/d= 1·04, 95% CI= 0·97, 1·10) nor circulating levels of EPA &DHA biomarkers (n=5 cohorts; RR per 3% of total fatty acids = 0·94, 95% CI= 0·75, 1·17). Both dietary ALA (n=7 studies; RR per 0·5 g/d = 0·93, 95% CI = 0·83, 1·04) and circulating ALA biomarker levels (n=6 studies; RR per 0·1% of total fatty acid = 0·90, 95% CI = 0·80, 1·00, P=0·06) were associated with non-significant trend towards lower risk of DM | No publication bias, but substantial heterogeneity between fish oil studies | The findings do not support either major harms or benefits of fish/seafood or EPA&DHA on development of DM. ALA consumption showed a n.s. trend towards diminished risk. |
II a |
| Wallin A 2012 [53] | Systematic review & meta-analysis of PCs | Diabetes T2 | Diabetes T2 incidence | n-3 PUFA, mostly fish oil, and fish | 16 PCs | 527'441 | Adults | 6- 19 yrs. | For each serving per week increment in fish consumption, the RRs (95% CIs) of type 2 diabetes were 1.05 (1.02–1.09), 1.03 (0.96–1.11), and 0.98 (0.97– 1.00) combining U.S., European, and Asian/Australian studies, respectively | Heterogeneous results due to geographical differences | There were differen-ces of risk of DM between geographi-cal regions with observed associati-ons of fish consum- ption and dietary intake of LC n-3 FA. | II a |
| Alhazmi A 2012 [54] | Systematic review & meta-analysis of PCs | Diabetes T2 | Relative Risk of diabetes T2 | Macronutrient intake | 22 PCs | >500'000 | Adults | 4.6- 20 yrs. | High intake of dietary carbohydrate was associated with an increased type 2 diabetes risk (RR= 1.11, 95% CI: 1.01 to 1.22, p=0.035); however, this effect was not observed in an analysis stratified by gender. Intake of total fat, SFA, MUFA & PUFA was not associated with diabetes risk | No studies fulfilled all requirements for a high-quality study free of bias | Fat and individual fatty acid intake was not associated with DM T2 risk | II a |
| Mansoor N 2016 [55] | Meta-analysis of RCTs | Obesity & CV risk factors | Weight loss, lipids | Low fat versus low carb | 11 RCTs | 1'369 | Adults, overweight-obese | 6 months | Participants on LoFat diets compared to LoCarb diets lost more weight (WMD –2·17 kg; 95% CI –3·36, –0·99) and triglycerides (WMD –0·26 mmol/l; 95% CI –0·37, –0·15), but had a greater increase in HDL-cholesterol (WMD 0·14 mmol/l; 95% CI 0·09, 0·19) and LDL-cholesterol (WMD 0·16mmol/l; 95% CI 0·003, 0·33) | Heterogeneity was moderate to high for all variables | The beneficial changes of LoCarb diets must be weighed against the possible detrimental effects of increased LDL-cholesterol | I a |
| Tobias DK 2015 [56] | Meta-analysis of RCTs | Obesity | Weight loss, serum triglycerides | Low fat versus other dietary interventions | 53 RCTs | 68128 | Adults, overweight-obese, formerly obese | >1 yr. | In weight loss trials, low-carbohydrate interventions led to significantly greater weight loss than did low-fat interventions (18 comparisons; WMD 1.15 kg [95% CI 0.52 to 1.79 | Incomplete outcome data was a high potential source of bias for 39 trials because of drop-out and loss-to-follow-up rates exceeding 5% | Higher-fat, low-carbohydrate dietary interventions led to a slight but significant, greater long-term weight loss than did low-fat interventions | I a |
| Sackner-Bernstein J, 2015 [57] | Meta-analysis of RCTs | Obesity | Weight loss, CV risk factors | Low fat versus low carb | 17 RCTs | 1'797 | Adults, overweight-obese | 8 wks.- 2 yrs. | Compared with low fat diet, low carbohydrate was associated with significantly greater reduction in weight (Δ = -2.0 kg, 95% CI: -3.1, -0.9) and significantly lower predicted risk of atherosclerotic cardiovascular disease events (p<0.03) | No patient-level data; frequent loss of follow-up | LoCarb diet appears to achieve greater weight loss and reduction in predicted risk of ASCVD events compared with LoFat diet | I a |
| Hooper L 2015 (Cochrane) [58] | Meta-analysis of RCTs & of PCs | Weight gain | Change of body weight, Lipids | Total fat intake | 32 RCTs, 25 PCs | 54'000 (RCTs) | Adults, not aiming to lose weight | Median: 5 yrs. | Eating less fat (compared with usual diet) resulted in a mean weight reduction of 1.5 kg (95% CI -2.0 to -1.1 kg), but greater weight loss results from greater fat reductions. The size of the effect on weight does not alter over time and is mirrored by reductions in body mass index (BMI) (-0.5 kg/m2, 95% CI -0.7 to -0.3) and waist circumference (-0.3 cm, 95% CI -0.6 to -0.02) | There was a high risk of performance bias due to lack of blinding; most RCTs were at unclear risk of reporting bias; some trials had high attrition rates | Lowering the proportion of fat in food leads to a small but noticeable decrease in body weight, body mass index and waist circumference in both, adults and children. The effect did not change over time | I a & II a |
Table 2: Meta-analyses on dietary fat or fatty acid intake in relation to diabetes type 2 and obesity.
