Research Article
Volume 1 Issue 3 - 2018
The Correlation Between Serums Follicular Stimulating Hormone, Body Mass Index, Year Since Menopause and Serum Estradiol in Thai Surgically Menopausal Women: A Cross-Sectional Study
Department of Obstetrics and Gynecology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
*Corresponding Author: Techatraisak K, Head of Division of Gynecologic Endocrinology, Department of Obstetrics and Gynecology, Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand.
Received: January 29, 2018; Published: February 08, 2018
Abstract
Background: The correlations of FSH, BMI and timing of oophorectomy on estradiol level have never been evaluated in Thai surgically menopausal women.
Aim: To study the correlation between serum follicular stimulating hormone (FSH), body mass index (BMI), year since menopause (YSM) and serum estradiol (E2) level in Thai surgical menopause.
Materials and Methods: Medical charts were reviewed from 2,000 consecutive surgically menopausal women enrolled at the Menopause Clinic, Faculty of Medicine Siriraj Hospital, from 1996-2015. Women with uncertain data of oophorectomy status, oophorectomy performed after natural menopause, receiving menopausal hormonal therapy or cessation of hormonal therapy less than 3 months were excluded. Data extracted were ages at surgery, at registration, at hormonal assays, indication for surgery, and weight and height at the time of assays of FSH and E2. Cases with FSH < 40 IU/ml were also excluded as this implied of ovarian remnant syndrome. 150 women were available for final analysis.
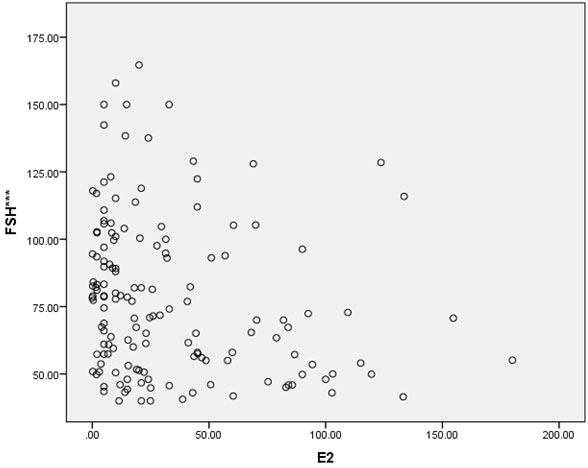
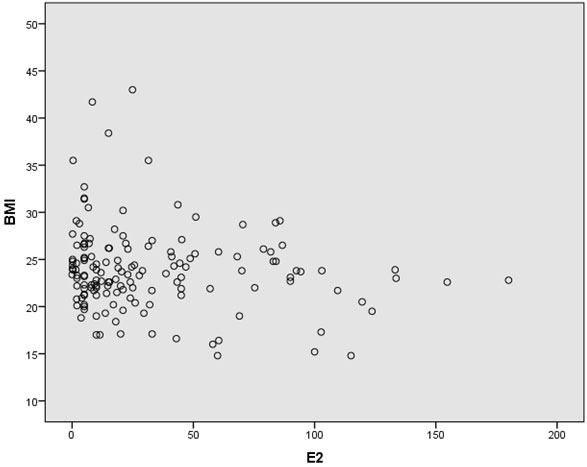
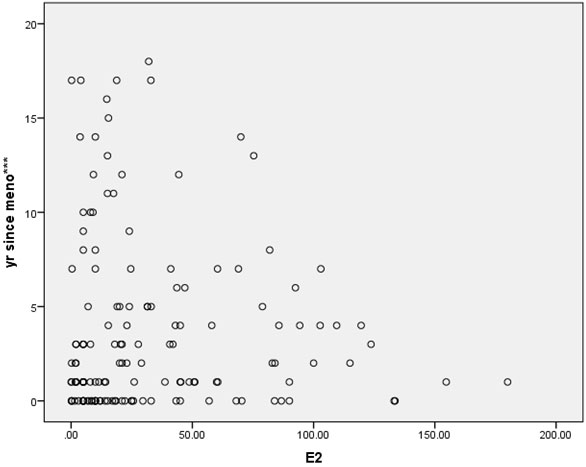
Results: The mean age of surgical menopause was 43.02 ± 5.39 year, with the average YSM when hormonal assays were performed of 3.69 ± 4.61 year. The mean BMI was 23.89 ± 4.43 kg/m2. The mean serum FSH and E2 levels were 78.19 ± 28.95 IU/L and 32.89 ± 38.33 pg/ml, respectively. Only the serum FSH level was inversely correlated with the serum E2 level with high significance (r = -0.208, p = 0.011). The BMI and the YSM failed to show significant correlation with the serum E2 level in this study (p = 0.053 and p = 0.547, respectively).
