Review Article
Volume 2 Issue 2 - 2018
Prognosis of Adult Critically Ill Cancer Patients admitted to adult Intensive Care Unit: A Systematic Review
1Department of Intensive Care Nursing, School of Nursing, Tehran University of Medical Sciences
2Mekelle University College of Health Science, Ayder Comprehensive Hospital, Ethiopia
3Kabul Medical University of Science, Nursing and Midwifery Faculty, Critical Nursing Care Department
2Mekelle University College of Health Science, Ayder Comprehensive Hospital, Ethiopia
3Kabul Medical University of Science, Nursing and Midwifery Faculty, Critical Nursing Care Department
*Corresponding Author: Frank Kiwanuka, Department of Intensive Care Nursing, School of Nursing, Tehran University of Medical
Sciences, Iran.
Received: July 03, 2018; Published: July 27, 2018
Abstract
Background:Regarding the nature of life threatening illnesses and adverse effects from cancer treatment, there is often pessimism about admitting critically ill cancer patients to intensive care units (ICU). The objective of this review was to determine the mortality rate and prognostic factors among critically ill cancer patients (CICP) admitted to adult Intensive care units. Such evidence could inform practice on admission of patients without excluding those who are likely to benefit and preventing futile care on the other hand for those who are unlikely to benefit.
Methods: The methodology of this review conforms to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA). Databases including: PubMed, Google Scholar, Public Library of Science and Cochrane Library were searched for studies on adult cancer patients admitted to ICU from 2007 to 2017 in peer-reviewed journals in English Language. The search was done using a combination of keywords “prognosis,” “mortality rate,” “intensive care units,” cancer patient,” and “neoplasms.” The review protocol was registered in Prospero (CRD42016980887).
Results: 51 studies were included in the final analysis with a total number of 354,133 patients. ICU mortality (ICU-MR) ranges from 3% to 84.1%. The average ICU-MR was 18% based on a total sample of 354,133 (95% CI 0.245-0.005). Patients with Hematological tumors (HM) have higher ICU-MR compared to those with solid cancers. ICU-MR in this sub population ranges from 33% to 84.1% with a weighted mean ICU-MR of 62.8% based on a sample of 62,788. ICU Mortality rates among CICPs admitted to ICU with solid cancers ranges from 21.4% to 72.7%.
Conclusion: Giving a full ICU-trail for Critically Ill Cancer Patients despite prognostic indices is supported. Following the ICU-trail, attention should be given to prompt timing on when to shift the focus of care towards palliation and symptom management. This could help in preventing futile interventions and not just prolonging life of CICPs admitted in ICU. Lastly, we recommend that more studies need to be conducted in Low and Middle income countries
Key word: Prognosis; critically ill cancer patients; Intensive care unit; critical care; Intensive care; Adult cancer patients
Introduction
Regarding the nature of life threatening illness and complications of cancer and chemotherapy, patients with cancer admitted to intensive care units (ICU) often present with negative prognosis. However, with advances in care and drug therapies, the prognosis for this sub population of patients admitted in the ICU has improved in some cancer sub groups. Various studies have examined the prognosis for various sub groups of cancer patients. Herein, we aim to explore the prognosis of cancer patients with respect to malignancy mainly focusing on studies published in the last decade.
Various studies have reported that outcomes of cancer patients admitted to ICU have improved over the last two decades [1-4, 6, 7]. However, in some cancer sub populations this might not be the case since some studies have noted contrary to former inference. Different outcomes have been reported depending on the complication warranting admission to ICU such as cardiac arrest [5]. Regarding general in hospital mortality among critically ill cancer patients, it is relatively high compared to non-cancer patients. It ranges between 40 to 92% [8]. While survival rates have improved from as low as 10% to 69% across various cancer sub populations [1-4, 7]. Comparatively, mortality in ICU among cancer patients is generally higher than in-hospital mortality. For instance: a systematic review of studies on survival among patients with solid tumor admitted to ICU reported an ICU-Mortality rate (ICU-MR) ranging from 4.5 to 85%. The review reported an overall average ICU-MR of 31.2% basing on a sample of 25,339 patients with solid tumors [8]. Various factors are associated with mortality in ICU among cancer patients. These include; median time from physiological derangement to intervention prior to ICU admission, severity of the illness, Eastern Cooperative Oncology Group performance status, hematological malignancies, stem- cell transplantation, presence of abnormal variables, presence of infection, need for mechanical ventilation, vassopressors and low PaO2/ FiO2 ratios [7,8].
Noteworthy, differences exist in ICU-MR among cancer sub-group depending on the reason warranting admission to ICU. For instance: cancer patients who are admitted as medical, as opposed to surgical admissions have increased risk of ICU mortality of between two- and fourfold [8]. Going further, difference still exists in surgical cancer patients. For instance: emergency surgery and planned surgeries [9]. Regarding this last point, it would be important to emphasize that some scholars [10] wonder about the admission in intensive care for postoperative monitoring of a programmed surgical procedure. This may not be true for mainly thoracic and some abdominal oncology surgery (pancreas, etc.) The major predictors of poor prognosis include; sepsis, organ failure, need for mechanical ventilation and vasopressors [8,9,11]. Sepsis increases ICU mortality by fivefold. Mechanical ventilation increases ICU mortality by around six fold. Use of vasopressors increases the risk of both ICU mortality and in hospital mortality rates [8].
Acute respiratory failure (ARF) is the leading complication rendering ICU admission in patients with cancer [4,6,12] especially those with hematological cancers. There is a high mortality rate due to ARF in the sub group of Hematological cancer patients [6,13,14]. This has been attributed to co-morbidities such as severe pulmonary dysfunction and nosocomial infections such as ventilator-associated pneumonia [15]. Mortality due to ARF has been estimated at 31.1% at day 28. Independent predictors of mortality due ARF include age, more than one line of chemotherapy, time between respiratory symptoms onset and ICU admission > 2 days, oxygen flow at admission and extra-respiratory symptoms [12].