List of meta-analyses published between 2012 and 2017.
List of meta-analyses published between 2012 and 2017.
These findings show that consumption of total fat or saturated fat intake has not been clearly associated with diabetes type 2 risk. Increased consumption of MUFA, olive oil and in some instances of n-6 PUFA have been associated with diminished diabetes risk and with improved metabolic control in patients with established diabetes when carbohydrates were replaced by MUFA. Regarding high versus low consumption of plant-derived n-3 PUFA, some studies suggested a diminished risk of developing type 2 diabetes and decreased insulin resistance but the findings were not consistent.
Seafood-derived n-3- PUFA have not been shown to reduce diabetes type 2 risk in Western populations. Regarding overweight and obesity, lowering the proportion of fat in the diet resulted in a small but noticeable decrease of body weight. When fat reduction was compared to carbohydrate reduction in weight loss trials, the latter was somewhat more efficacious to reduce weight than the former.
| Source | Study category | Disease | End point | Main nutritional theme | No. of included studies | No. of subjects | Subject group | Duration | RR (95%CI) | Limitations | Conclusions | LOE |
| Brennan SF 2017 [59] | Systematic review & meta-analysis of PCs | Breast cancer | Survival from breast cancer | Dietary fat, SFA | 15 PCs | 29241 | Women with breast cancer | 16 yrs. | There was no difference in risk of breast-cancer-specific death or all-cause death in the highest versus lowest category of total fat intake. Breast-cancer-specific death (n=4; HR=1.51; 95% CI: 1.09, 2.09; p < 0.01) was higher for women in the highest versus lowest category of saturated fat intake | Heterogeneity between studies; small sample size | Saturated fat intake was negatively associated with breast cancer survival | II a |
| Zhao J 2016 [60] | Systematic review & meta-analysis of PCs or case control studies | Endometrial cancer | Risk of new cancer | Dietary fat, SFA, MUFA, PUFA | 7 PCs & 14 case controls | approx. 15'000 | Women | 1 mo.- 10 yrs. | Endometrial cancer risk was significantly increased by 5% per 10% kilocalories from total fat intake (P=0.02) and by 17% per 10g/1000 kcal of saturated fat intake (P<0.001). 3 cohort studies showed significant inverse association between MUFA & cancer risk (odds ratio=0.84, 95% confidence interval= 0.73–0.98). No significant associations were found for PUFAs | Measurement error linked to the nature of food frequen-cy question-naire | High intake of total fat and SFA was associated with increased endometrial cancer risk. In addition, dietary MUFA was associated with decreased risk in cohort studies | II a |
| Cao Y 2016 [61] | Systematic review & meta-analysis of PCs | Breast cancer | Risk of new cancer | Dietary fat, SFA, PUFA, MUFA | 24 PCs | 38262 & 1.4 Mio controls | Women | 2- 25 yrs. | No association was observed between animal fat, vegetable fat, SAFA, MUFA, PUFA, n-3 PUFA, n-6 PUFA and risk of breast cancer | No subgroups of cancer types. FFQ are subject to error. | Dietary total fat and fatty acids might be not associated with risk of breast cancer | II a |
| Xia H, 2015 [62] | Systematic review & meta-analysis of PCs or case control studies | Breast cancer | Risk of new cancer | Dietary SFA | 24 PCs & 28 case controls | 35651 BC, 1.8 Mio controls | Women | Not stated | The associations between dietary SFA intake and risk of BC were 1.18 for case–control studies (high vs low intake, 95% confidence interval [CI]=.03–1.34) and 1.04 for cohort studies (95% CI=0.97–1.11) | Possible bias in case control studies (selection & recall) | A relationship was found between SFA intake and incidence of BC in case–control studies, and of postmenopausal BC risk in case–control but not in cohort studies | II a |