Conclusion: This study showed negative correlation between serum FSH and serum E2 in surgical menopause. No significant correlation between BMI or YSM and serum E2 was identified.
Keywords: FSH; BMI; Year since menopause; Estradiol; Surgical menopause; Cross-section study
Abbreviations: Estrone: E1; Estradiol: E2; Androstenedione: ASD; Follicular Stimulating Hormone: FSH; Body Mass Index: BMI; Years since Menopause: YSM
Introduction
After natural menopause, recently reported at the median age of 51 year in North America [1], many changes in sex hormone profile occur. In natural menopause, ovaries cease to produce estradiol (E2). While androstenedione (ASD) is the principle steroid secreted by the postmenopausal ovaries [2, 3]. The circulating level of ASD is about one-half of that seen before menopause [4]. However, most of this ASD is derived from the adrenal gland, with only a small amount secreted from the ovary. Additionally, E2 is still continuously produced in a number of extra-gonadal sites, mainly in adipose tissue [5].
The average circulating E2 level after natural menopause is approximately 10-20 pg/ml [6]. Whereas the circulating level of Estrone (E1) in naturally postmenopausal women is higher than that of E2, approximately 30-70 pg/ml [7]. Mean E2 levels started to drop about two years before menopause, decreased rapidly after then and plateaued by two years after the final menstrual period [8]. Because the biological potency of E2 is greater than that of E1, thus E2 has more clinical importance. In previous studies, secretion of E2 by the postmenopausal ovary does not appear to be an important contribution since its circulating levels are similar, in postmenopause, both before and after oophorectomy [9,10].
In natural menopause, serum E2 decreases and serum follicular stimulating hormone (FSH) increases with increasing age in midlife women. And ethnic differences was reported to also influence E2 and FSH levels over time [11]. In that report, body mass index (BMI) had an effect on E2 and FSH concentration, and also varied by menopausal status. Nevertheless, hormonal states associated with menopause may vary depend upon whether menopause was natural or surgical. Surgical menopause (premenopausal bilateral oophorectomy with or without hysterectomy), has increased recently and globally due to gynecological conditions, such as ovarian cyst or endometriosis. This type of menopause is also a consequence of cancer surgery in the children, adolescents or women in reproductive ages.
Prophylactic bilateral oophorectomy for the prevention of later ovarian cancer in women carrying Y chromosome materials or BRCA1/2 mutations has also become inevitably more common. For surgical menopause, cessation of ovarian hormone before the average age of menopause is known to cause more subsequent morbidity and mortality than women with natural menopause. This type of menopause also generally results in 40-50% decrease in serum estrogens and testosterone. In surgical menopause, estrogens were significantly decreased as compared to natural menopause. Also, bilateral oophorectomy was also significantly associated with 25% lower testosterone level than women with natural menopause (p < 0.001) [12]. Nevertheless, no distinction in that report was made regarding timing of oophorectomy (before or after natural menopause).
Many studies support the concept that in postmenopausal women, the circulating E2 is derived from the peripheral conversion of E1, which in turn is the product of the peripheral aromatization of circulating ASD. Weight gain resulting from reduced physical function was reported to be associated with hysterectomy [13]. Body weight, therefore, has a positive correlation with the circulating levels of E1 and E2, probably due to the ability of fat to aromatize androgens. Post-surgical decreased in activity also resulted in abdominal and adipose tissue disruption [14].
