Research Article
Volume 1 Issue 2 - 2017
Effect of Simulated In Vitro Digestion on Antioxidant Activity of Processed Cicer Arietinum and Amaranthus Caudatus Greens
1Research Scholar (Former), Department of Food Science and Nutrition, University of Mysore, Mysore 570 006, India
2Department of Food Science and Nutrition, University of Mysore, Mysore 570 006, India
2Department of Food Science and Nutrition, University of Mysore, Mysore 570 006, India
*Corresponding Author: Prof. Jamuna Prakash, Former Professor, Department of Food Science and Nutrition, University of Mysore,
Mysore 570 006, India.
Received: May 09, 2017; Published: May 17, 2017
Abstract
The effects of simulated in vitro digestion on antioxidant activity (AA) of food mix prepared with dehusked chickpea (Cicer arietinum) and amaranth greens (Amaranthus caudatus) were explored. Cooked mixes were stored in freezer (wet), refrigerator and at room temperature (RT) (dry) and analyzed for AA as such using three different assay techniques (pre-digestion) and after subjecting to in vitro digestion with pepsin and pancreatin enzymes (post-digestion). Total antioxidant assay revealed that stored dry mixes retained > 90% of AA, and frozen mix, 75%. The post-digestion AA retention ranged from 44-56% with frozen sample exhibiting lesser values. Reducing power and DPPH assay showed a higher retention of AA in frozen followed by refrigerated and RT samples. Retention of post-digestion AA was highest in refrigerated samples followed by RT and frozen samples. Stored and digested samples had lesser AA in comparison to their respective controls, however, despite reduction, considerable AA was still retained post-digestion indicating that processed and stored foods also have antioxidant potential.
Keywords: Legume and green mix; Total antioxidant activity; Reducing power; Free radical scavenging activity; Post-digestion antioxidant activity
Introduction
Foods as provided by nature are a source of nutrients and many bioactive components needed for normal functions and preserving health. Antioxidant components in particular are known for prevention and cure of many lifestyle disorders. During processing, some of these components are lost and at the physiological level, a part of original constituents may be available to the body after going through the process of digestion and absorption. Destruction of bioactive components may also be higher in highly processed foods as they undergo harsher treatments of temperature, or are stored for a longer time (Oghbaei and Prakash, 2013; 2017). During food preparation many changes affect food quality. The quality of food, with regard to nutrition, microbial safety and sensory aspects depends on a range of variables from farm to plate, including the quality of the raw material, processing techniques, packaging and cooking methods (Boekel., et al. 2010).
Effects of processing on macro and micro nutrients and other constituents of foods and their subsequent availability has been a subject of research for many years. Beside changes in the content of bioactive compounds, some studies have shown that various heat treatments can increase the total antioxidant capacity of different food groups (Pellegrini., et al. 2003). Sprouts of wheat, buck wheat, oats and corn showed increased antioxidant activity after thermal processing (Dewanto., et al. 2002; Kwon., et al. 2007; Randhir and Shetty, 2007). It is observed that as a result of heat exposure many new flavour compounds are formed predominantly due to Maillard reaction (Boekel, 2006). Antioxidants components and activity in foods are greatly influenced by processing techniques and qualitative and quantitative changes due to processing such as dehulling, cooking, storage, etc have been observed (Oghbaei and Prakash, 2013, 2015; 2016a; 2016b, 2017). Finally, the process of digestion modifies the antioxidant components in foods and decides their bioavailability in human system. There are no reports in literature exploring the retention of antioxidant activity in processed and stored food mixes after digestion (in vitro), which would indicate their availability for possible antioxidant function in human system. Hence, the present study aims to investigate the effect of processing (cooking and dehydration), storage duration (1-3 months) and temperature on antioxidant activity of a food mix prepared using a legume and greens and treated with digestive enzymes to mimic the human digestion system. The processing variables chosen represented the processed convenience foods available in the market.
Materials and Methods
Materials
The food materials used for the study, dehusked form of chickpea (Cicer arietinum) and fresh leaves of amaranth (Amaranthus caudatus) were purchased from a local source and cleaned before use. The chemicals and solvents used were of analytical grade. The enzymes used for the study pepsin (Batch No. 3-0060), pancreatin (Batch No. 0-0864), dialysis tubing (molecular mass cut off, 8000 Kda, D-9777, Lot 16H1545) were procured from Sigma Aldrich Co. USA. Glass double distilled water was used and all experiments were carried out in triplicate.
The food materials used for the study, dehusked form of chickpea (Cicer arietinum) and fresh leaves of amaranth (Amaranthus caudatus) were purchased from a local source and cleaned before use. The chemicals and solvents used were of analytical grade. The enzymes used for the study pepsin (Batch No. 3-0060), pancreatin (Batch No. 0-0864), dialysis tubing (molecular mass cut off, 8000 Kda, D-9777, Lot 16H1545) were procured from Sigma Aldrich Co. USA. Glass double distilled water was used and all experiments were carried out in triplicate.