Most ICUs have developed protocols for admitting critically ill patients. However often times the decision to admit patients is based on decisions between the informal care givers and the primary care physician. Prompt admission to ICU has been associated with better outcomes [16].
Closing doors of ICUs to CICPs should not be a way to avoid providing non-beneficial care. Whilst widely opening the doors of ICUs to critically ill cancer patients should not ultimately result in making more decisions of withholding and withdrawing life support. Evidence on prognosis of CICPs admitted to ICU could guide in triaging CICPs who could benefit from ICU admission. This formed the basis of this review. This review aims to provide evidence for genuine decision-making process so as to maintain or even improve outcomes in these patients without only prolonging the dying process.
Methods
Review Questions
This review utilized the PICO acronym; the population is adult cancer patients (P), the intervention (I) is admission to ICU, the comparative (C) is difference in ICU-MR across tumor sub groups and the outcome (O) is mortality rate in ICU. The following questions guided the review.
This review utilized the PICO acronym; the population is adult cancer patients (P), the intervention (I) is admission to ICU, the comparative (C) is difference in ICU-MR across tumor sub groups and the outcome (O) is mortality rate in ICU. The following questions guided the review.
- What is the mortality rate of critically ill cancer patients admitted to the intensive care unit?
- What factors are associated with ICU-MR among CICPs admitted to intensive care units?
Several approaches have been developed to conduct systematic reviews on a broader range of questions. We conducted this review according to the reporting guidelines by PRISMA.[17]
Scoping search: To ensure that there are no systematic literature reviews already available or in progress that have already addressed the review question, a scoping search was done on the Center for Reviews and Dissemination (CRD), Cochrane Central and Library, TRIP Database and the Campbell Collaboration. No review in recent years addressed the review question. The review protocol was registered in the Center for Reviews and Dissemination (ID: CRD42016980887).
Eligibility criteria
Type of studies: We included studies that assessed prognosis in adult cancer patients that were admitted to the ICU. We also included studies that evaluated outcomes of sub populations of cancer patients. We included studies that were published in English language, published in peer reviewed journals and were conducted in the last 10 years (Up to 04.11.2017). Initial search dates for the literature were January 1, 2007 through December 26, 2017. We excluded studies that were conducted in the ICU on cancer that did not report on outcomes of ICU admission at least in the next three after discharge.
Type of studies: We included studies that assessed prognosis in adult cancer patients that were admitted to the ICU. We also included studies that evaluated outcomes of sub populations of cancer patients. We included studies that were published in English language, published in peer reviewed journals and were conducted in the last 10 years (Up to 04.11.2017). Initial search dates for the literature were January 1, 2007 through December 26, 2017. We excluded studies that were conducted in the ICU on cancer that did not report on outcomes of ICU admission at least in the next three after discharge.
Types of participants:AdultCancer patients and any sub-type of cancer for example hematological malignancies, solid tumor cancers etc.
Type of prognostic outcomes: Studies that reported on mortality due to any cause or survival were included. We exclude studies that did not report at least on the criteria defined above.
Data sources
Databases: We searched the Cochrane database, Pubmed, Google Scholar and Public Library of Science (PLOS). We also scanned references of included pertinent studies for additional studies (Table 1).
Databases: We searched the Cochrane database, Pubmed, Google Scholar and Public Library of Science (PLOS). We also scanned references of included pertinent studies for additional studies (Table 1).
Search:Literature search was done using combinations of medical sub-heading (MEsH) which included: prognosis, outcomes, neoplasms, cancer, malignancy, intensive care, intensive care unit, critical care, intensive care and ICU with a time limit of ten years (Table 1).
| Database | Search terms | Limits | Number of Matches |
| Pubmed 04.11.2017 |
("Neoplasms"[Mesh] and "Prognosis"[Majr]) and "Intensive Care Units"[Mesh] and ("2007/01/01"[PDAT]: "2017/12/31"[PDAT]) | Years 2007-2017 | 8 |
| Public Library of Science (PLoS) 04.11.2017 |
everything:"prognosis of critically ill cancer patients admitted to Intensive care unit" | Years 2007-2017 | 0 |
| Google scholar 04.11.2017 |
Advanced search Articles with all of the word: “prognosis of critically ill cancer patients admitted to intensive care unit” |
Years 2007-2017 search by relevance Include “patients and citations’ where my words occur: “in the title of the article” |
18,000 pages |
| Cochrane Library 04.11.2017 |
MeSH Terms “Prognosis” “Cancer Patients” “Intensive Care Unit” | Years 2007-2017 | 5 |
Table 1: Description of the database search.
Data collection process and analysis
Selection of studies: The three authors independently inspected the abstract of each identified reference and obtained the full text of relevant articles were applicable. They independently reviewed the articles and applied the inclusion criteria. We documented the justification for exclusion of studies from the review. We named studies by first author and year of publication (with the addition of a, and b for different studies from the same author and year). All authors were responsible for excluding duplicates and studies based on publication and language limitation. Where there was doubt about inclusion of a certain study, the authors discussed the study and reached consensus. Selected studies were categorized into two categories; the first categories of studies were those that meet eligibility criteria. These were kept in the spreadsheet while the other categories of studies excluded were kept in a different spread sheet for future narrative review.
Selection of studies: The three authors independently inspected the abstract of each identified reference and obtained the full text of relevant articles were applicable. They independently reviewed the articles and applied the inclusion criteria. We documented the justification for exclusion of studies from the review. We named studies by first author and year of publication (with the addition of a, and b for different studies from the same author and year). All authors were responsible for excluding duplicates and studies based on publication and language limitation. Where there was doubt about inclusion of a certain study, the authors discussed the study and reached consensus. Selected studies were categorized into two categories; the first categories of studies were those that meet eligibility criteria. These were kept in the spreadsheet while the other categories of studies excluded were kept in a different spread sheet for future narrative review.
