Review Article
Volume 1 Issue 6 - 2017
Bioactive Constituents of Kokum and its Potential Health Benefits
1PG Institute of Post-Harvest Management, Killa – Roha, Dist. Raigad, Maharashtra, India
2Department of Technology (Food Technology), Shivaji University, Kolhapur - 416 004, Maharashtra, India
2Department of Technology (Food Technology), Shivaji University, Kolhapur - 416 004, Maharashtra, India
*Corresponding Author: PG Institute of Post-Harvest Management, Killa – Roha, Dist. Raigad, Maharashtra, India.
Received: August 27, 2017; Published: October 12, 2017
Abstract
The main focus of the review is to explore bioactive constituents of Kokum (Garcinia indica Choisy) and their health benefits. Kokum generally grown in tropical region and kokum has many bioactive compounds such as anthocyanin, Hydroxyl citric acid and Garcinol, those possesses several nutraceutical activities. Among the bioactive compound anthocyanin, this has a great potential as a natural colorant and having free radical scavenging activity. The hydroxyl citric acid (HCA) is major acid present in kokum, which is used as an anti-obesity ingredient in pharmaceutical preparations. Another bioactive compound garcinol which is a polyisoprenylated benzophenone derivative has antioxidant and chelating activity. This paper reviewed potential health benefits bioactive components present in kokum.
Keywords: Kokum; Bioactive compounds; Anthocyanins; HCA; Garcinol
Introduction
Kokum (Garcinia indica Choisy) is one of the underexploited tree spices. It is mostly found in Konkan region of Maharashtra, Goa, Karnataka, Kerala and Surat district of Gujarat on the West Coast of India, as well as it is found in some extent in the forests of Assam, Meghalaya and West Bengal. In spite of it’s incredible medicinal and nutritive properties, kokum is not cultivated systematically on orchard of other fruit such as mango, cashew nut etc. It is mostly found as a kitchen garden plant or mixed crop in plantations of coconut, areca nut, as roadside plants or in forest [1-2].
The locally Kokum has different names such as, Kokum in Hindi, while bheranda, bhiranda, kokamba, kokambi, ratamba, ratambi, tambada amba in Marathi. In Tamil it is known as murgal, murgal-mara and in Malayalam it is known as kaattampi kokkam, in Kannada it is called murgina, punarpuli, devana huli. Tintali in Oriya and Kokum or bhirind in Gujarati and Konkani. In Sanskrit it is known with different names such as vrikshamia, amlabija, amlapura, amlashaka. In French, Italian and Spanish the name is spelt as cocum and in Portuguese it is known as brindao or brindonna [2-4].
The precise statistics regarding area production and productivity is not available as kokum is not planted in an organized manner. As per a baseline survey carried out in 2010, kokum is grown on about 1000 ha area in the Konkan region with production of about 4500 MT fruits. According to the survey conducted earlier by Chief Conservator of Forest out of the total 46,600 Kokum trees in the state of Maharashtra; 43,000 trees existed in Ratnagiri and Sindhudurg Districts of Maharashtra state of India. It was also reported that in South Konkan region of Maharashtra produces, 1674 MT of dried Kokum rind, 757 MT of Kokum syrup and 40 MT of Kokum butter.
Kokum is a tropical evergreen tree, related to the mangos teens. A slender tree with sloping branches, it reaches heights of 15m (50 ft). The thin bark is lined and the leaves 1/2 oblong. The fruit is round in shape, dark purple in colour and about 4 cm (1 in) in diameter with 5-8 seeds. The kokum fruits and its different parts are shown in figure 1.
Kokum fruit contains various bioactive compounds, those possesses antioxidant, anti-bacterial and antifungal properties. Scientific research documented its activity against several cancer cell lines, including breast cancer, liver cancer and leukaemia. In addition, kokum also exhibits antihistamine and anti-inflammatory properties. Traditionally, kokum has been utilized from many years as a medicine for diarrhoea treatment, skin infection and wounds healing. Some meditational activities of kokum are shown in table 1. Dried kokum fruit rinds are widely used in cooking as they impart a sweetish-tangy flavour to the food. The fruits contain citric acid, acetic acid, malic acid, ascorbic acid, hydroxyl citric acid and garcinol.