Methods
Preparation of food mix
The food was prepared using chick pea (Cicer arietinum) and amaranth leaves (Amaranthus caudatus). The processing variables chosen were dehydration, storage under room temperature and under refrigeration (dry mix) and frozen storage (wet mix). These represented ready-to-eat frozen food mix or dehydrated convenience products which need reconstitution. One part of legume (100g) and two parts of greens (200g) were mixed in a glass bowl, and 100 ml water was added to facilitate cooking process. The mix was heated in a pressure cooker for 20 min. After cooking, the contents were transferred to mixer and homogenized well. The ground mass was divided into three parts, one part as such was retained for immediate analysis, second part was stored in freezer in airtight containers till further use, and last portion was dried for 24h at 40°C. The dried mix was further divided into two parts and stored in airtight jars under refrigeration and at room temperature for 105 days. All samples were stored in separate jars made with polyethylene terephthalate and drawn on day 35 (First month), 70 (Second month) and 105 (Third month) for analysis. The room, fridge and freezer temperatures were at 28, 5 and -17°C during storage period respectively. The frozen samples and refrigerated samples were thawed at room temperature before use.
Preparation of food mix
The food was prepared using chick pea (Cicer arietinum) and amaranth leaves (Amaranthus caudatus). The processing variables chosen were dehydration, storage under room temperature and under refrigeration (dry mix) and frozen storage (wet mix). These represented ready-to-eat frozen food mix or dehydrated convenience products which need reconstitution. One part of legume (100g) and two parts of greens (200g) were mixed in a glass bowl, and 100 ml water was added to facilitate cooking process. The mix was heated in a pressure cooker for 20 min. After cooking, the contents were transferred to mixer and homogenized well. The ground mass was divided into three parts, one part as such was retained for immediate analysis, second part was stored in freezer in airtight containers till further use, and last portion was dried for 24h at 40°C. The dried mix was further divided into two parts and stored in airtight jars under refrigeration and at room temperature for 105 days. All samples were stored in separate jars made with polyethylene terephthalate and drawn on day 35 (First month), 70 (Second month) and 105 (Third month) for analysis. The room, fridge and freezer temperatures were at 28, 5 and -17°C during storage period respectively. The frozen samples and refrigerated samples were thawed at room temperature before use.
Analysis
Moisture content of fresh and dry mixes was determined initially (AOAC, 2005). This was needed for the purpose of computing the antioxidant activity data on dry weight basis as moisture was a variable in samples. Antioxidant activity was determined in pre and post-digested aqueous extract of all mixes by using in vitro simulated digestion system with 3 different assays. Brief account of the methods used is given below.
Moisture content of fresh and dry mixes was determined initially (AOAC, 2005). This was needed for the purpose of computing the antioxidant activity data on dry weight basis as moisture was a variable in samples. Antioxidant activity was determined in pre and post-digested aqueous extract of all mixes by using in vitro simulated digestion system with 3 different assays. Brief account of the methods used is given below.
Estimation of antioxidant activity (AA)
Total antioxidant activity by phosphomolybdenum method-This assay is based on the reduction of Mo (VI) to Mo (V) by the sample analysate and the subsequent formation of green phosphate/Mo (V) complex at acidic pH (Prieto., et al. 1999).
Total antioxidant activity by phosphomolybdenum method-This assay is based on the reduction of Mo (VI) to Mo (V) by the sample analysate and the subsequent formation of green phosphate/Mo (V) complex at acidic pH (Prieto., et al. 1999).
Free radical scavenging activity using DPPH -DPPH, a commercial oxidizing radical is reduced by antioxidants. The disappearance of the DPPH radical absorption at a characteristic wavelength is monitored by decrease in optical density (Singh., et al. 2002).
Ferric Reducing antioxidant Power-Inthis assay, Fe3+/ferricyanide complexis reduced to the ferrous form by antioxidants. The Fe2+ formed is monitored by measuring the formation of Perl’s Prussian blue at 700 nm (Oyaizu, 1986).
Simulated in vitro digestion of food mix
Food mixes were digested with pepsin and pancreatin simulating the physiological digestion procedure. Known amount of sample was suspended in requisite buffer and enzymes were added and the mixture incubated in shaker water bath. The pH adjustments were made as required and the mixture was transferred to a semi-permeable membrane. The digested dialysate was centrifuged and the supernatant aqueous solution used for estimation of post-digestion AA (Luten., et al. 1996).
Food mixes were digested with pepsin and pancreatin simulating the physiological digestion procedure. Known amount of sample was suspended in requisite buffer and enzymes were added and the mixture incubated in shaker water bath. The pH adjustments were made as required and the mixture was transferred to a semi-permeable membrane. The digested dialysate was centrifuged and the supernatant aqueous solution used for estimation of post-digestion AA (Luten., et al. 1996).
Statistical analysis
The data was analyzed statistically to test the difference between samples if any, using Students ‘T’ test, analysis of variance, and posthoc Tukey’s test for multiple comparisons. Since moisture was a variable in samples, moisture free values were used for all statistical comparisons for validity.
The data was analyzed statistically to test the difference between samples if any, using Students ‘T’ test, analysis of variance, and posthoc Tukey’s test for multiple comparisons. Since moisture was a variable in samples, moisture free values were used for all statistical comparisons for validity.