Data extraction and management: Data was extracted into a piloted data-extraction spreadsheet. For each study, we recorded the sample size and dichotomized prognostic measures into mortality rate and prognostic factors. Data extracted included mortality, survival rates, and prognostic factors for each study that was included in the study.
Data items: The primary outcomes were mortality rate from any cause and prognostic factors.
For included studies we assessed the following
Incomplete outcome data: We recorded the number of participants enrolled in the study and number of participants evaluated at the end of the study.
Incomplete outcome data: We recorded the number of participants enrolled in the study and number of participants evaluated at the end of the study.
Quality assessment: Critical appraisal of individual studies was done by assessing whether the article fulfilled the criteria for inclusion, language limitation, was an empirical study and published in peer-reviewed journal. The commonest reasons for rejection were: studies having focus on other aspects other than the primary outcomes and the study not including adult cancer patients. Methodological rigor was appraised in collaborative discussions among the authors to ensure inclusion criteria consistency. Critical evaluation of methodological rigor in individual studies was done by assessing whether the study methodology, data collection and data analysis were explicitly performed.
Summary measures:The principal summary measures were the study setting, samples size and recruitment measure. Due to of study designs included, intensive care unit mortality rates were considered in this review.
Risk of bias across studies:The prognostic factors that were considered in this review were those obtained in studies after adjusting for confounders. Therefore the study highlighted independent prognostic factors obtained after multivariate regression analysis. In addition, the included studies were of a variety of different study designs therefore to assess the risk of reporting bias, we constructed a funnel plot to assess the effect of small sample sizes on the main outcomes.
Synthesis of results:Synthesis of aggregate data was done through tabulation of key aspects of the included studies, and narrative description of characteristics across studies. Meta-analysis is unlikely to be appropriate, due bias was assessed according to generic concepts of selection, performance, attrition and detection biases appropriate to each study design. Selection bias was assessed according to the sampling method.
Additional analysis of sub groups:Due to the heterogeneity of cancer sub populations, sub groups were categorized according to the malignancy sub types. As a result we we developed appropriate subgroups after the first scan of the full text of selected studies.
Results
Study characteristics
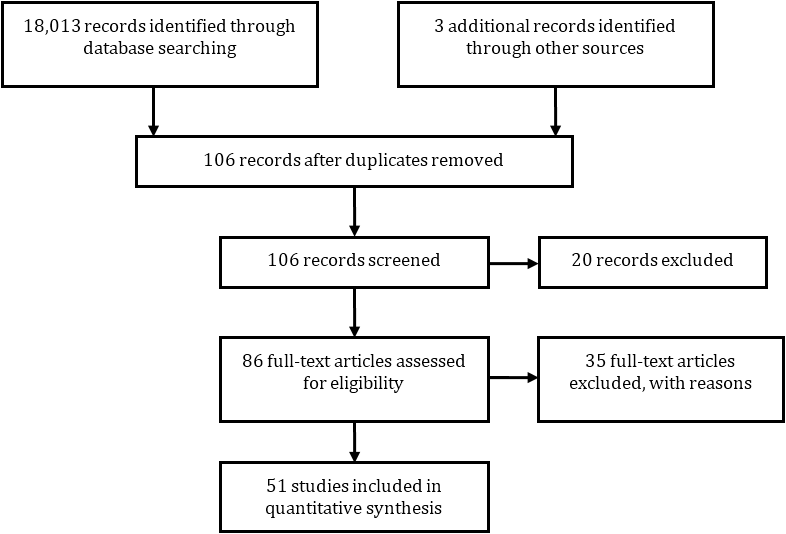
Fifty one studies were included in the analysis after removing the duplicates and studies which did not meet inclusion criteria (Figure 1). All studies included in the analysis were quantitative in nature [2-7, 9, 11-16, 18-20, and 22-64]. Studies fell into two categories: those that reported a case mix of all cancers, and those that reported on specific patient subpopulations.
Fifty one studies were included in the analysis after removing the duplicates and studies which did not meet inclusion criteria (Figure 1). All studies included in the analysis were quantitative in nature [2-7, 9, 11-16, 18-20, and 22-64]. Studies fell into two categories: those that reported a case mix of all cancers, and those that reported on specific patient subpopulations.
Twenty two studies included involved mixed cancer patients while 29 studies were on specific tumor type or tumor types. Most studies reported on a case mix of cancer sites and where cancer site-specific outcomes were reported, Studies on cancer patients with HM were the commonest homogeneous studies. Fifteen studies involved only patients with Hematological malignancies, five studies involved patients with only solid tumors, seven studies involve patients with only lung cancer while only one study accessed only gynecological tumors [15].
Two studies involved international intensive care units [26,51]. The study of Azoulay was conducted in France and Belgium while that of Soares [9] was a multinational cohort study with ICUs in Europe and South America. With respect to information or measurement biases, it is possible that studies misclassified whether or not patients had cancer, or misclassified the site of the primary tumor. However, because no additional sources of information were available to validate the classification, we were unable to evaluate the extent, if any, of such biases. As all studies reported total and not cause-specific mortality, misclassification of outcomes is unlikely, although the timing of death may have been incorrect.
Across studies outcome measurement bias exists since there could have been misrepresented in studies that only included patients with specific interventions such as Acute Respiratory Distress syndrome and Acute Kidney injury requiring specific interventions such as Invasive mechanical ventilation and dialysis. The total number of the patients was 354,133. The study of Monique [60] had the highest number of patients with 251,748 participants while Park’s study had the least number of participants [46]. Patients comprised of age from 30 years to 80 years, and a mixture of both genders.