| Han J 2015 [63] | Meta-analysis of observational studies | Gastric cancer | Risk of new cancer | Dietary fat | 22 studies | approx. 8500 cases & 500'000 controls | Adults | Not stated | The S-RR was 1.18 with highest intake versus lowest intake of total fat (95% CI: 0.999–1.39; n = 28; P< 0.001). There were positive associations between SAFA intake (SRR = 1.31; 95%CI: 1.09–1.58; n = 18;P<0.001), and inverse association between PUFA intake (SRR = 0.77; 95%CI: 0.65–0.92; n = 16; P = 0.003) | Case control studies may introduce recall and selection bias, FFQ, measurement errors etc. | Intake of total fat is potentially positively associated with gastric cancer risk, and specific subtypes of fats account for different effects | II a |
Table 3: Meta-analyses on dietary fat or fatty acid intake in relation to certain types of cancer.
List of meta-analyses published between 2012 and 2017.
List of meta-analyses published between 2012 and 2017.
These studies show that high intake of total fat and of SFA was associated with increased risk of cancer of breast, endometrium and stomach in some but not all observational studies.
| Source | Study category | Disease | End point | Main nutritional theme | No. of included studies |
No. of subjects | Subject group | Duration | RR (95%CI) | Limitations | Conclusion | LOE |
| Grosso G 2016 [64] | Review & meta-analysis of observational studies | Depression | Risk of new disease | n-3 PUFA & fish | 31 observational studies | 255’076 sub-jects, 20’000 cases with depression | Adults | Not stated | Pooled risk estimates of depres- sion for extreme categories of both total n-3 PUFA and fish-de-rived n-3 PUFA [EPA&DHA] resulted in decreased risk for the highest compared with the low-est intake (RR=0.78, 95% CI:0.67, 0.92and RR=0.82, 95% CI:0.73, 0.92, respectively. | Design of the studies included and confound-ding due to lack adjustment for certain variables | Dietary n-3 PUFA intake is associated with lower risk of depression | II a |
| Zhang y, 2016 [65] | Meta-analysis of PCs | Dementia, Parkinson disease | Risk of new disease | n-3 PUFA & fish | 21 PCs | 18‘1580 subjects, 4438 with cognitive impairment | Elderly adults, mostly >65 yrs. | 2.1-21 yrs. | A 1-serving/wk. increment of die-tary fish was associated with lower risks of dementia (RR: 0.95; 95% CI: 0.90, 0.99; P = 0.042, I2 = 63.4%) and Alzhei-mer D. (RR: 0.93; 95% CI: 0.90, 0.95; P = 0.003, I2 = 74.8%). Pooled RRs of Mild Cognitive Impairment and Parkinson Disease were 0.71 (95% CI: 0.59, 0.82; P = 0.733, I2 = 0%) and 0.90 (95% CI: 0.80, 0.99; P = 0.221), respectively, for an 8-g/d increment of PUFA intake. A 0.1-g/d increment of dietary DHA intake was associated with lower risks of dementia (RR: 0.86; 95% CI: 0.76, 0.96; P=0.001). | Vitamin E intake appeared as the most-frequent confounding factor | Marine-derived DHA was associated with lower risk of dementia and Alzheimer disease but without a linear dose-response relation | II a |
| Appleton KM, 2015 (Cochrane) [66] | Meta-analysis of RCTs | Depression | Risk of new disease | n-3 PUFA & fish | 25 RCTs | 1’438 | Adults | wks.- months | For the placebo comparison, n-3 PUFA supplementation results in a small to modest benefit for de-pressive symptomology, com-pared to placebo: standardised mean difference (SMD) -0.30 (95% confidence interval (CI) -0.10 to -0.50 | The quality of the evidence for all outcomes was judged as low to very low. | Possible benefit in severe depression (not in mild symptomatology) | I a |
| Cooper RE, 2015 [67] | Meta-analysis of RCTs | Cognitive Impairment | Symptoms | Omega-3 PUFA | 24 RCTs | Adults & children (with ADHD & related disorders) | n-3 PUFA supplementation, in the whole sample and the TD and ADHD+RD subgroup, did not show improvements in any of the cognitive performance measures. In those with low n-3 PUFA status, supplementation improved short-term memory. | There is some evidence that n-3 PUFA supplementation improves cognition in those who are n-3 PUFA deficient, but not in those who were sufficient. | I a |
Table 4: Meta-analyses on dietary fat or fatty acid intake in relation to other endpoints (neurologic, psychiatric).