Result and Discussion
The AA of aqueous extracts of food mix as such and in dialysate which underwent the process of digestion, was estimated by three different methods to study the effect of storage on antioxidant potential of processed chickpea and amaranth greens food mix. The analysis was done initially (0 day) and on day 35, 70 and 105 of storage. The results of the study are presented in Tables 1-2 and Figure 1-2.
Total antioxidant activity
Pre and post-digestion total antioxidant activity (TAA) in aqueous extracts of mixes and their post digestion retention are presented in Table 1. In dry form of food mix, TAA was 38156 µgmol/g and reduced to 34943 and 35312 µgmol/g after 3 months of storage in ambient and under refrigeration respectively. Both values were significantly different from 0 day value. The frozen sample also retained TAA during 1st month, after which reduction was insignificant (the moisture content of frozen food mix was high as it was fresh sample). Post-digestion TAA in dry food mix reduced continually, however, reduction was small and analysis of variance showed that the changes were significant only between 0 day and at the end of storage period. Sample stored at -17ºC showed slight reduction of TAA (1837 µgmol/g) during period of storage. Retention of AA during storage has been studied by different workers. Van der Sluis., et al. (2001) reported that cold storage/storage at controlled atmosphere conditions did not affect the AA of the apple samples. TAA in orange carrots remained relatively constant during chill storage conditions, whereas a highly significant decrease in modified atmosphere packed purple carrots was observed (Alasalvar., et al. 2005).
Pre and post-digestion total antioxidant activity (TAA) in aqueous extracts of mixes and their post digestion retention are presented in Table 1. In dry form of food mix, TAA was 38156 µgmol/g and reduced to 34943 and 35312 µgmol/g after 3 months of storage in ambient and under refrigeration respectively. Both values were significantly different from 0 day value. The frozen sample also retained TAA during 1st month, after which reduction was insignificant (the moisture content of frozen food mix was high as it was fresh sample). Post-digestion TAA in dry food mix reduced continually, however, reduction was small and analysis of variance showed that the changes were significant only between 0 day and at the end of storage period. Sample stored at -17ºC showed slight reduction of TAA (1837 µgmol/g) during period of storage. Retention of AA during storage has been studied by different workers. Van der Sluis., et al. (2001) reported that cold storage/storage at controlled atmosphere conditions did not affect the AA of the apple samples. TAA in orange carrots remained relatively constant during chill storage conditions, whereas a highly significant decrease in modified atmosphere packed purple carrots was observed (Alasalvar., et al. 2005).
Retention of TAA in post-digested samples stored at different temperatures were also expressed as percent of pre-digested activity for easier comparison and results show that differences were statistically insignificant (Table 1). Frozen sample showed slight variations between 44.1 to 53.1% during 0 day and 3 months storage. For dry mixes values ranged between 45.7-56.7% for sample stored at 5ºC and 50.9-56.3% for sample stored at 28ºC. Klimczak., et al. (2007) studied effect of storage on antioxidant components and their activity in orange juice. The decrease in the content of polyphenols and vitamin C upon storage was reflected by the decrease in the antioxidant capacity of orange juices. Small changes in flavanone content were observed, indicating its high stability upon storage.
| Storage Temperature | 0 Day (Control) |
First Month | Second Month | Third Month |
| Pre Digestion | ||||
| 28°C | 38156a ± 204 | 35760ab ± 534 | 35450ab ± 904 | 34943a ± 754 |
| 5°C | 38156a ± 204 | 36355ab ± 785 | 35844ab ± 857 | 35312b ± 441 |
| -17°C | 12352a ± 55 | 10448b ± 413 | 10000b ± 468 | 9357b ± 78 |
| Post Digestion | ||||
| 28°C | 21381a ± 1093 | 20137a ± 2529 | 19049ab ± 841 | 18040b ± 2181 |
| 5°C | 21381a ± 1093 | 20599a ± 2437 | 19835a ± 1278 | 16124b ± 1312 |
| -17°C | 5960a ± 721 | 5249a ± 306 | 5307a ± 486 | 4123a ± 201 |
| Percent Retention-post Digestion | ||||
| 28°C | 56.0a ±2.0 (A) | 56.3a ± 5.0 (A) | 54.5a ± 1.7 (A) | 50.9a ± 4.3 (A) |
| 5°C | 56.0a ± 2.0 (A) | 56.7a ± 4.7 (A) | 55.3a ± 2.5 (A) | 45.7a ± 2.6 (A) |
| -17°C | 48.2a ±4.1 (B) | 50.2a ±2.1 (A) | 53.1a ±3.4 (A) | 44.1a ±0.0 (A) |
Rows with different superscript are statistically different. Percent retention post-digestion calculated by
dividing post digestion activity by pre digestion activity and multiplying by 100. Capital letters in
parenthesis indicate inter-sample differences as influenced by temperature.