Overall ICU mortality rates
ICU mortality was reported in 51 studies, ranging from 3% to 84.1%. The average ICU-MR was 18% based on a total sample of 354,133 (95% CI 0.245-0.005). Patients with Hematological tumors have the highest intensive care unit mortality rates (ICU-MR). ICU-MR in this sub population ranges from 33% 5 to 84.1%. The weighted average ICU-MR was 62.8% based on a sample of 62,788.
ICU mortality was reported in 51 studies, ranging from 3% to 84.1%. The average ICU-MR was 18% based on a total sample of 354,133 (95% CI 0.245-0.005). Patients with Hematological tumors have the highest intensive care unit mortality rates (ICU-MR). ICU-MR in this sub population ranges from 33% 5 to 84.1%. The weighted average ICU-MR was 62.8% based on a sample of 62,788.
Three studies by Monique [60], Bos [22] and Slatore [50] study contributed more than half of this sample. Bos and Monique’s studies had a case mix of all cancer patients while Slatore’s study comprised of only cancer patients with Hematological malignancies. Among HM, ICU-MR in patients with Acute Leukemia is 67% [55]. Among patients with AML, ICU-MR ranges between 52% [38] to 55% [49]. The average mortality of patients with Myeloma is 40% [47]. The weighted mean ICU-MR among patients with AML is 54.4% out of a sample of 515.
ICU-MR among patients with only solid tumor subpopulation was reported in five studies [30,39,44 & 45]. Mortality rates among solid tumor patient sub-population ranges from 21.4% [44] to 72.7% [30]. The weighted mean ICU-MR in this patient sub group is 35.9% out of a sample of 3,561 patients. This is lower than that among CICPs with Hematological malignancies. This finding is similar to that reported in a review of Puxty [8]. However; the samples used to compute the mean mortality rate in this study are less skewed than those of Puxty.
ICU-MR in the sub-population of lung tumors ranges from 22% to 58.6% with a weighted mean ICU-MR OF 35.5% out of a sample of 2,982. Soubani’s review of cancer patients with lung cancer reported similar findings [20]. Regarding gynecological malignancies, ICU-MR in this subpopulation was 17.3% however it was assessed in only one study.
Predictors of mortality in ICU
Due to scanty information we were not certain about validity mortality rates reported in the studies. Independent prognostic factors were considered to cater for confounders (Table 2).
Due to scanty information we were not certain about validity mortality rates reported in the studies. Independent prognostic factors were considered to cater for confounders (Table 2).
| Patient population | Independent Predictors of ICU mortality | Mortality rates |
| Solid tumors | Advanced refractory cancer Poor baseline Eastern Cooperative Oncology Group performance status Physiological status scores Poor ECOG-PS Metastatic disease Poor APACHE II Scores Thrombocytopenia |
Range 21.4%-72.7% Weighted mean MR 36.7% out of a sample of 3561 |
| Lung cancer sub group | Logistic organ dysfunction Need for Invasive Mechanical Ventilation Multiple organ failure Need for vasopressors Poor APACHE II scores, ECOG-PS & mSOFA score Presence of cancer-related complications Cancer remission Thrombocytopenia Renal failure Respiratory conditions |
Range 22%-58.6% Weighted mean MR 35.9% out of a sample of 2982 |
| Hematological malignancies | Time between onset of symptoms and ICU admission Logistic Organ Dysfunction Poor functional status Advanced refractory cancer Poor baseline Eastern Cooperative Oncology Group performance status Multiple organ failure Need for vasopressors Length of hospital stay prior to ICU admission Acute Leukemia A cumulative positive fluid balance higher than 1100 ml/24 h |
Range 33%- 84.1% Weighted mean MR 62.8% out of a sample of 62788 |
| AML sub group | Poor SAPS II Scores Direct admission to the ICU of patients with high-risk AML |
Range 55% to 52% Weighted mean MR 54.4% out of a sample of 515 |
Table 2: Predictors of ICU-Mortality and mortality rate among different cancer sub groups.
HM: Hematological Malignancies, ST: Solid Tumors, Allo-HSCT: Allo- Hematopoietic stem cell transplant; AML: Acute myelogenous leukemia; ALL: Acute lymphocytic leukemia; APACHE Acute Physiology and Chronic Health Evaluation; APS: Acute Physiology Score. SAPS: Simplified Acute Physiology Score; SOFA: Sequential Organ Failure Assessment; mSOFA,-modified Sequential Organ Failure Assessment; MV: Mechanical Ventilation; ECOG-PS: Eastern Cooperative Oncology performance scale- Eastern Cooperative Oncology Group performance status
HM: Hematological Malignancies, ST: Solid Tumors, Allo-HSCT: Allo- Hematopoietic stem cell transplant; AML: Acute myelogenous leukemia; ALL: Acute lymphocytic leukemia; APACHE Acute Physiology and Chronic Health Evaluation; APS: Acute Physiology Score. SAPS: Simplified Acute Physiology Score; SOFA: Sequential Organ Failure Assessment; mSOFA,-modified Sequential Organ Failure Assessment; MV: Mechanical Ventilation; ECOG-PS: Eastern Cooperative Oncology performance scale- Eastern Cooperative Oncology Group performance status
Hematological patients: Studies have reported various independent predictors of ICU-MR in this patient sub population. Mortality related factors include: need for IMV, poor functional status, multiple organ failure, need for vasopressors, poor APACHE scores, time to admission in ICU, admission due to an event related to myeloma progress, mSOFA scores, sepsis, acute leukemia, direct admission of patients with high risk AML with physiological disturbances, raised serum urea at time of admission and patients who underwent RI Allo-HSCT have high mortality rates in ICU. Predictors of ICU mortality among patients with acute myeloid Leukemia include poor SAPS II score and direct admission of patients with high risk AML (Table 2). Predictors of ICU mortality among patients with Acute Leukemia include poor APACHE score and type of therapy (BMT preparative regimen).