| Pharmacological properties | Phytochemicals | References |
| Antibacterial | Garcinol, isogarcinol and xanthochymol | [20, 24-25, 35, 37-39] |
| Anticlastogenic effect | Garcinol | [28] |
| Antidiabetic activities | Cyanidin 3-glucoside | [40-41] |
| Antineoplastic and Chemopreventive effects | Garcinol and isogarcinol, Cyanidin-3-glucoside | [22,28, 30-31, 36-37,42,48] |
| Antifungal activity | 1. Aqueous extract possess antifungal action on Candida albicans and Penicillium sp 2. The chloroform extract from spent rinds inhibits the growth of and production of aflatoxin by Aspergillus flavus |
[37, 49] |
| Anti-glycation activities | Garcinol | [19] |
| Anti-obesity activity | Hydroxycitric acid acid Cyanidin 3-glucoside | [18,50] |
| Antioxidant effects | Garcinol Cyanidin-3-glucoside | [19,23,28, 47-48, 49,51] |
| Cardioprotective effects | Cyanidin-3-glucoside | [52] |
| Gastroprotective effects | Garcinol | [19, 32-33] |
| Inhibition of carbonyl Content | Garcinol | [53] |
| Inhibitory effects on elastase and Hyaluronidase | Methanolic extract of kokum rind as well as the ethyl acetate and water fraction possess anti-hyaluronidase and anti-elastase activities in vitro. | [54] |
| Inhibition of lipid peroxidation | Garcinol | [19,23,53] |
| Neuroprotection | Garcinoland Cyanidin-3-glucoside | [28, 55-56] |
Table 1: Summary of reported nutraceutical activities of bioactive compounds of Kokum.
Ayurvedic and Industrial applications of kokum
In traditional medicine, kokum has a long history of uses in the Indian Ayurveda. The leaves and fruits are well known for their sour and astringent taste, thermogenic, constipating and digestive. The herbal preparations made from kokum rinds are used in the treatment of inflammatory ailments, for rheumatic pains and bowel complaints. The fruit is considered to be antihelmintic and cardiotonic [4]. The Kokum agal (Juice) and squash made out of the rind is used to cure various diseases such as piles, haemorrhoids, colic problems, ulcers, inflammations, treat sores, dermatitis, diarrhoea, dysentery, ear infection, etc. It is also used to facilitate digestion and to prevent over perspiration or hyper perspiration [4]. Kokum is a natural antacid and the preparation rind, yogurt and salt is supposed to relieve gastric ulcerations and burning sensation [4]. The Kokum butter is useful in dysentery, diarrhoea, phthisis pulmonalis and scorbutic diseases. Application of kokum butter on the skin is known to possess wound healing property and to be useful in ameliorating ulcerations, fissures of the lips, hands, chapped skin and inflammatory sores [4].
In traditional medicine, kokum has a long history of uses in the Indian Ayurveda. The leaves and fruits are well known for their sour and astringent taste, thermogenic, constipating and digestive. The herbal preparations made from kokum rinds are used in the treatment of inflammatory ailments, for rheumatic pains and bowel complaints. The fruit is considered to be antihelmintic and cardiotonic [4]. The Kokum agal (Juice) and squash made out of the rind is used to cure various diseases such as piles, haemorrhoids, colic problems, ulcers, inflammations, treat sores, dermatitis, diarrhoea, dysentery, ear infection, etc. It is also used to facilitate digestion and to prevent over perspiration or hyper perspiration [4]. Kokum is a natural antacid and the preparation rind, yogurt and salt is supposed to relieve gastric ulcerations and burning sensation [4]. The Kokum butter is useful in dysentery, diarrhoea, phthisis pulmonalis and scorbutic diseases. Application of kokum butter on the skin is known to possess wound healing property and to be useful in ameliorating ulcerations, fissures of the lips, hands, chapped skin and inflammatory sores [4].
The kokum rinds are commercially used to prepare concentrated syrups, which on appropriate dilution gives the ready to use cool health drinks especially during the off-season. The local community of Goa, also uses the rinds for prepare wine. Dried rinds are powdered and marketed to be used as acidulant for traditional curries [5]. Kokum butter isolated from the seeds is in great demand in confectionery, medicines and cosmetic industries. Kokum butter has fatty acid and triacylglycerol compositions, tolerance toward milk fat and solidification properties similar to those of cocoa butter [6].
These properties are considered ideal in confectionary industry and kokum is used as a replacement to cocoa butter in the preparation of chocolates [6]. Studies have also shown that kokum butter when used along with cocoa butter increases the heat-resistance property of cocoa butter and chocolate and is helpful in preventing the heat induced softening and loss of consistency of chocolates [6-7]. The kokum butter is also of use in the production of soaps and candle [5,8].