Table 1: Pre and post digestion total antioxidant activity and its retention in aqueous extracts of curry mix during storage (μmol/g)
Table 1: Pre and post digestion total antioxidant activity and its retention in aqueous extracts of curry mix during storage (μmol/g)
| Storage Duration | Pre-Digestion | Post-Digestion | ||||
| Temperature (ºC) | -17 | 5 | 28 | -17 | 5 | 28 |
| Reducing power, mg sample required for 0.500 nm activity | ||||||
| 0 day | 19.92a | 15.75a | 15.75a | 38.32a | 31.64a | 31.64a |
| 1st M | 22.13b | 16.19a | 16.56a | 43.28b | 24.92a | 27.59ab |
| 2nd M | 21.85b | 17.39a | 19.56b | 54.48c | 39.67a | 40.00ab |
| 3rd M | 21.97b | 17.79a | 21.45b | 53.05c | 31.80a | 33.18b |
| Free Radical Scavenging Activity, IC50 Values | ||||||
| 0 day | 6.65a | 6.17a | 6.17a | 22.61a | 21.37a | 21.37a |
| 1st M | 6.98ab | 7.48b | 7.80b | 31.19b | 23.34a | 24.05ab |
| 2nd M | 8.46ab | 8.74c | 9.56c | 33.00b | 23.78a | 28.49b |
| 3rd M | 9.72b | 9.78d | 10.63d | 33.11b | 25.23a | 28.82b |
*: 4th concentration used for calculation. See Figure 1 and 2.
Cells within column with different letter are statistically different.
Table 2: Inter-sample comparisons of antioxidant activity determined by reducing power assay and Free radical scavenging activity.
Cells within column with different letter are statistically different.
Table 2: Inter-sample comparisons of antioxidant activity determined by reducing power assay and Free radical scavenging activity.
When effect of temperature on percent retention of TAA following digestion was analyzed, it can be seen that during 1st, 2nd and 3rd
month, temperature did not affect percent retention but on 0 day percent retention of frozen sample was significantly lesser than dried
sample. These differences were not significant on subsequent storage.
Reducing power assay
Figure. 1 depicts the result of AA of pre and post-digested aqueous extracts of food mixes which were stored at 28, 5, -17ºC during storage. The results discussed are for highest concentration of samples used (dehydrated sample, pre and post-digestion 16 mg; fresh sample, pre and post-digestion 35.6 and 51.0 mg respectively). Important observations were as follows: In extracts of mixes stored at 28ºC, 0 day (control) and 1st month, the AA were very close with values of 0.50, 0.48 and 0.51, 0.49 nm, whereas in 2nd and 3rd month, it reduced to 0.40, 0.37 and 0.44, 0.41 nm respectively. Storage for longer time (2-3 months) significantly reduced activity of sample stored at 28ºC (refer to Table 2). The differences between lowest pre-digestion and highest post-digestion activity was 0.13 nm. This could be due to large molecular size of antioxidant components which did not move through the dialysis tube. The maximum (0.21 nm) and minimum (0.20 nm) post-digestion activity was observed after 1st and 2nd months respectively, (the differences were insignificant). Sample stored at 5ºC behaved similar to the sample stored at 28ºC, but the difference between activity of sample at higher (0 day and 1st month, 0.50 and 0.49 nm) and lower level (2nd and 3rd month, 0.46 and 0.45 nm) were lesser than portion stored at 28ºC. It showed that storage of dry food mix under refrigeration maintained better activity which could be due to retaining more antioxidant components or providing less opportunity for using them in oxidation reaction during storage.
Figure. 1 depicts the result of AA of pre and post-digested aqueous extracts of food mixes which were stored at 28, 5, -17ºC during storage. The results discussed are for highest concentration of samples used (dehydrated sample, pre and post-digestion 16 mg; fresh sample, pre and post-digestion 35.6 and 51.0 mg respectively). Important observations were as follows: In extracts of mixes stored at 28ºC, 0 day (control) and 1st month, the AA were very close with values of 0.50, 0.48 and 0.51, 0.49 nm, whereas in 2nd and 3rd month, it reduced to 0.40, 0.37 and 0.44, 0.41 nm respectively. Storage for longer time (2-3 months) significantly reduced activity of sample stored at 28ºC (refer to Table 2). The differences between lowest pre-digestion and highest post-digestion activity was 0.13 nm. This could be due to large molecular size of antioxidant components which did not move through the dialysis tube. The maximum (0.21 nm) and minimum (0.20 nm) post-digestion activity was observed after 1st and 2nd months respectively, (the differences were insignificant). Sample stored at 5ºC behaved similar to the sample stored at 28ºC, but the difference between activity of sample at higher (0 day and 1st month, 0.50 and 0.49 nm) and lower level (2nd and 3rd month, 0.46 and 0.45 nm) were lesser than portion stored at 28ºC. It showed that storage of dry food mix under refrigeration maintained better activity which could be due to retaining more antioxidant components or providing less opportunity for using them in oxidation reaction during storage.