Solid tumors: Predictors of mortality risk in this patient category include: need for vassopressors, metastatic disease [38], Poor ECOG-PS [30,35], poor APACHE II Scores [44] and Thrombocytopenia [29]. ICU-MR in the patient sub category of Lung tumors is predicted by poor LOD score, need for invasive mechanical ventilation, multiple organ failure, need for vassopressors, Poor ECOG-P, poor mSOFA scores, Sepsis, Metastasis, presence of cancer related complications, cancer remission, thromcytopenia, renal failure and hypoalbuminemia (Table 2). With respect to gynecological malignancies, independent predictors of ICU-MR include need for vassopressors and APACHE II Score and sepsis [15].
Discussion
This review describes literature from various studies that reported on mortality and prognostic factors among adult cancer patients admitted to intensive care units. The studies that reported on patients with solid tumors were characterized by a case mix of different tumor types. Given the paucity of the published studies, we did not do further scrutiny of studies since this could even be less informative. However, even with these limitations, we were able to include as many credible studies as possible.
Globally, as the population continues to live longer and increased exposure to risk factors of cancer in some countries, the number of cancer cases is expected to increase. Proportionally, the demand for ICUs is also expected to increase. This signifies the need for information to guide ICU admission policies. These are expected to be drawn largely from evidence. Our review adds to existing literature. It draws evidence from a study mix from international studies and highlights the ways in which this information on ICU-MR could be use to guide interventions.
Intensive care requires knowledgeable clinicians on basic pathology, diagnosis and therapy of cancer problems in a collaborative manner [66]. In addition, they must also be knowledgeable of the ICU-MR and prognostic factors. In this review, the ICU-MR ranged from 3% to 84.1% across studies. The wide difference in mortality could be attributed the difference in mortality rate between patients suffering from hematological malignancies and those of solid tumors. Indeed, patients with hematological cancers have significantly higher mortality rate than those with solid tumors. The difference in mortality rate is attributable to various factors. For instance: patients suffering from solid tumors are often admitted in ICU following planned surgery, which is rare among those with hematological malignancies [9]. Secondly, neutropenia is a common reason for admission in ICU. In this regard, few patients with solid tumors are neutropenic or transient and shallow [68] which significantly lowers their rate of admission and hence have lower mortality in ICU compared to their counterparts suffering from HM. Thirdly, there is significant difference in the prevalence of kidney failure and other forms of organ failure across cancer patients subpopulations [69,70].
Our findings are congruent with those reported in a review by Puxty [8] three years ago. The average ICU mortality rate was 18% (95% CI 0.245-0.005). This is lower than that reported in previous review of 31.2% [21]. This signifies the fact that cancer patients should be considered for ICU admission. Previous reviews have also recommended the admission of CICPs to ICU and improved survival in this patient sub-population [66]. However, the review of Darmon [66] mainly utilized traditional narrative approaches written by experts, which normally present overviews and are liable to haphazard and biased conclusions. Since our systematic review included a comprehensive search strategy, appraisal and synthesis of research evidence, it can be used as shortcuts in the evidence-based process.
This review adds information on the ICU mortality and prognostic factors among CICPs admitted in ICU. The ICU-MR is lower than previously reported, suggesting that ICU admission of CICPs should not entirely be based on multifactorial derangements in this ICU patient sub group. However, giving an ICU-trail for CICPs with poor prognostic indices is supported as suggested in earlier studies [3].
Following the ICU-trail, attention should be given to prompt timing on when to shift focus of care towards palliation and symptom management. This will help in preventing futile interventions and not just prolonging life in CICPs admitted in ICU. Specifically, while based on limited evidence, we recommend considering palliative care in critically ill patients with advance stage solid tumors. This is based on the fact that as recently demonstrated by Zerbib and colleagues [71], chemotherapy in patients suffering from solid cancers in ICU often gives disappointing results compared to short, medium and long term benefits in those suffering hematological malignancies with exception of those suffering from multiple organ failure.
Studies reported on various factors associated with ICU mortality. Need for, and duration of invasive mechanical ventilation is associated with ICU mortality across studies. These were the commonest predictors of ICU mortality across studies. Poor physiological scores across different tools such as; APACHE II, mSOFA and SAPS has been associated with ICU mortality [8,11,35], however different studies report on different tools. Organ failure with at least more than two failing organs is associated with mortality across studies and this had the strongest effect after need for mechanical ventilation.
Only one homogeneous study conducted in Mexico has reported on outcomes of cancer patients with gynecological malignancies [15], with an ICU-MR of 17.3%. Predictors of ICU-MR included need for vassopressors and APACHE II Score. This finding may not be generalizable to all CICPs in this sub group from other settings. We recommend more research in this patient sub group to build on existing literature.
Predictors of mortality in ICU mortality remain consistent over the last decade despite advancement in technology and therapy. However, the decision to admit CICPs shouldn’t be solely based on these factors. We recommend additional studies focusing on guidance on when to shift focus of care towards palliation and symptom control. Metastatic disease has been reported in previous studies as a predictor of ICU mortality [8, 54]. However, inconsistencies exist across studies with regards to association of metastatic disease and ICU mortality. This could be attributed to the intensity of care in ICU given to CICPs with this index. Patients with hematological malignancies have been reported to have the highest ICU mortality across studies. Studies have reported various predictors of ICU-MR in this patient sub population such as: need for IMV, poor functional status, multiple organ failure, need for vasopressors, poor APACHE scores, time to admission in ICU, admission due to an event related to myeloma progress, mSOFA scores, sepsis, acute leukemia, direct admission of patients with high risk AML with physiological disturbances, raised serum urea at time of admission and patients who underwent RI Allo-HSCT have high mortality rates in ICU.