Nutraceutical properties of Kokum
Kokum contains three major bioactive compounds namely anthocynin, hydroxycitric acid and garcinol and all of these possesses nutraceutical properties. All of these compounds are present in the rinds of Kokum. They play beneficial role in human health since they have anti-cancer and anti-obesity properties (table 1).
Kokum contains three major bioactive compounds namely anthocynin, hydroxycitric acid and garcinol and all of these possesses nutraceutical properties. All of these compounds are present in the rinds of Kokum. They play beneficial role in human health since they have anti-cancer and anti-obesity properties (table 1).
Anthocyanins
The two major anthocyanin pigments found in Kokum are characterized as cyanidin-3-glucoside and cyanidin-3-sambubioside. They have been identified by thin layer chromatography as well as HPLC, mass and NMR spectroscopy [5,9]. Anthocyanins constitute approximately 2.4% of the total fruit biomass. These pigments can scavenge free radicals and are water soluble. They can be extracted from the fruit rind by hydraulic press using 1% acidified water as a solvent [5]. The monomeric anthocyanins in Kokum can be measured using pH differential method [10].
The two major anthocyanin pigments found in Kokum are characterized as cyanidin-3-glucoside and cyanidin-3-sambubioside. They have been identified by thin layer chromatography as well as HPLC, mass and NMR spectroscopy [5,9]. Anthocyanins constitute approximately 2.4% of the total fruit biomass. These pigments can scavenge free radicals and are water soluble. They can be extracted from the fruit rind by hydraulic press using 1% acidified water as a solvent [5]. The monomeric anthocyanins in Kokum can be measured using pH differential method [10].
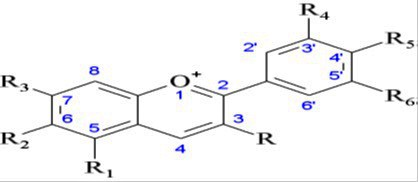
Anthocyanins are group of important compounds which are part of flavonoids and responsible for red and purple colours in fruits. Chemically, anthocyanins are based on a C-15 skeleton with a chromane ring having a second aromatic B-ring in position 2 (C6-C3-C6). Usually one or more sugar molecules are attached to different hydroxylated positions of basic structure. Substituted glycosides of salts of phenyl-2- benzopyrilium (anthocyanidins) are anthocyanins [11]. Basic structure of anthocyanidin is shown in Figure 3 where different R could be -H, - OH or -OCH3. Figure 2 also shows the most accepted nomenclature [11].
This basic structure of anthocyanidin pigment is responsible for a number of different colour compounds produced by chemical combination with glycosides and acyl groups. The most common sugar groups occurring in nature are glucose, rhamnose, xylose, galactose, arabinose and fructose while common acyl groups are coumaric, caffeic, ferulic, p-hydroxy benzoic, synapic, malonic, acetic, succinic, malic, oxylic etc. Substitution of hydroxyl and methoxyl groups has influence on different shades of colours of anthocyanins. Increase in the number of hydroxyl groups gives more bluish shade while increase in methoxyl groups increases redness. Depending upon the number and position of hydroxyl and/or methoxyl groups there are total 17 anthocyanidins of which 6 are the most common ones, namely cyanidin, delphinidin, pelargonidin, malvidin, peonidin, petunidin. Cyanidins have hydroxyl groups attached at 3,5,7,3’ and 4’ position. It gives magenta and crimson shades [11]. Cyanidin-3-glucoside found in Kokum has hydroxyl groups attached at the corresponding positions and glycosidic linkage at position 3.
The other major pigment cyanidin-3-sambubioside is similar in structure and has disaccharide sambubiose attached instead of glucose.
Anthocyanins have been shown to possess strong antioxidant activity. Given their wide distribution in nature, daily intake of anthocyanins is 25 to 215 mg/person depending upon gender and age [11]. Anthocyanins prevent ascorbic acid oxidation, scavenge free radicals, show inhibitory effects against oxidative enzymes and reduce the risk of cancer and heart diseases [12]. The 3’ and 4’ –OH in B-ring determine radical scavenging capacity with a saturated 2,3- double bond. Different glycosylation and hydroxylation positions determine their 12 potentials as an antioxidant [13]. With increase in hydroxyl groups in B-ring, antioxidant activity increases when present as glucosides. Corresponding aglycones have weaker activities [14]. Azevedo., et al. [14] showed antioxidant properties of anthocyanins with DPPH, FRAP and oxygen consumption assays. They showed radical scavenging activity and reducing capacity increased with the number of hydroxyl groups present in B-ring. 3’ and 4’ –OH groups are important in preventing ascorbic acid oxidation by anthocyanins-metal chelation [15].