Highly significant positive correlations of ideal body weight and both E2 (r = 0.472, p < 0.001) and E1 (r = 0.383, p < 0.001) was found [4]. Less significant positive correlation of E2 level (r = 0.18, p < 0.03) with BMI was also reported [9]. On the contrary, one previous study in 150 naturally menopausal women followed up for six years showed no correlation between log E2 level and BMI (p = 0.6) [8]. And/or the abrupt change in estrogen and androgen production resulting from oophorectomy may increase the risk for weight gain. One previous study in surgically menopausal women who usually underwent bilateral oophorectomy at a much younger age, also assumed that the production of extra-gonadal site estrogen and androgen started in approximately 12-18 months later than that in naturally menopausal women [15].
For this reason, years since menopause (YSM) may affect estrogen and androgen production in surgical menopause. This study focused on the controversial correlation between serum E2 level and FSH, BMI, YSM in surgical menopause. And also compare serum E2 levels after oophorectomy from other previously reports. The study was approved by Siriraj Ethical Review Board (COA number Si 815/2016).
Materials and Methods
This retrospective cross-sectional study was conducted in postsurgical menopausal women attending the Menopause Clinic, Department of Obstetrics and Gynecology, Faculty of Medicine Siriraj Hospital. The subjects were women who underwentbilateral oophorectomy with or without hysterectomy before a naturalmenopause. Medical charts retrieved from 2,000 consecutive cases of surgical menopause who enrolled at our clinic for the first time from 1996-2015 were reviewed. Subjects with unknown or uncertain data ofoophorectomy status were excluded. Women who oophorectomy wasperformed after a natural menopause, receiving menopausal hormonal therapy or stopping therapy less than three months were also excluded.
Data extracted were age at surgery, at registration, at hormonal assays, indication for gynecologic surgery,weight and height at the time of hormonal assays. BMI was calculated as weight (kg) divided by height (m2). YSM was calculated in full calendar year since bilateral oophorectomy. Hormonal assays were assessed by the Electrochemiluminescense Immunoassay (ECLIA) method at the Clinical Pathology Department. Onlywomen whose serum FSH and E2 simultaneously tested were recruited for the analysis. As these two hormonal assays were not routinely performed in our clinic, 176 cases with the results of both assays were identified. Outliers of E2 levels were excluded (E2 ≤ 0.02 pg/ml, n = 2). Also, subjects with FSH < 40 IU/mlwere excluded as this implied recent oophorectomy or ovarian remnant syndrome. Finally, 150 cases were available for final analysis.
Calculations and Statistical Analysis
The Statistics Package for the Social Sciences Program (SPSS 18.0 statistical package) was used for data analysis. A Pearson correlation analysis was used to demonstrate the correlations between serum FSH, BMI, YSM and serum E2 level, with a p-value less than 0.05 considered as statistically significant.
The Statistics Package for the Social Sciences Program (SPSS 18.0 statistical package) was used for data analysis. A Pearson correlation analysis was used to demonstrate the correlations between serum FSH, BMI, YSM and serum E2 level, with a p-value less than 0.05 considered as statistically significant.
Results
In this cross-sectional study, approximately two-thirds of the patients were gravidity = 0 (62%), and parity = 1 (64.7%). Most of the patients (92%) never miscarried. Overall baseline characteristics, hormonal profiles and indications for surgery are shown in Table 1. The mean age of surgical menopause was 43.02 ± 5.39 year with a wide age range of 24-56 year. The average YSM when hormonal assays were performed was 3.69 ± 4.61 year, also with a wide range of 0-18 year. The mean BMI was 23.89 ± 4.43 kg/m2, which was higher than the cut-off value for Asian population (≤ 23 kg/m2). The BMIs ranged from underweight (18.20 kg/m2) to morbid obesity (43.02 kg/m2). The mean serum FSH and E2 levels were 78.19 ± 28.95 IU/L and 32.89 ± 38.33 pg/ml, respectively. The BMI and the YSM failed to show significant correlations with the E2 level in this study (p = 0.053 and p = 0.547, respectively). Only the FSH level was inversely correlated with the E2 level with high significance (r = -0.208, p = 0.011) as shown in Table 2. The scattered plots between the FSH levels, the BMIs, the YSMs and the E2 levels are also shown in Figure 1-3 respectively.