However, differences exist with regards to characteristics of CICPs admitted to ICU and this could affect the conclusion of generalizing factors to the entire cancer population. For instance in the study of Yeo involving a case mix of patients with HM, acute leukemia increases the ICU-MR by 34% [20]. Indeed, this is also reflected in the study of Thakkar [54] involving only patients with acute Leukemia. The difference in mortality and associated prognostic factors is expected with varying characteristics of ICU and characteristics of patients admitted to ICU. Therefore caution should be taken when making decisions in situations where cancer types and ICU expertise differs.
Even though there are limited RCTs in this area, questions on prognosis of CICPs admitted in ICU can be answered by meta-analysis and systematic reviews. This formed the basis of this review. Future studies should focus on prognostication of survival in ICU on specific clearly defined cancer sub groups. Future research should also focus on comparison of both cancer and none cancer patients admitted to ICU. This will allow justified conclusions on specific exposure to poor ICU prognosis associated with having cancer.
This is the first systematic review in last decade to report specifically on ICU mortality of cancer patients admitted to ICU. The review protocol of this review followed guidelines outlined in the PRISMA guideline for systematic reviews [17]. Therefore the findings of this study are methodologically reproducible unlike narrative reviews.
This review adds to existing literature which can guide ICU practitioners by providing condensed literature from a span of original research finding. In so doing it provides pooled information on ICU mortality while highlighting prognostic factors on specific tumors.
Limitation of the study
Owing to the case mix of the study participants and settings included in this study, therefore a large difference exists in the characteristics of study populations included in this paper. This predisposes this review to confounding bias since different patient characteristics have different effects on mortality for example the type of complication rendering ICU admission could have varying outcomes. For Instance; cancer patients admitted to ICU following cardiac arrest had a high mortality of 74% [2] compared to those who had undergone surgery. In addition, there are significant variations in the sample sizes used in the studies. This makes the precision of the findings of the studies included wide and making the level of confidence narrow. Lastly, most studies included in our review are from developed countries with a few studies from Brazil, as such the reported data cannot be extrapolated to low and middle income countries which in fact form the majority globally.
Owing to the case mix of the study participants and settings included in this study, therefore a large difference exists in the characteristics of study populations included in this paper. This predisposes this review to confounding bias since different patient characteristics have different effects on mortality for example the type of complication rendering ICU admission could have varying outcomes. For Instance; cancer patients admitted to ICU following cardiac arrest had a high mortality of 74% [2] compared to those who had undergone surgery. In addition, there are significant variations in the sample sizes used in the studies. This makes the precision of the findings of the studies included wide and making the level of confidence narrow. Lastly, most studies included in our review are from developed countries with a few studies from Brazil, as such the reported data cannot be extrapolated to low and middle income countries which in fact form the majority globally.
Conclusion and implication of key findings
Survival of cancer patients could have improved over the last decade. Owing to the global increase in exposure to cancer risk factors, aging population and increase in industrialization especially in developing countries; the number of cancer patients is expected to increase hence the increase in demand for ICU. Therefore, information that guides ICU practitioners is of outmost important. This information is expected to guide admission policy in ICU which is provided for in this review. Regarding allo-BMT recipients, more progress still needs to be made to define who may benefit from admission to ICU. Regarding, treatment cessation decisions, or treatment limitations, these should be made considering the cancer subgroup as there seems to be significant difference between patients suffering solid cancers and hematological patients admitted to ICU.
Future studies in this arena would be improved by detailing additional variables such as type of complication rendering ICU admission, clearly defined tumor types, comparisons of both cancer and non-cancer patients admitted to ICU and ICU characteristics. These comparisons will produce more generalizable finding and comparative evaluation of outcomes.
References
- Azoulay E., et al. “Improved survival in cancer patients requiring mechanical ventilatory support: impact of noninvasive mechanical ventilatory support”. Critical Care Medicine 29.3 (2001): 519-525.
- Azoulay E., et al. “Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium-a groupe de recherche respiratoire en reanimation onco-hematologique study”. Journal of Clinical Oncology 31.22 (2013): 2810-2818.
- Benoit DD., et al. “Has survival increased in cancer patients admitted to the ICU? We are not sure”. Intensive Care Medicine 40.10 (2014): 1576-1579.
- Bird GT., et al. “Outcomes and prognostic factors in patients with haematological malignancy admitted to a specialist cancer intensive care unit: a 5 yr study”. British Journal of Anaesthesia 108.3 (2012): 452-459.
- Champigneulle B., et al. “What is the outcome of cancer patients admitted to the ICU after cardiac arrest? Results from a multicenter study”. Resuscitation 92 (2015): 38-44.
- Geerse DA., et al. “Prognosis of patients with haematological malignancies admitted to the intensive care unit: Sequential Organ Failure Assessment (SOFA) trend is a powerful predictor of mortality”. European Journal of Internal Medicine 22.1 (2011): 57-61.
- Song JU., et al. “Early intervention on the outcomes in critically ill cancer patients admitted to intensive care units”. Intensive Care Medicine 38.9 (2012): 1505-1513.
- Puxty K., et al. “Survival in solid cancer patients following intensive care unit admission”. Intensive Care Medicine 40.10 (2014): 1409-1428.
- Soares M., et al. “Characteristics and outcomes of patients with cancer requiring admission to intensive care units: a prospective multicenter study”. Critical Care Medicine 38.1 (2010): 9-15.
- Gillies MA., et al. “Current research priorities in perioperative intensive care medicine”. Intensive care medicine 43.9 (2017): 1173-1186.
- Sawicka W., et al. “The effectiveness of the APACHE II, SAPS II and SOFA prognostic scoring systems in patients with haematological malignancies in the intensive care unit”. Anaesthesiology Intensive Therapy 46.3 (2014): 165-169.
- Cheng Q., et al. “The prognostic factors for patients with hematological malignancies admitted to the intensive care unit”. Springerplus 5.1 (2016): 2038.