Anthocyanins also have effect on lipid peroxidation. They are better agents against lipid peroxidation than α-tocopherol. Anthocyanins also have scavenging properties against – OH and O2 -. Bioflavonoids such as leucoanthocyanidins, catechins, flavonols etc. along with anthocyanins such as cyanidin-3-glucoside have shown activity to improve permeability and strength of capillaries, to accelerate the ethanol metabolism and to reduce inflammations and eremitic reactions [11].
Hydroxycitric acid
Hydroxycitric acid (HCA) is a major acid found in kokum. HCA is also found in other Garcinia species such as G. cambogia, G. atrovirdis etc. [16]. Kokum can contain up to 23% of HCA on dry basis. The major part is found in leaves and rinds as HCA and some quantity is present as HCA lactone. HCA which is also called as Garcinia acid can be separated from rinds by thermal as well as some non-thermal methods. HCA has been separated as sodium salt by combination of aqueous NaOH and methanol extraction and then neutralizing with HCl. Acetone is used to obtain pure crystals of HCA. HCA has also been separated by a thermal method in which HCA is extracted with deionized water and then concentrated by osmotic membrane distillation with hydrophobic polypropylene membrane. This method being non thermal avoids degradation of HCA and also HCA lactone formation [5]. HCA has also been produced from microbes like Streptomyces sp. U121 and Bacillus megaterium G45C [17].
Hydroxycitric acid (HCA) is a major acid found in kokum. HCA is also found in other Garcinia species such as G. cambogia, G. atrovirdis etc. [16]. Kokum can contain up to 23% of HCA on dry basis. The major part is found in leaves and rinds as HCA and some quantity is present as HCA lactone. HCA which is also called as Garcinia acid can be separated from rinds by thermal as well as some non-thermal methods. HCA has been separated as sodium salt by combination of aqueous NaOH and methanol extraction and then neutralizing with HCl. Acetone is used to obtain pure crystals of HCA. HCA has also been separated by a thermal method in which HCA is extracted with deionized water and then concentrated by osmotic membrane distillation with hydrophobic polypropylene membrane. This method being non thermal avoids degradation of HCA and also HCA lactone formation [5]. HCA has also been produced from microbes like Streptomyces sp. U121 and Bacillus megaterium G45C [17].
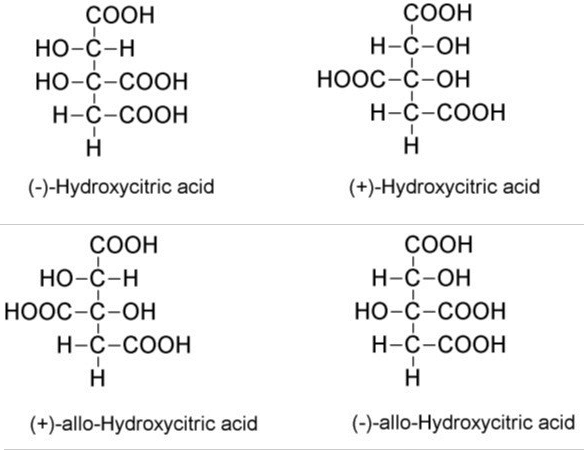
HCA has hydroxyl groups at the second and third carbon atom. HCA has two asymmetric carbons and thus two pairs of diastereoisomers. Out of the four, (-)- hydroxycitric acid is found in Garcinia species. Free HCA is readily converted to HCA lactone while evaporation or concentration. Structures of HCA and its isomers are shown in Figure 3. There are two corresponding lactones for each pair namely, (-)-hydroxycitric acid lactone and (+)-allo-hydroxycitric acid lactone. Since HCA is unstable in its free form, it is commercially available as a calcium salt. Potassium hydroxycitrate is also formed by KOH treatment of HCA [18].
(-)-HCA has inhibitory effect on ATP: citrate lyase (ATP: citrate oxaloacetate lyase, EC 4.1.3.8). This enzyme plays influential role in fatty acid synthesis from carbohydrates. It catalyses cleavage of citrate to acetyl-CoA and oxaloactate.