| Characteristics | Overall (Mean ± SD) | Overall (Median) | Overall (Range) |
| Age at menopause (year) | 43.02 ± 5.39 | 44.0 | 24-56 |
| Age at registration (year) | 46.43 ± 6.62 | 47.0 | 25-66 |
| Age at hormonal assays (year) | 46.68 ± 6.77 | 47.0 | 25-66 |
| Year since menopause (year) | 3.69 ± 4.61 | 2.0 | 0-18 |
| Serum FSH level (IU/L) | 78.19 ± 28.95 | 72.60 | 40.00-164.68 |
| Serum E2 level (pg/ml) | 32.89 ± 38.33 | 18.90 | 0.02-180.00 |
| BMI (kg/m2) | 23.89 ± 4.43 | 23.55 | 18.20-43.00 |
| Indication for oophorectomy | Cases (%) | ||
| Myoma | 72 (48.0) | ||
| Endometriosis | 16 (10.7) | ||
| CINIII/CA Cervix | 14 (9.3) | ||
| Myoma + Endometriosis | 12 (8.0) | ||
| Adenomyosis + Endometriosis | 7 (4.7) | ||
| Endometrial hyperplasia | 7 (4.7) |
Table 1: The demographic characteristics the laboratory results, and the indications of gynecologic surgery of all 150 patients.
| Parameter | Serum E2 level | p-value |
| Serum FSH level | ||
| Correlation | -208* | 0.011 |
| BMI | ||
| Correlation | -0.153 | 0.053 |
| Year since menopause | ||
| Correlation | 0.050 | 0.547 |
Table 2: Correlations between the serum FSH level, the BMI, the year since menopause and the serum E2 level in this study.
*significant at < 0.05 level
*significant at < 0.05 level
Discussion
In this report, the overall mean age at surgery was 43.02 ± 5.39 year. The mean YSM, which was also the mean time of hormonal assays since surgery, was 3.69 ± 4.61year. This meant that the hormonal results were analysed when the patients were mainly in an early postsurgical stage. The average level of E2 in this study was slightly higher than the levels of both natural and surgical menopause (10-20 pg/ml) previously published elsewhere [3,12,16]. However, in natural menopause, the ovary still produces a small amount of estrogens and androgens [17]. This small ovarian function may also be responsible for a relatively lower E2 level in surgical menopause than in natural menopause.
One previous small study in peri/postmenopausal women in our clinic in 2006 reported different findings [18]. The average age at menopause of 32 cases in the surgically menopausal group in that study was 42.27 ± 6.29 year, comparable with the average age in this study. While the average YSM in the surgically menopausal group was 5.59 ± 5.03 year, slightly more than that in the present report. Our previous result also showed that the E2 level in the surgically menopausal group (63.05 ± 136.39 pg/ml) was significantly higher than that of 43 women in the naturally menopausal group (25.05 ± 37.66 pg/ml, p = 0.001).
However, the average serum E2 in the surgically menopausal women in the present study was much lower (32.89 ± 38.33 pg/ml), which was close to previously reported elsewhere of 10-20 pg/ml. The serum FSH levels in our previous study of the perimenopausal, the naturally menopausal and the surgically menopausal group were not significantly different (p = 0.566). However, in that previous study, there were significantly negative correlation between the overall levels of FSH and E2. This present study showed the similar result. In our previous study, no significance between serums FSH or E2 with YSM was observed. This study of a much larger sample of 150 women showed the similar result. Nevertheless, BMI was not studied in our previous report.
On the contrary, in one other study in surgical menopause, the estrogen and androgen levels were significantly decreased as compared to natural menopause. And these physiological changes occurred quickly after surgical than natural menopause [19]. The other results of one cross-sectional study from the Netherlands comparing 35 premenopausal bilateral oophorectomy and 40 naturally postmenopausal women showed the lower E2 level without statistical significance in surgical than that in natural menopause (52 ± 17pmol/L vs 45 ± 15 pmol/L, respectively). Although the average age of women with surgical menopause was much lower than that with natural menopause (45.9 vs 58.5 year) with short time since menopause (1.8 vs 4.1 month) [20].