- Mokart D., et al. “Critically ill cancer patients in the intensive care unit: short-term outcome and 1-year mortality”. Acta Anaesthesiologica Scandinavica 56.2 (2012): 178-189.
- Turkoglu M., et al. “Acinetobacter baumannii infection in patients with hematologic malignancies in intensive care unit: risk factors and impact on mortality”. Journal of Critical Care 26.5 (2011): 460-467.
- Namendys-Silva SA., et al. “Outcomes of critically ill gynecological cancer patients admitted to intensive care unit”. American Journal of Hospice and Palliative Medicine 30.1 (2013): 7-11.
- Kwak YG., et al. “Risk factors for device-associated infection related to organisational characteristics of intensive care units: findings from the Korean Nosocomial Infections Surveillance System”. Journal of Hospital Infection 75.3 (2010): 195-199.
- Moher D. “Guidelines for reporting health care research: advancing the clarity and transparency of scientific reporting”. Canadian Journal of Anesthesia 56.2 (2009): 96-101.
- Mokart D., et al. “Delayed intensive care unit admission is associated with increased mortality in patients with cancer with acute respiratory failure”. Leuk Lymphoma 54.8 (2013): 1724-1729.
- Adam AK and Soubani AO. “Outcome and prognostic factors of lung cancer patients admitted to the medical intensive care unit”. European Respiratory Journal 31.1 (2008): 47-53.
- Yeo CD., et al. “Prognostic factors in critically ill patients with hematologic malignancies admitted to the intensive care unit”. Journal of Critical Care 27.6 (2012): e1-6.
- Soubani AO and Ruckdeschel JC. “The Outcome of Medical Intensive Care for Lung Cancer Patients The Case for Optimism”. Journal of Thoracic Oncology 6.3 (2011): 633-638.
- Bos MM., et al. “Outcomes of cancer patients after unplanned admission to general intensive care units”. Acta Oncologica 51.7 (2012): 897-905.
- Andréjak C., et al. “Admission of advanced lung cancer patients to intensive care unit: a retrospective study of 76 patients”. BMC cancer 11.1 (2011): 159.
- Anisoglou S., et al. “Outcome of lung cancer patients admitted to the intensive care unit with acute respiratory failure”. Hippokratia 17.1 (2013): 60-63.
- Aygencel G., et al. “Prognostic factors in critically ill cancer patients admitted to the intensive care unit”. Journal of critical care 29.4 (2014): 618-626.
- Azoulay E., et al. “Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium-a groupe de recherche respiratoire en réanimation onco-hématologique study”. Journal of Clinical Oncology 31.22 (2013): 2810-2818.
- Bonomi MR., et al. “Outcomes of elderly patients with stage IIIB–IV non-small cell lung cancer admitted to the intensive care unit”. Lung Cancer 77.3 (2012): 600-604.
- Caruso P., et al. “Short‐and long‐term survival of patients with metastatic solid cancer admitted to the intensive care unit: prognostic factors”. European Journal of Cancer Care 19.2 (2010): 260-266.
- Cherif H., et al. “Predictors of short and long-term outcome in patients with hematological disorders admitted to the intensive care unit for a life-threatening complication”. Supportive Care in Cancer 15.12 (2007): 1393-1398.
- Christodoulou C., et al. “Performance status (PS): a simple predictor of short-term outcome of cancer patients with solid tumors admitted to the intensive care unit (ICU)”. Anticancer Research 27.4C (2007): 2945-2948.
- Fu S., et al. “Outcome analyses after the first admission to an intensive care unit in patients with advanced cancer referred to a phase I clinical trials program”. Journal of Clinical Oncology 29.26 (2011): 3547-3552.
- Gristina GR., et al. “Noninvasive versus invasive ventilation for acute respiratory failure in patients with hematologic malignancies: a 5-year multicenter observational survey”. Critical Care Medicine 39.10 (2011): 2232-2239.
- Hampshire PA., et al. “Admission factors associated with hospital mortality in patients with haematological malignancy admitted to UK adult, general critical care units: a secondary analysis of the ICNARC Case Mix Programme Database”. Critic care 13.4 (2009): R137.
- Hawari FI., et al. “The effect of implementing high-intensity intensive care unit staffing model on outcome of critically ill oncology patients”. Critical Care Medicine 37.6 (2009): 1966-1971.
- Kopterides P., et al. “General prognostic scores in outcome prediction for cancer patients admitted to the intensive care unit”. American Journal of Critical Care 20.1 (2011): 56-65.
- Lecuyer L., et al. “The ICU trial: a new admission policy for cancer patients requiring mechanical ventilation”. Critical Care Medicine 35.3 (2007): 808-814.
- Lengliné E., et al. “Intensive care unit management of patients with newly diagnosed acute myeloid leukemia with no organ failure’. Leuk & Lymph 53.7 (2012): 1352-1359.
- McGrath S., et al. “ICU and 6-month outcome of oncology patients in the intensive care unit”. QJM: An International Journal of Medicine 103.6 (2010): 397-403.
- Mendoza V., et al. “The hospital-survival and prognostic factors of patients with solid tumors admitted to an ICU”. American Journal of Hospice and Pall Med® 25.3 (2008): 240-243.
- Mokart D., et al. “Prognosis of neutropenic patients admitted to the intensive care unit”. Intensive Care Medicine 41.2 (2015): 296-303.
- Mokart D., et al. “Delayed intensive care unit admission is associated with increased mortality in patients with cancer with acute respiratory failure”. Leuk & lymph 54.8 (2013): 1724-1729.
- Ñamendys-Silva SA., et al. “Prognostic factors in patients with systemic lupus erythematosus admitted to the intensive care unit”. Lupus 18.14 (2009): 1252-1258.
- Ñamendys-Silva SA., et al. “Outcome of critically ill patients with hematological malignancies”. Annals of Hematology 92.5 (2013): 689-695.