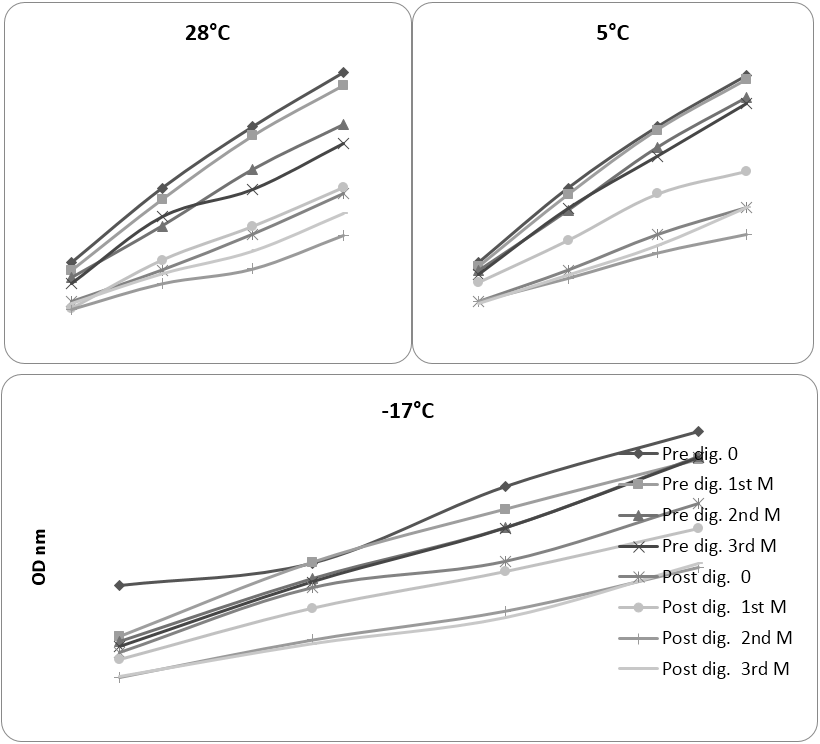
Pre and post-digestion concentration for 28 and 5ºC (mg): A: 4.0, B: 8.0, C: 12.0, D: 16.0. Pre and post digestion
concentration for -17ºC (mg): A: 8.9, B: 17.8, C: 26.7, D: 35.6 and A: 12.7, B: 25.5, C: 38.2, D: 51.0 respectively.
Figure 1: Pre and post-digestion antioxidant activity (reducing power) in curry mix stored at different temperature before and during 3 months storage.
Figure 1: Pre and post-digestion antioxidant activity (reducing power) in curry mix stored at different temperature before and during 3 months storage.
Pattern of reducing power activity in frozen mix was different from dried mixes. There was no major difference between lowest pre-digestion and highest post-digestion reducing power activity in frozen mix, while in dry mixes it was significant. For frozen portion, 0 day reducing power (0.891 nm) was significantly higher than 1st, 2nd and 3rd months of storage. Fresh 0 day and 3rd month sample exhibited maximum and minimum activity after digestion (0.665 and 0.485 nm respectively), which were significantly different. Post-digestion activity reduced continually and differences between 0 day and 1st month and between 1st month and 2nd and 3rd month were significant. Hunter and Fletcher (2002) studied effect of processing and storage on antioxidant compounds and activity (FRAP method) of spinach and peas. It was observed that frozen vegetables had similar AA equivalent to the vegetable purchased fresh from super market and much higher level compared to jarred and canned vegetables. AA decreased on storage of sample in ambient and chilled temperature with higher loss for ambient storage. To enable valid comparison between the effects of all three storage temperature together, the amount of sample in mg quantity required to obtain 0.500 nm OD for reducing power was calculated and results are presented in Table 2.
The important observations were:
- Pre and post-digestion extracts of dehydrated mixes required lesser quantity of sample to exhibit reducing power equivalent to 0.500 nm OD which indicates higher AA than fresh form.
- Comparison of pre and post-digestion at 0 day showed that approximately double the amount of samples were required for producing same activity which show significant losses during digestion procedure.
- After 3 months, pre and post-digestion reducing power activity of the sample stored at 5ºC exhibited highest activity followed by sample stored at room temperature and frozen storage.
The statistical analysis of effect of temperature on reducing power assay (Table 2) showed that, sample stored at 5ºC did not change statistically during storage study. Post-digestion activity showed slight variations. Activity in 1st month (0.32 nm) was highest and 2nd month (0.20 nm) showed least activity, however, there was no significant difference among activity during storage.
Calculating percent retention of AA (Table 3) showed that pre-digestion retention was highest at -17ºC (90%) followed by 5ºC (89%) and 28ºC (74%). The post-digestion activity retention was as follows: 5ºC (101%), 28 (87%) and -17ºC (72%) respectively. Danesi and Bordoni (2008) reported that freezing increased AA in green vegetables whereas; the activities decreased or were not affected in yellow/red vegetables. They concluded that frozen cooked green vegetables show a higher AA but cooked red/yellow vegetables after freezing showed decreased activity. Though vitamins, carotenoids and phenolic compounds act synergistically in expressing antioxidant potential, they are differently sensitive to processing.
| Condition | Reducing power | DPPH | ||||
| Temperature (ºC) | -17 | 5 | 28 | -17 | 5 | 28 |
| Pre- digestion | 74.4b ± 4.4 | 89.4ab ± 0.5 | 90.7a ± 1.7 | 58.0a ± 0.5 | 63.1a ± 1.3 | 68.9a ± 4.4 |
| Post- digestion | 87.9a ± 13.5 | 101.4a ± 13.4 | 72.2a ± 1.1 | 74.2ab ± 2.1 | 84.9a ± 4.9 | 63.5b ± 1.8 |
Values in rows with different superscript are significantly different.