In contrast, one study in Bangladesh also reported significantly higher mean E2 level (p < 0.001) in 30 surgical menopause aged 45-55 year than that in 30 natural menopause [21]. Another recently published data from India [22], comparing the serum E2 levels between surgical and natural menopause (n = 50 each), showed that the mean serum E2 of the surgical menopause (10.72 ± 2.30 pg/ml) was significantly lower than that of the natural menopause (18.18 ± 2.59 pg/ml, p < 0.001). The result of E2 level in surgically menopausal women in India was different from previously published data from our clinic and the finding from our present study, as mentioned earlier. This might imply that ethnic different may play a role in E2 production in postmenopausal status.
The prospective 10 years follow-up of 1,962 women from a community-based multiple sites across the United States (the SWAN Study), reported that BMI increased the annual rate of change of 0.19kg/m2 for women after bilateral oophorectomy. And this rate of increase was significantly more rapid after hysterectomy with or without bilateral oophorectomy compared to that of 0.08kg/m2 in women after natural menopause. Also, in that study, hysterectomy with bilateral oophorectomy was associated with more increases in BMI in hysterectomy with bilateral oophorectomy than that with ovarian conservation or natural menopause [23].
As mentioned earlier for postmenopausal women, the serum E2 level was mainly derived from the peripheral conversion of E1, which was derived from ASD. And also the finding that obese older women were at risk for the development of endometrial cancer than thinner women. Thus, we hypothesized that the peripheral conversion of E2 were proportionally associated with fat mass. Also, the relation between BMI and the percentage of body fat were reported to be different across ethnic groups by body composition analyses. Large variations were observed among Asian populations and also white populations. Hong Kong Chinese, Indonesian, Singaporean, urban Thai and young Japanese women had lower BMIs at a given body fat compared with Europeans [24]. As a consequence, there should be some correlation between BMI and serum E2 level in postmenopausal women. Nevertheless, our present study failed to show a correlation between BMI and E2 level.
The strength of this present study was that the number of cases analysed were also much higher than in many previous reports. Hormonal assays were simultaneously performed with the same laboratory technique, although with various YSMs. Also, the average YSM at the time of hormonal assays was after the assumed starting period of extra-gonadal site estrogen production after surgery. The women in our clinic was homogeneous Indochina descendants. However, there were some limitations in the present study. Only 150 of 2,000 surgically menopausal women were available for the final analysis, since both FSH and E2 assays were not routinely performed in surgically menopausal women in our clinic as mentioned before. The average age of the women at the time of hormonal analyses was relatively young with the average short time since menopause. Also, the median BMI of women in this study was defined as overweight or obese for Asian women.
Conclusion
The results of this study showed a negative correlation between serum FSH and E2 in Thai surgically menopausal women. The data failed to show significant correlation between serum E2 level and BMI, or YSM. Controversial data on the correlation between BMI and serum E2 level still exists. Further studies with a larger number of women with more variations in BMI and YSM are needed to clarify this correlation. The effect of aromatase activity at a different age of a patient, which affects conversion of serum androgen to E2 in different BMI group of menopausal women should also be further studied.
Acknowledgement
The authors would like to thank Mr. Suttiphol Udompunturak, the statistician, for assistance of data analysis.
The authors would like to thank Mr. Suttiphol Udompunturak, the statistician, for assistance of data analysis.
References
- North American Menopause Society. Menopause Practice: A Clinician’s Guide. 3rd edition. Cleveland, OH: North American Menopause Society (2007).
- Grodin JM., et al. “Source of estrogen production in postmenopausal women”. The Journal of Clinical Endocrinology & Metabolism 36.2 (1973): 207-214.
- Fritz MA and Speroff L. “Menopause and perimenopause transition”. Clinical Gynecologic Endocrinology and Infertility 8th edition. Marc A. Fritz and Leon Speroff (editors). Lippincott Williams & Wilkins, a Wolters Kluwer business (2011): 634-636.