- Namendys-Silva SA., et al. “Prognostic factors in critically ill patients with solid tumours admitted to an oncological intensive care unit”. Anaesthesia and Intensive Care 38.2 (2010): 317-324.
- Park SY., et al. “Outcome and predictors of mortality in patients requiring invasive mechanical ventilation due to acute respiratory failure while undergoing ambulatory chemotherapy for solid cancers”. Supportive Care in Cancer 21.6 (2013): 1647-1653.
- Peigne V., et al. “Continued survival gains in recent years among critically ill myeloma patients”. Intensive Care Medicine 35.3 (2009): 512-518.
- Pène F., et al. “Temporal changes in management and outcome of septic shock in patients with malignancies in the intensive care unit”. Critical Care Medicine 36.3 (2008): 680-686.
- Rosolem MM., et al. “Critically ill patients with cancer and sepsis: clinical course and prognostic factors”. Journal of Critical Care 27.3 (2012): 301-307.
- Schellongowski P., et al. “Prognostic factors for intensive care unit admission, intensive care outcome, and post-intensive care survival in patients with de novo acute myeloid leukemia: a single center experience”. Haematologica 96.2 (2011): 231-237.
- Slatore CG., et al. “Intensive care unit outcomes among patients with lung cancer in the surveillance, epidemiology, and end results–Medicare registry”. Journal of Clinical Oncology 30.14 (2012): 1676-1691.
- Soares M., et al. “Intensive care in patients with lung cancer: a multinational study”. Annals of oncology 25.9 (2014): 1829-1835.
- Song J-U., et al. “Risk factors to predict outcome in critically ill cancer patients receiving chemotherapy in the intensive care unit”. Supportive Care in Cancer 19.4 (2011): 491-495.
- Taccone FS., et al. “Characteristics and outcomes of cancer patients in European ICUs”. Critical Care 13.1 (2009): R15.
- Thakkar SG., et al. “Survival and predictors of outcome in patients with acute leukemia admitted to the intensive care unit”. Cancer 112.10 (2008): 2233-2240.
- Toffart A-C., et al. “Use of intensive care in patients with nonresectable lung cancer”. Chest journal 139.1 (2011): 101-108.
- Townsend WM., et al. “Improved intensive care unit survival for critically ill allogeneic haematopoietic stem cell transplant recipients following reduced intensity conditioning”. British Journal of Haematology 161.4 (2013): 578-586.
- Trinkaus M., et al. “Predictors of mortality in patients undergoing autologous hematopoietic cell transplantation admitted to the intensive care unit”. Bone marrow Transplantation 43.5 (2009): 411-415.
- van Vliet M., et al. “Trends in admission prevalence, illness severity and survival of haematological patients treated in Dutch intensive care units”. Intensive Care Medicine 40.9 (2014): 1275-1284.
- Wohlfarth P., et al. “Prognostic factors, long-term survival, and outcome of cancer patients receiving chemotherapy in the intensive care unit”. Annals of hematology 93.10 (2014): 1629-1636.
- Bos MM., et al. “Outcomes of cancer patients after unplanned admission to general intensive care units”. Acta Oncologica 51.7 (2012): 897-905.
- Park MD., et al. “Outcomes in critically ill patients with heamatological malignancies who received renal replacement therapy for acute kidney injury in an intensive care unit”. Journal of Critical Care 26.1 (2011): 107.e1107.e6.
- Oeyen SG., et al. “Long-term outcomes and quality of life in critically ill patients with hematological or solid malignancies: a single center study.” Intensive Care Medicine 39.5 (2013): 889-898.
- Kim YJ., et al. “Who should be admitted to the intensive care unit? The outcome of intensive care unit admission in stage IIB-IV lung cancer patients”. Medical Oncology 31.3 (2014): 847.
- Chou K-T., et al. “Hospital outcomes for patients with stage III and IV lung cancer admitted to the intensive care unit for sepsis related acute respiratory failure”. Journal of Palliative Medicine 15.11 (2012): 1234-1239.
- DE Almeida JP., et al. “Positive fliud balance is associated with reduced survival in critically ill patients with cancer”. Acta Anaesthesiologica Scandinavica 56.6 (2012): 712-717.
- Azoulay E., et al. “The Intensive Care Medicine research agenda on critically ill oncology and hematology patients”. Intensive care medicine 43.9 (2017): 1365-1382.
- Schellongowski P., et al. “Critically ill patients with cancer: chances and limitations of intensive care medicine-a narrative review”. ESMO Open 1.5 (2016).
- Schnell D., et al. “Management of neutropenic patients in the intensive care unit (NEWBORNS EXCLUDED) recommendations from an expert panel from the French Intensive Care Society (SRLF) with the French Group for Pediatric Intensive Care Emergencies (GFRUP), the French Society of Anesthesia and Intensive Care (SFAR), the French Society of Hematology (SFH), the French Society for Hospital Hygiene (SF2H), and the French Infectious Diseases Society (SPILF)”. Annals of Intensive Care 6.1 (2016): 90.
- Kemlin D., et al. “Acute kidney injury in critically ill patients with solid tumours”. Nephrology Dialysis Transplantation (2018).
- Darmon M., et al. “Acute kidney injury in critically ill patients with haematological malignancies: results of a multicentre cohort study from the Groupe de Recherche en Reanimation Respiratoire en OncoHematologie”. Nephrology Dialysis Transplantation 30.12 (2015): 2006-2013.
- Zerbib Y., et al. “Urgent Chemotherapy for Life-Threatening Complications Related to Solid Neoplasms”. Critical care medicine 45.7 (2017): e640-e648.
Citation:
Frank Kiwanuka., et al. “Prognosis of Adult Critically Ill Cancer Patients admitted to adult Intensive Care Unit: A Systematic
Review”. Medical Research and Clinical Case Reports 2.2 (2018): 184-196.
Copyright: © 2018 Frank Kiwanuka., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.