Retention of activity calculated from value of 0 day and 3rd month.
Table 3: Pre and post-digestion percent retention antioxidant activity in mixes stored at different temperature after 3 months storage.
Retention of activity calculated from value of 0 day and 3rd month.
Table 3: Pre and post-digestion percent retention antioxidant activity in mixes stored at different temperature after 3 months storage.
Antioxidant activity by free radical scavenging assay (DPPH method)
The antioxidant potential was analyzed as radical scavenging activity in terms of percent DPPH inhibition. Pre and post-digestion free radical scavenging properties (FRSA) of sample stored under different temperatures are presented in Figure 2. For mixes stored in different temperature, pre-digestion FRSA was partially lost during storage, differences between 0 day and end of storage study were significant (refer Table 2 for statistics).
The antioxidant potential was analyzed as radical scavenging activity in terms of percent DPPH inhibition. Pre and post-digestion free radical scavenging properties (FRSA) of sample stored under different temperatures are presented in Figure 2. For mixes stored in different temperature, pre-digestion FRSA was partially lost during storage, differences between 0 day and end of storage study were significant (refer Table 2 for statistics).
For mixes stored at 28 and 5ºC, 0 day FRSP was in range of 25.5-81.1%, for samples with lower to higher concentrations respectively. After 3 months of storage at 28 and 5ºC, the range decreased to 11.9-47.0 and 16.3-51.1% respectively. As the concentrations between pre and post-digestion and among samples were different, IC50 values were calculated for better comparison and results are presented in Table 2. [IC50 values indicate the amount of sample required to exhibit 50% of FRSA and hence can be used for inter sample comparison in case of differing concentrations]. Comparing IC50 values between pre and post-digestion revealed that FRSA reduced markedly due to digestion. The quantity of sample stored at different temperature for producing same activity after digestion was more than 3 fold. Considering highest concentration, changes in FRSA of post-digestion extracts in mixes stored at 28 and 5ºC between start and end of storage was significant. Same trend was observed in reducing power assay.
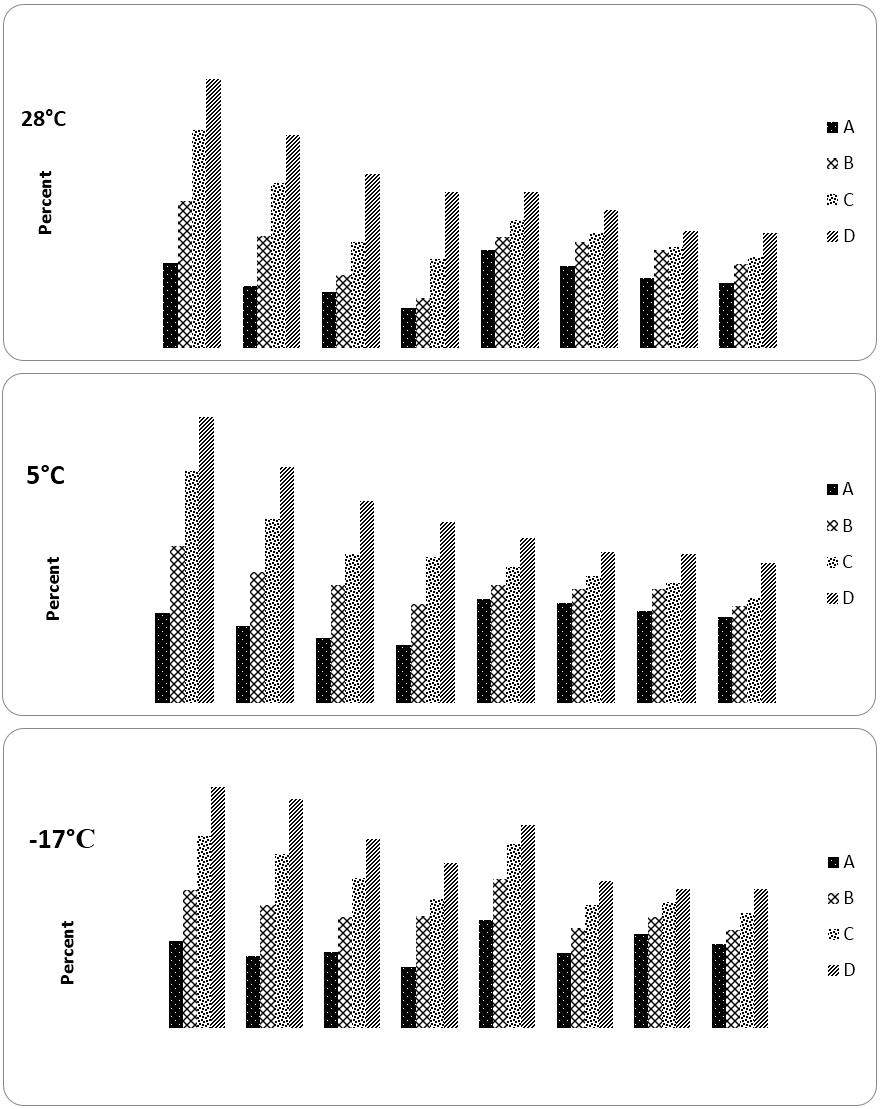
Pre and post-digestion concentration for 28 and 5 ºC (mg): A: 2.5, B: 5.0, C: 7.5, D: 10.0
and A: 12.5, B15.0, C: 17.5, D: 20.0 respectively. Pre and post digestion concentration for
-17ºC (mg): A: 6.3, B: 12.7, C: 19.1, D: 25.5 and A: 2.2, B: 4.4, C: 6.6, D: 8.9 respectively
Figure 2: Pre and post-digestion antioxidant activity (DPPH) in curry mix stored at different temperature before and during 3 months storage.