The main findings of observational studies suggest that intake of long-chain n-3 fatty acids is associated with diminished incidence of cognitive impairment in elderly subjects, decreased risk of dementia and decreased risk of severe depression. Randomised controlled trials confirmed an improvement of cognition only in subjects which were n-3 PUFA deficient. show that high intake of total fat and of SFA was associated with increased risk of cancer of breast, endometrium and stomach in some but not all observational studies.
The main findings of observational studies suggest that intake of long-chain n-3 fatty acids is associated with diminished incidence of cognitive impairment in elderly subjects, decreased risk of dementia and decreased risk of severe depression. Randomised controlled trials confirmed an improvement of cognition only in subjects which were n-3 PUFA deficient.
Recent publications of large trials not reviewed in the meta-analyses of Tables 1-4
The PURE study showed that across 18 countries from 5 continents increased fat consumption was associated with lower total and cardiovascular disease mortality (Dehghan M., et al. [16]. The data showed there was a large socio-demographic and economic heterogeneity between these 18 countries with widely discrepant rates of total mortality. Countries with higher levels of income and education had both, higher rates of fat consumption and higher life expectancy. Therefore, there is a considerable likelihood of residual confounders- that other factors explained the higher life expectancy in countries with higher fat consumption.
The PURE study showed that across 18 countries from 5 continents increased fat consumption was associated with lower total and cardiovascular disease mortality (Dehghan M., et al. [16]. The data showed there was a large socio-demographic and economic heterogeneity between these 18 countries with widely discrepant rates of total mortality. Countries with higher levels of income and education had both, higher rates of fat consumption and higher life expectancy. Therefore, there is a considerable likelihood of residual confounders- that other factors explained the higher life expectancy in countries with higher fat consumption.
The question whether high consumption of pro-inflammatory (n-6 polyunsaturated fatty acids) exert negative health effect is still debated. An new approach to this topic was taken by May-Wilson S., et al. [17]. These authors showed in a study using Mendelian randomisation analysis that a pro-inflammatory fatty acid profile (due to genetic factors) affected colorectal cancer risk. In particular, decreased risk of colon cancer was associated with high serum MUFAs and PUFA (linoleic) concentrations, and increased risk with high serum PUFA (arachidonic acid) and SFA (stearic acid) concentrations.
In a re-evaluation of the traditional diet-heart hypothesis, Ramsden., et al. analysed data of the Minnesota Coronary Experiment (1968-73) [18]. The authors concluded that available evidence from randomized controlled trials shows that replacement of saturated fat in the diet with linoleic acid effectively lowers serum cholesterol but does not translates a lower risk of death from coronary heart disease or all causes. Findings from the Minnesota Coronary Experiment add to growing evidence that incomplete publication has contributed to overestimation of the benefits of replacing saturated fat with vegetable oils rich in linoleic acid.
In order to assess the relationship between consumption of n-6 PUFA and total and cause specific mortality, Wu JH., et al. measured circulating n-6 PUFA in the Cardiovascular Health Study [19]. The authors found that circulating levels of LA, the major dietary n-6 PUFA, was related to lower total mortality and especially subtypes of CVD mortality in older adults. Other circulating n-6 PUFA, including AA, were not significantly associated with total or CVD mortality.