The ultimate source of carbon for fatty acids is acetyl-CoA and acetyl-CoA is an important molecule in formation of fats from carbohydrates. Thus, by limiting the availability of acetyl-CoA, (-)-HCA plays important role in regulating fatty acid synthesis. The knowledge of powerful inhibition of ATP: citrate lyase by HCA helps in the study of citrate cleavage reaction [18]. HCA, in some cases, has been observed to stimulate fatty acid synthesis. HCA inhibits lipogenesis only when cytoplasmic acetyl-CoA is produced by citrate cleavage enzyme otherwise if the alternate source of acetyl-CoA is available, for example, acetate, it will activate fatty acid synthesis (18). Since HCA regulates the ATP: citrate lyase enzyme and thus citrate cleavage reaction it acts as an anti-obesity agent. Due to its regulatory effect it is also known as weight controlling agent. HCA can be used to increase activity of carnitine palmitoyl transferase (CPT 1). CPT 1 is a rate limiting factor in fat burning and thus weight loss. HCA limits production of acetyl-CoA which in turn reduces production of malonyl-CoA. Malonyl-CoA has inhibitory effect on CPT 1. Thus, by reducing malonyl-CoA formation HCA works as a weight reducing agent. But since malonyl-CoA is also important in transmitting insulin signal in the cells, HCA may have adverse effect on insulin sensitivity (18).
Garcinol
Kokumfruit contains 1.5% of polyisoprenylated benzophenone derivative called Garcinol. Garcinol is a yellow coloured, fat soluble pigment found in the rinds of Kokum at level of 2-3%. In fact, all Garcinia species have some amount of garcinol [19-20]. Garcinol can be separated from the fruit rinds by ethanol or hexane extraction [2, 19].
Kokumfruit contains 1.5% of polyisoprenylated benzophenone derivative called Garcinol. Garcinol is a yellow coloured, fat soluble pigment found in the rinds of Kokum at level of 2-3%. In fact, all Garcinia species have some amount of garcinol [19-20]. Garcinol can be separated from the fruit rinds by ethanol or hexane extraction [2, 19].
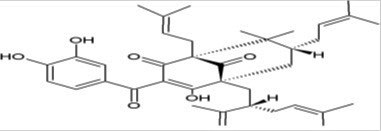
Garcinol is a polyisoprenylated benzophenone derivative and contains phenolic hydroxyl groups. This makes it active antioxidant. It is also called as camboginol, a triisoprenylated chalcone. It has β-diketone moiety and thus resembles a known antioxidant viz. curcumin [22]. Molecular weight of Garcinol is 602 (C38H50O6) and its melting point is 122°C [5]. It is crystallized out from hexane extract of the fruit rind. The absorption spectral data and molecular formula indicate relation to isomeric xanthochymol and in terms of optical rotation to cambogin. The 1,3-diketone system is enolisable since presence of two isomeric trimethyl ethers. The UV spectrum of garcinol shows that 1,3-diketone system is conjugated to the 3,4-dihydroxybenzoyl moiety. The IR spectrum of trimethyl ethers shows there is presence of saturated carbonyl group and two α, β-unsaturated carbonyl groups. Some features of the garcinol molecule indicate it can be derivable from Maclurin (2,4,6,3',4'- pentahydroxybenzophenone) and five isoprenyl units [2]. The general structure of garcinol is shown in Figure 4.
Garcinol has been studied for its anti-cancer, anti-ulcer, anti-oxidative and antiglycation activity [5].
The antioxidant activity of Kokum syrup, aqueous and boiled extract has been measured by various techniques such as ORAC, FRAP, ABTS etc. and it is shown that these preparations have very good antioxidant potential due to presence of garcinol and anthocyanins [23]. Garcinol can scavenge alkyl-peroxyl radicals to form hydroperoxy derivative of garcinol and cambogin or isogarcinol. Isogarcinol has similar biological activities as garcinol and is potent antioxidant as well. These compounds can induce apoptosis in human leukemia HL-60 cells; inhibit NO radical generation and LPS-induced iNOS gene expression. Thus, garcinol has been shown to have better anti-tumor activity than curcumin [24-25]. Garcinol has been shown to possess antioxidant activity in H2O2-NaOH-DMSO system and radical scavenging activity against hydroxyl radical, methyl radical and superoxide anion. The emulsified garcinol suppresses superoxide anion similar to DL-α tocopherol (by weight), while it has three times greater free radical scavenging activity against 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals than DL-α- tocopherol by weight [19]. Garcinol is also shown to possess antioxidant activity against arachidonic acid metabolism and NO radical synthesis by modulation of arachidonic acid metabolism. Defective arachidonic acid metabolism and generation of NO radicals are involved in carcinogenesis and inflammation [26]. Liao., et al. [28] showed garcinol prevents NO radical accumulation in LPS induced inflammatory mediators such as iNOS and COX-2.