- Meldrum DR., et al. ”Changes in circulating steroids with aging in postmenopausal women”. Obstetrics & Gynecology 57.5 (1981): 624-628.
- Simpson ER. “Sources of estrogen and their importance”. The Journal of Steroid Biochemistry and Molecular Biology 86.3-5 (2003): 225-230.
- Judd HL., et al. “Origin of serum estradiol in postmenopausal women”. Obstetrics & Gynecology 59.6 (1982): 680-686.
- Judd HL., et al. “Endocrine function of the postmenopausal ovary: concentration of androgens and estrogens in ovarian and peripheral vein blood”. The Journal of Clinical Endocrinology & Metabolism 39.6 (1974):1020-1024.
- Burger HG., et al. “Prospectively Measured Levels of Serum Follicle-Stimulating Hormone, Estradiol, and the Dimeric Inhibins during the Menopausal Transition in a Population-Based Cohort of Women”. The Journal of Clinical Endocrinology & Metabolism 84.11 (1999): 4025-4030.
- Rannevik G., et al. “A longitudinal study of the postmenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density”. Maturitas 21.2 (1994):103-113.
- Vermeulen A and Verdonck L. “Factors affecting sex hormone levels in postmenopausal women”. Journal of Steroid Biochemistry 11.1 (1979): 899-904.
- Randolph JF., et al. “Change in estradiol and follular-stimulating hormone across the early menopausal transition: Effects of ethnicity and age”. The Journal of Clinical Endocrinology & Metabolism 89.4 (2004):1555-1561.
- Kotsopoulos J., et al. “The relationship between bilateral oophorectomy and plasma hormone levels in postmenopausal women”. Hormones and Cancer 6 (2015): 54-63.
- Sowers M., et al. “Physical functioning and menopause states”. Obstetrics & Gynecology 110.6 (2007): 1290-1296.
- Cooper R., et al. “Is there an association between hysterectomy and subsequent adiposity?” Maturitas 58.3 (2007): 296-307.
- Purohit A and Reed MJ. “Regulations of estrogen synthesis in postmenopausal women”. Steroid 67.12 (2002): 979-983.
- Laughlin GA., et al. “Hysterectomy, Oophorectomy, and Endogenous Sex Hormone Levels in Older Women: The Rancho Bernardo Study”. The Journal of Clinical Endocrinology & Metabolism 85.2 (2000): 645-651.
- Fogle RH., et al. “Ovarian androgen production in postmenopausal women”. The Journal of Clinical Endocrinology & Metabolism 92.8 (2007): 3040-3043.
- Angsuwathana S., et al. “Serum follicle stimulating hormone and estradiol in peri/postmenopausal women attending Siriraj menopause clinic: A retrospective study”. Journal of the Medical Association of Thailand 89.8 (2006):1101-1108.
- Bachmann G. “Physiologic aspects of natural and surgical menopause”. The Journal of Reproductive Medicine 46 (2001): 307-315.
- Korse CM., et al. “Estradiol and testosterone levels are lower after oophorectomy than after natural menopause”. Tumor Biology 30.1 (2009): 37-42.
- Kabir F., et al. “Osteoporosis in surgical menopause”. Journal of Bangladesh Society of Physiologist 6 (2011): 39-44.
- Naik RR., et al. “Comparison of serum estradiol in surgical and natural menopause”. IOSR Journal of Pharmacy and Biological Sciences 9.1(2014): 65-67.
- Gibson CJ., et al. “Body mass index following natural menopause and hysterectomy with and without bilateral oophorectomy”. International Journal of Obesity 37.6 (2013): 809-813.
- WHO Expert Consultation. “Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies”. Lancet 363.9403 (2004): 157-163.
Citation:
Techatraisak K., et al. “The Correlation Between Serums Follicular Stimulating Hormone, Body Mass Index, Year Since
Menopause and Serum Estradiol in Thai Surgically Menopausal Women: A Cross-Sectional Study”. Gynaecology and Perinatology 1.3
(2018): 158-165.
Copyright: © 2018 Techatraisak K., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.