Mini Review
Volume 1 Issue 1 - 2017
An Insight into Early Fetal Loss
Woman and Fetal Health Program, Dr Arab Medical Center, Jeddah, Saudi Arabia
*Corresponding Author: Hisham Arab, Woman and Fetal Health Program, Dr Arab Medical Center, Jeddah, Saudi Arabia.
Received: August 13, 2017; Published: August 21, 2017
Abstract
The medical literature has witnessed recently an influx of analogous reports from different parts of the world agreeing on the same concept of managing early fetal loss whether threatened or recurrent miscarriages. The use of oral and not vaginal progestogen has been recommended because it has 20 times progestogenic potency and without any estrogenic, androgenic or glucocorticoid effects. Beside its high oral tolerability and safety, it reduces the miscarriage rate by 30-50%.
Keywords: Abortion; Fetal; Threatened; Recurrent, Miscarriage; Dydrogesterone; Progesterone
Introduction
Science and technology work hand in hand to achieve new breakthroughs that will advance the medical care of our patients. Physicians have been struggling for years with the management of early fetal loss, whether threatened or recurrent miscarriages, because of the fact that forty to 50% of such cases are of unknown etiology. Various empirical treatment attempts have been tried without satisfactory results. However, the accumulated experimental and clinical data over the past couple of years about the beneficial effect of an oral progestogen is worth mentioning since many authoritative scientific bodies around the world are now recommending its use in such conditions, mainly unexplained recurrent miscarriages.The Indonesian Obstetric and Gynecologic Society Guidelines 2011 [1], The statement of the Australian and New Zeeland Royal College of Obstetricians and Gynecologists 2013 [2], the Saudi Society of Obstetrics and Gynecology Guidelines 2014 [3], and more recently The European Progestin Club Guidelines 2015 [4] have agreed on the following statement that “there is a significant reduction in the rate of miscarriage with the use of dydrogesterone”.
New Pathophysiology Enforces Old Pharmaceutical
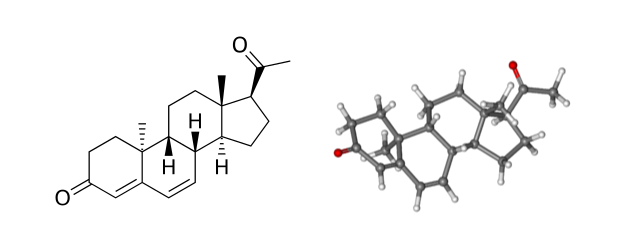
There is a newer concept now that links both immunologic and hormonal abnormalities to the problem of threatened as well as recurrent miscarriage. Dydrogesterone was found to be the only progestogen that has this dual effect to reverse such aberrations. When natural progesterone is treated with light technology a synthetic progestogen is developed; called Dydrogesterone. It has a great similarity to the natural progesterone as it lacks any estrogenic, androgenic, or mineralocorticoid effects. In fact it has even a better receptor affinity than micronized natural progesterone due to its curved retrosteroid structure (figure 1). The following are the reasons why oral dydrogesterone stands alone as a progestational agent that significantly reduces the rate of spontaneous miscarriages of unknown etiology.
There is a newer concept now that links both immunologic and hormonal abnormalities to the problem of threatened as well as recurrent miscarriage. Dydrogesterone was found to be the only progestogen that has this dual effect to reverse such aberrations. When natural progesterone is treated with light technology a synthetic progestogen is developed; called Dydrogesterone. It has a great similarity to the natural progesterone as it lacks any estrogenic, androgenic, or mineralocorticoid effects. In fact it has even a better receptor affinity than micronized natural progesterone due to its curved retrosteroid structure (figure 1). The following are the reasons why oral dydrogesterone stands alone as a progestational agent that significantly reduces the rate of spontaneous miscarriages of unknown etiology.
Figure 1: Dydrogesterone is a highly selective progestogen due to its unique structure
that allows high selectivity for in vivo activation of the progesterone receptors.