Thus, garcinol may have neuroprotective effects against brain injury. A recent study by Koeberle and co-workers [29] showed that garcinol interferes with two enzymes, 5-lipoxygenase and microsomal prostaglandin PGE2 synthase that play important role in inflammation and tumorigenesis. Garcinol and its oxidative products interact with colon cancer cells such as HT-29 and HCT-116 as well as normal immortalized intestinal cells such as IEC-6 and INT-407.
They have potent growth-inhibitory effects on all intestinal cells but more effective on cancer cells than normal ones. Thus, at certain concentration garcinol can be used to inhibit growth of cancer cells [27]. Another similar study on garcinol has shown inhibition of growth of human leukemia HL-60 cells suggesting its chemopreventive action [30]. Balasubramanyam., et al. [31] have shown non-specific inhibition of histone acetyltransferase by garcinol suggesting anti-HIV property. Above research shows that garcinol has very promising antioxidant, anticancer, anti-inflammatory properties. Garcinol is also reported to show some antimicrobial activity. It plays important role in treatment of gastric ulcers caused by Helicobacter pylori chronic infection. This bacterium along with cells from gastric mucous membrane produces hydroxyl radicals and superoxide anions.
Conventional antibiotics such as Clarithromycin have side effects and thus garcinol can be a good alternative [32-33]. Garcinol also showed antimicrobial activity against Staphylococcus aureus which was comparable to traditional antibiotic Vancomycin [34-35]. Yoshida and co-workers [36] reported, garcinol fortified diet decreases the incidence of tounge neoplasms and pre-neoplasms. It also induces apoptosis through the activation of caspases and thus works as anti-tumor agent [22]. There are numerous reported mechanisms through which garcinol acts as antioxidant, anti-inflammatory or anti-cancer agent as explained above.
References
- Padhye S., et al.“Emerging role of Garcinol, the antioxidant chalcone from Garcinia indica Choisy and its synthetic analogs”. Journal of Hematology and Oncology 2 (2009): 38-49.
- Chandran., et al. “Nature watch: The Kokum tree.” Resonance 1 (1996): 86-89.
- Baliga and Katiyar SK. “Chemoprevention of photocarcinogenesis by selected dietary botanicals”. Photochemistry Photobiological Sciences 5.2 (2006): 243-253.
- CHEMEXCIL. “Selected medicinal plants of India”. Basic Chemicals Pharmaceutical
and Cosmetic Export Promotion Council, Bombay 400 039, India (1992). - Nayak CA., et al. “Bioactive constituents present in Garcinia indica Choisy and its potential food applications: A review”. International Journal of Food Properties 13 (2010): 441-453.
- Reddy SY and J V Prabhakar. “Cocoa butter extenders from Kokum (Garcinia indica) and Phulwara (Madhuca butyracea) butter”. Journal of the American Oil Chemists' Society 71.2 (1994) 217-219.
- Maheshwari B and S Yella Reddy. “Application of kokum (Garcinia indica) fat as cocoa butter improver in chocolate”. Journal of the Science of Food and Agriculture 85.1 (2005): 135-140.
- Bhat, JD., et al. “Compendium and proceedings of 2nd national seminar on Kokum (Garcinia indica Choisy)”. Goa University (2005).
- Nayak CA., et al. “Characterization of anthocyanin from Garcinia indica choisy.” Food Chemistry 118.3 (2010): 719-724.
- Wrolstad RE., et al. “Hand book of Food Analytical Chemistry-Pigments, colorants, flavor, texture, and bioactive components”, Wiley Inter science Publications: NJ (2005): 5-70.
- Delgado-Vargas F., et al. “Natural pigments: carotenoids, anthocyanins, and betalains – characteristics, biosynthesis, processing and stability”. Critical Reviews in Food Science and Nutrition 40.3 (2000): 173-289.
- Briddle P and C.F.Timberlake “Anthocyanins as natural food colours—selected aspects”. Food Chemistry 58. 1-2 (1997) 103-109.
- Wang H., et al. “Total antioxidant capacity of fruits”. Journal of Agriculture Food Chemistry 44.3 (1996): 701-705.
- Tsushima M., et al. “Novel Marine di-Z-Carotenoids: Cucumariaxanthins A, B, and C from the Sea Cucumber Cucumaria japonica”. Journal of Natural Prododucts 59 (1996): 30-34.