Propositions for specific changes of current nutritional guidelines such as those published in Switzerland [4] The recommendation that saturated fatty acids should be less than 10% of total energy consumption should be changed to that there is no convincing reason to limit the consumption to this range of consumption The consumption of vegetable oils should not be limited, and a detailed recommendation regarding the type of vegetable oil should not be given. The recommendation for long-chain n-3 PUFA should be limited to subjects with established cardiovascular disease [7].
Conflict of interest
The author declares to have not conflict of interest in the subject of this publication.
The author declares to have not conflict of interest in the subject of this publication.
References
- Jensen MD., et al. “2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society”. Circulation 129.25-2 (2014): S102–138.
- US Department of Health and Human Services. “Dietary guidelines for Americans 2010”. (2010).
- Colombani P., et al. Fette in der Ernährung. Expertenbericht der Eidgenössischen Ernährungskommission (2006): 1–50.
- Keller U., et al. Fette in der Ernährung. Aktualisierte Empfehlungen der Eidgenössischen Ernährungskommission. (2012): 6.
- WHO. “Diet, nutrition and the prevention of chronic diseases”. WHO (2013).
- Visioli F and Bernardini E. “Extra virgin olive oil’s polyphenols: biological activities”. Current Pharmaceutical Design 17.8 (2011) : 786-804.
- Kromhout D., et al. The 2015 Dutch food-based dietary guidelines”. European Journal of Clinical Nutrition 70.8 (2016): 869–878.
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. “2015 – 2020 Dietary Guidelines for Americans”. (2015).
- Michas G., et al. “Dietary fats and cardiovascular disease: Putting together the pieces of a complicated puzzle”. Atherosclerosis 234.2 (2014): 320–328.
- Schwingshackl L., et al. “Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies”. The American Journal of Clinical Nutrition 105.6 (2017): 1462–1473.
- O’Sullivan TA., et al. “Food Sources of Saturated Fat and the Association with Mortality: A Meta-Analysis”. American Journal of Public Health 103.9 (2013): e31–42.
- Brassard D., et al. “Comparison of the impact of SFAs from cheese and butter on cardio metabolic risk factors: a randomized controlled trial”. The American Journal of Clinical Nutrition 105.4 (2017): 800–809.
- Thorning TK., et al. “Whole dairy matrix or single nutrients in assessment of health effects: current evidence and knowledge gaps”. The American Journal of Clinical Nutrition 105.5 (2017): 1033–1045.
- Mozaffarian D. “Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review”. Circulation 133.2 (2016): 187–225.
- Wu JHY., et al. “Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis”. British Journal of Nutrition 107(2012): S214–227.
- Dehghan M., et al. “Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study”. The Lancet 390.10107 (2017):2050–2062.
- May-Wilson S., et al. “Pro-inflammatory fatty acid profile and colorectal cancer risk: A Mendelian randomisation analysis”. European Journal of Cancer 84(2017): 228–238.
- Ramsden CE., et al. “Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968-73)”. BMJ 353 (2016): i1246.
- Wu JH., et al. “Circulating Omega-6 Polyunsaturated Fatty Acids and Total and Cause-Specific Mortality: The Cardiovascular Health Study”. Circulation 130.15 (2014): 1245–1253.
- Harcombe Z., et al. “Evidence from prospective cohort studies does not support current dietary fat guidelines: a systematic review and meta-analysis”. British Journal of Sports Medicine 51.24 (2017):1743–1749.
- 21. Micha R., et al. “Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: Systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE)” PLOS ONE. 12.4 (2017): e0175149.
- Micha R., et al. "Association between Dietary Factors and Mortality from Heart Disease, Stroke, and Type 2 Diabetes in the United States". JAMA 317.9 (2017): 912-924.
- Alexander DD., et al. "A Meta-Analysis of Randomized Controlled Trials and Prospective Cohort Studies of Eicosapentaenoic and Docosahexaenoic Long-Chain Omega-3 Fatty Acids and Coronary Heart Disease Risk". Mayo Clinic Proceedings 92.1 (2017): 15–29.