Figure 2: Pre and post-digestion antioxidant activity (DPPH) in curry mix stored at different temperature before and during 3 months storage.
The 0 day post-digestion FRSA was found to be 29.3 and after 3 months, mix stored at 28 and 5ºC showed FRSA of 19.6 and 24.2% respectively. On first day of storage pre and post-digestion IC50 of fresh mixes stored at -17ºC was 6.65 and 22.61 respectively. Pre-digestion reduction in FRSA was significant at end of 3 month storage whereas in dialysate significant reduction was observed during 1st month and differences thereafter were negligible. IC50 values for pre and post-digested aqueous extracts of curry mixes increased continually which indicates that as duration increased, FRSA decreased, hence a larger sample was needed to obtain 50% activity. A reduction in post-digestion AA has also been reported in a study with germinated green gram by authors (Oghbaei and Prakash, 2017).
Following are the salient observations regarding FRSA of mixes; (i) Pre and post-digestion FRSA was higher at 0 day than 1st, 2nd and 3rd month (lesser IC50), (ii) Fresh and dry food mix on 0 day exhibited IC50 of 6.65 and 6.17 mg for pre-digestion sample and (iii) after 3 months IC50 value of both frozen and dehydrated sample was lesser than the sample stored at 28ºC, which exhibited maximum loss of FRSA. Food mix stored at -17 and 5ºC during 1st, 2nd and 3rd month showed same pre-digestion IC50 range but the activity were higher than sample stored at ambient temperature. The dry sample stored at both 5 and 28ºC showed similar extent of activity up to 2nd months but after 3rd month the activity of sample stored at room temperature was less than refrigerated one (IC50 10.63 and 9.78 mg respectively).
Post-digestion FRSA of curry mix on 0 day was higher in dehydrated sample but the differences were negligible, as storage duration increased, the differences between IC50 of fresh and dehydrated sample increased and after 3 months the highest activity was exhibited by refrigerated sample followed by room temperature and frozen sample (IC50, 25.23, 28.82 and 33.11 mg respectively).
Percent retention of pre-digested sample FRSA, which was stored at -17, 5 and 28ºC was found to be 68.9, 63.1 and 58.0% respectively but post digestion values followed opposite trend and frozen sample exhibited least (63.5%) and refrigerated sample highest (84.9%) retention. It may be concluded that dry sample could provide better in vitro FRSP following 3 months of storage than fresh frozen one.
The effect of time and temperature on bioactive components and AA of two commercial orange juices was studied (Klimczak., et al. 2007). It was found that vitamin C and free and conjugated hydroxyl cinnamic acids were the most affected by both period and temperature of storage. After 6 months of storage at 18, 28 and 38°C the content of vitamin C decreased by 21, 31 and 81%, respectively. In the test with DPPH radical, there was a slight increase in antioxidant capacity during the first 2 months of storage at 18 and 28°C. In the same period at 38°C, the antioxidant activity decreased by 13%. The ferric reducing antioxidant power (FRAP) values also declined upon storage; but for juices stored at 28°C and 38°C, the changes were lower as compared to DPPH assay. At the end of storage at 18, 28 and 38°C, the FRAP values were reduced by 23, 34 and 57, respectively.
The antioxidant properties of polyphenols may change with respect to their oxidation state. In fact, although enzymatic and chemical oxidations of polyphenols are generally in charge for a decrease in their antioxidant properties, outcome of some studies have suggested that partially oxidized polyphenols can exhibit higher AA than the corresponding non-oxidized forms. This was attributed to the increased ability of partially oxidized polyphenols to donate a hydrogen atom from the aromatic hydroxyl group to a free unpaired electron (Nicoli., et al. 2000). Moreover, during heating, the increase of TAA values might be due to the formation of Maillard reaction products (MRPs) that possess AA.
All three methods of estimating AA, showed that mixes on 0 day, either fresh or dehydrated, exhibited higher activity than mixes stored for a month or more. Similar results were reported in an earlier study using green gram and amaranth mix by authors (Oghbaei and Prakash, 2013). It has been reported that conditions such as temperature, light, moisture, exposure to air, etc can affect antioxidant retention and stability during storage (Zerdin., et al. 2003).