- Azevedo J., et al. “Antioxidant properties of anthocyanidins, anthocyanidin-3-glucosides and respective portisins”. Food Chemistry 119.2 (2010): 518-523.
- Sarma AD., et al. “Antioxidant ability of anthocyanins against ascorbic acid oxidation”. Photochemistry 45.4 (1997): 671-674.
- Lewis YS., et al. “Acid in Garcinia Cambogia”. Current Science 33 (1964): 82-83.
- Yamada Y., et al. “Chemistry, physiological properties, and microbial production of hydroxycitric acid”. Applied Microbiology and Biotechnology 75.5 (2007): 977-982.
- Jena BS., et al. “Chemistry and Biochemistry of (-)-Hydroxycitric Acid from Garcinia”. Journal of Agricultural and Food Chemistry 50.1 (2002): 10-22.
- Yamaguchi F., et al. “Antioxidative and Anti-Glycation Activity of Garcinol from Garcinia indica Fruit Rind”. Journal of Agricultural and Food Chemistry 48.2 (2000): 2320-2325.
- Bakana P., et al. “Structure and chemotherapeutical activity of a polyisoprenylated benzophenone from the stem bark of Garcinia huillensis”. Journal of Ethnopharmacology 21.1 (1987): 75-84.
- Sahu A., et al. “Polyisoprenylated benzophenones from Garcinia pedunculata”. Phytochemistry 28. 4 (1989): 1233-1235.
- Pan MH., et al. “Induction of apoptosis by garcinol and curcumin through cytochrome Crelease and activation of caspases in human leukemia HL-60 cells”. Journal of Agricultural and Food Chemistry 49.3 (2001): 1464-1474.
- Mishra A., et al. “Antioxidant activity of Garcinia indica (kokam) and its syrup”. Current Science 91.1 (2006): 90-93.
- Sang S., et al. “Chemical studies on antioxidant mechanism of garcinol: Analysis of radical reaction products of garcinol with peroxyl radicals and their antitumor activities”. Tetrahedron 58.51 (2002): 10095-10102.
- Sang S., et al. “Chemical studies on antioxidant mechanism of garcinol: Analysis of radical reaction products of garcinol and their antitumor activities”. Tetrahedron 57.50 (2001): 9931-9938.
- Hong J., et al. “Modulation of arachidonic acid metabolism and nitric oxide synthesis by garcinol and its derivatives”. Carcinogenesis 27.2 (2006): 278-286.
- Hong J., et al. “Effects of garcinol and its derivatives on intestinal cell growth: Inhibitory effects and autoxidation-dependent growth-stimulatory effects”. Free Radical Biology and Medicine 42.8 (2007): 1211-1221.
- Liao CH., et al. “Effects of garcinol on free radical generation and NO production in embryonic rat cortical neurons and astrocytes”. Biochemical and Biophysical Research Communication 329.4 (2005): 1306-1314.
- Koeberle A., et al. “Identification of 5-lipoxygenase and microsomal prostaglandin E2 synthase-1 as functional targets of the anti-inflammatory and anti-carcinogenic garcinol”. Biochemical Pharmacology 77.9 (2009): 1513-1521.
- Matsumoto K., et al. “Cytotoxic benzophenone derivatives from Garcinia species display a strong apoptosis-inducing effect against human leukemia cell lines”. Biological Pharmaceutical Bulletin 26.4 (2003): 569-571.
- Balasubramanyam K. et al. “Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression”. The Journal of Biological Chemistry 279.32 (2004): 33716-33726.
- Chatterjee A., et al. “The bactericidal effects of Lactobacillus acidophilus, garcinol and Protykin compared to clarithromycin, on Helicobacter pylori”. Molecular & Cellular Biochemistry 243.1-2 (2003): 29-35.
- Chatterjee A., et al. “Antimicrobial effects of antioxidants with and without clarithromycin on Helicobacter pylori”. Molecular and Cellular Biochemistry 270.1-2 (2005): 125-130.
- Rukachaisirikul V., et al. “An antibacterial biphenyl derivative from Garcinia bancana MIQ”. Chemical and Pharmaceutical Bulletin (Tokyo) 53.3 (2005): 342-343.
- Iinuma M., et al. “Antibacterial activity of some Garcinia benzophenone derivatives against methicillin resistant Staphylococcus aureus”. Biological Pharmaceutical Bulletin 19.2 (1996):311-314.