- Pimpin L., et al. "Is Butter Back? A Systematic Review and Meta-Analysis of Butter Consumption and Risk of Cardiovascular Disease, Diabetes, and Total Mortality". PLOS ONE 11.6 (2016): e0158118.
- Souza RJ de., et al. "Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies". BMJ (2015): 351:h3978.
- Hooper L., et al. "Reduction in saturated fat intake for cardiovascular disease". Cochrane Database of Systematic Reviews 10.6 (2015): CD011737.
- Farvid MS., et al. "Dietary Linoleic Acid and Risk of Coronary Heart Disease: A Systematic Review and Meta-Analysis of Prospective Cohort Studies". Circulation 130.18 (2014): 1568–1578.
- Wen YT., et al. "Effects of Omega-3 fatty acid on major cardiovascular events and mortality in patients with coronary heart disease: A meta-analysis of randomized controlled trials". Nutrition, Metabolism & Cardiovascular Diseases 24.5 (2014): 470-475.
- Schwingshackl L and Hoffmann G. "Dietary fatty acids in the secondary prevention of coronary heart disease: a systematic review, meta-analysis and meta-regression". BMJ 4.4 (2014).
- Chowdhury R., et al. "Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis". Annals of Internal Medicine 160.6 (2014): 398–406.
- Goede J de., et al. "N-6 and N-3 Fatty Acid Cholesteryl Esters in Relation to Fatal CHD in a Dutch Adult Population: A Nested Case-Control Study and Meta-Analysis". PLOS ONE 8.5 (2013): e59408.
- Ramsden CE., et al. "Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis". BMJ (2013): e8707–e8707.
- Pan A., et al. "α-Linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis". The American Journal of Clinical Nutrition 96.6 (2012): 1262-1273.
- Kotwal S., et al. "Omega 3 Fatty Acids and Cardiovascular Outcomes: Systematic Review and Meta-Analysis." Circulation: Cardiovascular Quality and Outcomes 5.6 (2012): 808–818.
- Hooper L., et al. "Reduced or modified dietary fat for preventing cardiovascular disease". Cochrane Cochrane Database (2012).
- Schwingshackl L and Hoffmann G. "Monounsaturated fatty acids, olive oil and health status: a systematic review and meta-analysis of cohort studies". Lipids in Health and Disease 13.1 (2014): 154.
- Cheng P., et al. "Can dietary saturated fat be beneficial in prevention of stroke risk? A meta-analysis". Neurological Sciences 37.7 (2016): 1089-1098.
- Cheng P., et al. "BMI Affects the Relationship between Long Chain N-3 Polyunsaturated Fatty Acid Intake and Stroke Risk: a Meta-Analysis". Scientific Reports 5 (2015): 14161.
- Martínez-González MA., et al. "Olive oil consumption and risk of CHD and/or stroke: a meta-analysis of case–control, cohort and intervention studies". British Journal of Nutrition 112.2 (2014): 248–259.
- Larsson SC., et al. "Long-chain omega-3 polyunsaturated fatty acids and risk of stroke: a meta-analysis". European Journal of Epidemiology 27.12 (2012): 895–901.
- Chowdhury R., et al. "Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: systematic review and meta-analysis". BMJ (2012): e6698.
- Jovanovski E., et al. "The effect of alpha-linolenic acid on glycemic control in individuals with type 2 diabetes: A systematic review and meta-analysis of randomized controlled clinical trials"'. Medicine 96.21 (2017).
- Wu JHY., et al. "Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies". The Lancet Diabetes & Endocrinology 5.12 (2017): 965–974.
- Schwingshackl L., et al. "Olive oil in the prevention and management of type 2 diabetes mellitus: a systematic review and meta-analysis of cohort studies and intervention trials". Nutrition & Diabetes 7.4 (2017): e262.
- Lin N., et al. "What is the impact of n-3 PUFAs on inflammation markers in Type 2 diabetic mellitus populations?: a systematic review and meta-analysis of randomized controlled trials". Lipids in Health and Disease (2016) :133.
- Qian F., et al. "Metabolic Effects of Monounsaturated Fatty Acid–Enriched Diets Compared With Carbohydrate or Polyunsaturated Fatty Acid–Enriched Diets in Patients With Type 2 Diabetes: A Systematic Review and Meta-analysis of Randomized Controlled Trials".Diabetes Care 39.8 (2016):1448–1457.