Conclusions
Food mixes stored under refrigeration exhibited better AA after storage duration. Dry portion exhibited better AA than fresh frozen sample. Loss of AA in dehydrated samples were significant as determined by all assays. AA of digested sample was lesser in comparison to pre-digested samples indicating that there are losses of AA components and their activities during the process of digestion. However, considerable activity is still retained indicating that processed food mixes based on legume and curry retain their antioxidant activity during processing and storage at different temperature. These observations are important for consumers, who use processed foods frequently and derive a large part of their nutrients from processed foods.
Conflict of Interest
Authors have no conflict of interest regarding the research reported in this paper.
Authors have no conflict of interest regarding the research reported in this paper.
References
- Alasalvar C., et al. “Effect of chill storage and modified atmosphere packaging (MAP) on antioxidant activity, anthocyanins, carotenoids, phenolics and sensory quality of ready-to-eat shredded orange and purple carrots”. Food Chemistry 89.1 (2005): 69-76.
- A.O.A.C. “Official Method of Analysis,Determination of moisture, ash, protein and fat, Washington, DC, USA: “Association of Official Analytical Chemists (2005).
- Boekel V. “Formation of flavour compounds in the Maillard Reaction”. Biotechnology Advances24.2 (2006): 230-233.
- Boekel V., et al. "A review on the beneficial aspects of food processing". Molecular Nutrition and Food Research 54.9 (2010): 1215-1247.
- Danesi F and Bordoni A. “Effect of home freezing and Italian style of cooking on antioxidant activity of edible vegetables”. Journal of Food Science 73.6 (2008): 109-112.
- Dewanto V., et al. “Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity”. Journal of Agricultural Food Chemistry 50.10 (2002): 3010-3014.
- Hunter KJ and Fletcher JM. “The antioxidant activity and composition of fresh, frozen, jarred and canned vegetables”. Innovative Food Science and Emerging Technologies 3.4 (2002): 399-406.
- Klimczak I., et al. “Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices”. Journal of Food Composition and Analysis 20.3-4 (2007): 313-322.
- Kwon YI., et al. “Traditional diet of Americas for management of diabetes and hypertension”. Journal of Medicinal Foods 10 (2007): 266-275.
- Luten J., et al. “Interlaboratory trial on the determination of in vitro iron dialysability from food”. Journal of Science of Food and Agriculture 72.4 (1996): 415-424.
- Nicoli MC., et al. “Effect of enzymatic and chemical oxidation on the antioxidant capacity of catechin model systems and apple derivatives”. Journal of Agricultural Food Chemistry 48.10 (2000): 4576-4580.
- Oghbaei M and Prakash J. “Effects of processing and digestive enzymes on retention, bioaccessibility and antioxidant activity of bioactive components in food mixes based on legumes and green leaves”. Food Biosciences 4 (2013): 21-30.
- Oghbaei M and Prakash J. “Antioxidant components and their in vitro bioaccessibility in processed and stored chick pea and amaranth greens mix”. Croatian Journal of Food Technology, Biotechnology and Nutrition 10.1-2 (2015): 45-50.
- Oghbaei M and Prakash J. “Nutritional properties of Green gram germinated in mineral fortified soak water: I. Effect of dehulling on total and bioaccessible nutrients and bioactive components”. Journal of Food Science and Technology 54.4 (2017): 871-879.
- Oghbaei M and Prakash J. “Nutritional properties of Green gram germinated in mineral fortified soak water: II. Effect of cooking on total and bioaccessible nutrients and bioactive components”. Journal of Food Science and Technology 54.4 (2017): 880-889.
- Oghbaei M and Prakash J. “Effect of digestive enzymes treatment on antioxidant properties of germinated green gram fortified with minerals”. Cereal Chemistry 94.2 (2017): 284-290.
- Oyaizu M. “Studies on product of browning reaction produced from glucose amine”. Japan Journal of Nutrition 44 (1986): 307-315.
- Pellegrini N., et al. “Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays”. Journal of Nutrition 133.9 (2003): 2812-2819.
- Prieto P., et al. “Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of Vitamin E”. Analytical Biochemistry 269.2 (1999): 337-34.
- Randhir R. and Shetty K. “Mung beans processed by solid-state bioconversion improves phenolic content and functionality relevant for diabetes and ulcer management”. Innovative Food Science and Emerging Technology 8.2 (2007): 197-204.
- Singh R., et al. “Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models”. Journal of Agricultural Food Chemistry 50.1 (2002): 81-86.
- Van der Sluis AA., et al. “Activity and concentration of polyphenolic antioxidants in apple: effect of cultivar, harvest year, and storage conditions”. Journal of Agricultural Food Chemistry 49.8 (2001): 3606-3613.
- Zerdin K., et al. “The vitamin C content of orange juice packed in an oxygen scavenger material”. Food Chemistry 82.3 (2003): 387-395.
Citation:
Morteza Oghbaei and Jamuna Prakash. “Effect of Simulated In Vitro Digestion on Antioxidant Activity of Processed Cicer
Arietinum and Amaranthus Caudatus Greens”. Nutrition and Food Toxicology 1.2 (2017): 53-62.
Copyright: © 2017 Morteza Oghbaei and Jamuna Prakash. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.