- Yoshida K., et al. “Dietary garcinol inhibits 4-nitroquinoline 1-oxide-induced tongue carcinogenesis in rats”. Cancer Letters 221.1 (2005): 29-39.
- Varalakshmi KN., et al. “Antimicrobial and cytotoxic effects of Garcinia indica fruit rind extract.” American-Eurasian Journal of Agricultural & Environmental Sciences 7.2 (2010): 652-656.
- Pasha C., et al. “Antisalmonella activity of selected medicinal plants”. Turkish Journal of Biology 33 (2009): 59-64.
- Negi PS and G K Jayaprakasha “Control of foodborne pathogenic and spoilage bacteria by Garcinol and Garcinia indica extracts and their antioxidant activity”. Journal of Food Science Education 69.3 (2006): 61-65.
- Kirana Hand BP Srinivasan “Aqueous extract of Garcinia indica choisy restores glutathione in type 2 diabetic rats”. Journal of Young Pharmacists 2.3 (2010): 265-268.
- Sasaki R., et al. “Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice”. Biochemical Pharmacology 74.11 (2007): 1619-1627.
- Chen CS., et al. “Nicotine-induced human breast cancer cell proliferation attenuated by garcinol through down-regulation of the nicotinic receptor and cyclin D3 proteins”. Breast Cancer Research and Treatment 125.1 (2011): 73-87.
- Chen G., et al. “Cyanidin-3- glucoside reverses ethanol-induced inhibition of neurite outgrowth: Role oglycogen synthase kinase 3 Beta”. Neurotoxicity Research 15.4 (2009): 321-331.
- Ahmad A., et al. “Apoptosisinducing effect of garcinol is mediated by NF-kappaB signaling in breast cancer cells”. Journal of Cellular Biochemistry 109.6 (2010): 1134-1141.
- Prasad S., et al. “Garcinol Potentiates TRAIL-Induced Apoptosis through Modulation of Death Receptors and Antiapoptotic Proteins”. Molecular Cancer Therapeutics 9.4 (2010): 856-868.
- Cooke D., et al. “Effect of cyanidin-3-glucoside and an anthocyanin mixture from bilberry on adenoma development in the ApcMin mouse model of intestinal carcinogenesis— relationship with tissue anthocyanin levels”. International Journal of Cancer 119.9 (2006): 2213-2220.
- Ding M. et al. “Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity”. The Journal of Biological Chemistry 281.25 (2006):17359-17368.
- Tanaka T., et al. “Prevention of colonic aberrant crypt foci by dietary feeding of garcinol in male F344 rats”. Carcinogenesis 21.6 (2000): 1183-1189.
- Selvi, AT., et al. “Inhibition of growth and aflatoxin production in Aspergillus flavus by Garcinia indica extract and its antioxidant activity”. Food Microbiology 20.4 (2003): 455-460.
- Tsuda T., et al. “Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice.” The Journal of Nutrition 133.7 (2003): 2125-2130.
- Elisia I and Kitts DD. “Anthocyanins inhibit peroxyl radical-induced apoptosis in Caco-2 cells”. Molecular and Cellular Biochemistry 312 (1-2) (2008): 139-145.
- Xu JW., et al. “Upregulation of endothelial nitric oxide synthase by cyanidin-3-glucoside, a typical anthocyanin pigment.” Hypertension 44.2 (2004): 217-222.
- Kolodziejczyk J., et al. “Effects of garcinol and guttiferone K isolated from Garcinia cambogia on oxidative/nitrative modifications in blood platelets and plasma”. Platelets 20.7 (2009): 487-492.
- Sahasrabudhe A., et al. “Anti-hyaluronidase, anti-elastase activity of Garcinia indica.” International Journal of Botany 6.3 (2010): 299-303.
- Chen PN., et al. “Mulberry anthocyanins, cyanidin 3-rutinoside and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line”. Cancer Letters 235.2 (2006): 248-259.
- Lenta BN., et al. “Leishmanicidal and cholinesterase inhibiting activities of phenolic compounds from Allanblachia monticola and Symphonia globulifera”. Molecules 12.8 (2007): 1548-1557.
Citation:
Rahul C Ranveer and Akshya K Sahoo. “Bioactive Constituents of Kokum and its Potential Health Benefits”. Nutrition and
Food Toxicology 1.6 (2017): 236-244.
Copyright: © 2017 Rahul C Ranveer and Akshya K Sahoo. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.




































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.