- Imamura F., et al. “Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials”. PLOS Medicine 13.7 (2016): e1002087.
- Abbott KA., et al. "Do ω-3 PUFAs affect insulin resistance in a sex-specific manner? A systematic review and meta-analysis of randomized controlled trials". The American Journal of Clinical Nutrition 104.5 (2016): 1470–1484.
- Chen C., et al. "Effects of Omega-3 Fatty Acid Supplementation on Glucose Control and Lipid Levels in Type 2 Diabetes: A Meta-Analysis". PLOS ONE 10.10 (2015): e0139565.
- Aronis KN., et al. "Effects of trans fatty acids on glucose homeostasis: a meta-analysis of randomized, placebo-controlled clinical trials". The American Journal of Clinical Nutrition 96.5 (2012): 1093–1099.
- Zheng JS., et al. “Marine N-3 Polyunsaturated Fatty Acids Are Inversely Associated with Risk of Type 2 Diabetes in Asians: A Systematic Review and Meta-Analysis”. PLOS ONE 7.9 (2012): 4452.
- Zhou Y., et al. “Association of fish and n-3 fatty acid intake with the risk of type 2 diabetes: a meta-analysis of prospective studies”. British Journal of Nutrition 108.3 (2012): 408-417.
- Wallin A., et al. “Fish Consumption, Dietary Long-Chain n-3 Fatty Acids, and Risk of Type 2 Diabetes: Systematic review and meta-analysis of prospective studies”. Diabetes Care 35.4 (2012): 918-929.
- Alhazmi A., et al. “Macronutrient Intakes and Development of Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cohort Studies”. Journal of the American College of Nutrition 31.4 (2012): 243-258.
- Mansoor N., et al. “Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: a meta-analysis of randomised controlled trials”. British Journal of Nutrition 115.3 (2016): 466-479.
- Tobias DK., et al. “Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis”. The Lancet Diabetes & Endocrinology 3.12 (2015): 968-979.
- Sackner-Bernstein J., et al. “Dietary Intervention for Overweight and Obese Adults: Comparison of Low-Carbohydrate and Low-Fat Diets. A Meta-Analysis”. PLOS ONE 10.10 (2015): 0139817.
- Hooper L., et al. “Effects of total fat intake on body weight”. Cochrane Database of Systematic Reviews (2015).
- Brennan SF., et al. “Dietary fat and breast cancer mortality: A systematic review and meta-analysis”. Critical Reviews in Food Science and Nutrition 57.10 (2017): 1999-2008.
- Zhao J., et al. “Dietary fat intake and endometrial cancer risk: A dose response meta-analysis”. Medicine 95.27 (2016): 4121.
- Cao Y., et al. “Dietary total fat and fatty acids intake, serum fatty acids and risk of breast cancer: A meta-analysis of prospective cohort studies”. International Journal of Cancer 138.8 (2016): 1894-1904.
- Xia H., et al. “Meta-analysis of Saturated Fatty Acid Intake and Breast Cancer Risk”. Medicine 94.52 (2015): 2391.
- Han J., et al. “Dietary Fat Intake and Risk of Gastric Cancer: A Meta-Analysis of Observational Studies”. PLOS ONE 10.9 (2015): 0138580.
- Grosso G., et al. “Dietary n-3 PUFA, fish consumption and depression: A systematic review and meta-analysis of observational studies”. Journal of Affective Disorders 205 (2016): 269-81.
- Zhang Y., et al. “Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies”. The American Journal of Clinical Nutrition 103.2 (2016): 330-340.
- Appleton KM., et al. “Omega-3 fatty acids for depression in adults”. The Cochrane Database of Systematic Reviews 11 (2015): 004692.
- Cooper RE., et al. “Omega-3 polyunsaturated fatty acid supplementation and cognition: A systematic review and meta-analysis”. Journal of Psychopharmacology 29.7 (2015): 753-763.
Citation:
Ulrich Keller. “Health Aspects of Nutritional Fats and Oils. A Review of Recent Findings”. Nutrition and Food Toxicology 2.6
(2018): 488-516.
Copyright: © 2018 Ulrich Keller. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.