Combined Endocrine and Immunological Features:
Like any other progesterone it enhances the implantation process and reduces the myometrial tone with improvement of uterine blood flow. However, the additional feature of modulating the immune response to hamper the materno-fetal rejection has only been shown with dydrogesterone alone. The process of miscarriage is characterised by an abundance of pro-inflammatory cytokines like IL-2, TNFα, TNFβ, IFNγ released by T-helper cells type1 (Th1) with diminished activity of the Th2 cell which produces anti-inflammatory cytokines like IL-4,5,6,10, and 13 [5]. Dydrogesterone was found to reverse this process either directly or indirectly through its effect on the lymphocytes and the release of progesterone-induced blocking factor (PIBF) [6]. Women suffering from threatened miscarriage usually have lower level of PIBF in their urine. Those who received Dydrogesterone treatment showed increased PIBF to normal levels and enjoyed lesser chance of miscarriage [7].
Like any other progesterone it enhances the implantation process and reduces the myometrial tone with improvement of uterine blood flow. However, the additional feature of modulating the immune response to hamper the materno-fetal rejection has only been shown with dydrogesterone alone. The process of miscarriage is characterised by an abundance of pro-inflammatory cytokines like IL-2, TNFα, TNFβ, IFNγ released by T-helper cells type1 (Th1) with diminished activity of the Th2 cell which produces anti-inflammatory cytokines like IL-4,5,6,10, and 13 [5]. Dydrogesterone was found to reverse this process either directly or indirectly through its effect on the lymphocytes and the release of progesterone-induced blocking factor (PIBF) [6]. Women suffering from threatened miscarriage usually have lower level of PIBF in their urine. Those who received Dydrogesterone treatment showed increased PIBF to normal levels and enjoyed lesser chance of miscarriage [7].
Superior to Vaginal Micronized Progesterone (VMP)
Dydrogesterone has 1.5 times better affinity to progesterone receptors than VMP. It also has approximately 5.6 times better oral availability than micronized progesterone. When compared to VMP it has a faster onset of action with longer stable effect [8]. Moreover, the PROMISE Trial, which included 836 women from 36 sites from England and 9 sites from Netherland, was published recently and indicated clearly that administration of VMP in the first trimester to women with recurrent miscarriages of unknown etiology did not result in higher rate of live births. Oral Dydrogesterone clinical trials, on the other hand, are still coming with more confirmatory evidence of its beneficial role in the management of both recurrent and threatened miscarriages [9]. Several Cochrane systematic reviews have shown that dydrogesterone use in similar patients has resulted in 30–50% reduction in miscarriage rate. These studies are summarised systematically in the Saudi and the European guidelines (3,4).
Dydrogesterone has 1.5 times better affinity to progesterone receptors than VMP. It also has approximately 5.6 times better oral availability than micronized progesterone. When compared to VMP it has a faster onset of action with longer stable effect [8]. Moreover, the PROMISE Trial, which included 836 women from 36 sites from England and 9 sites from Netherland, was published recently and indicated clearly that administration of VMP in the first trimester to women with recurrent miscarriages of unknown etiology did not result in higher rate of live births. Oral Dydrogesterone clinical trials, on the other hand, are still coming with more confirmatory evidence of its beneficial role in the management of both recurrent and threatened miscarriages [9]. Several Cochrane systematic reviews have shown that dydrogesterone use in similar patients has resulted in 30–50% reduction in miscarriage rate. These studies are summarised systematically in the Saudi and the European guidelines (3,4).
The safety Oral Dydrogesterone
Clinical experience does not provide evidence of a causal link between maternal Dydrogesterone use during pregnancy and birth defects. All clinical studies that used Dydrogesterone in the first trimester have indicated the same. Realistically, It is a commonly used drug by women in almost 100 countries around the world to regulate their periods or to support their luteal phase with a high chance of incidental use in early pregnancies, apart from the miscarriage indications. Nevertheless, a recent report estimated that over the past 50 years (1960 - 2014) >20 million pregnancies were exposed to dydrogesterone without increased fetal malformations. One poorly designed study [10] tried to link it with higher incidence of congenital heart disease but more likely this will turn like the hypospadias accusation that started in 1999 and was refuted by a strong evidence in the study of Kallen in 2010 [11].
Clinical experience does not provide evidence of a causal link between maternal Dydrogesterone use during pregnancy and birth defects. All clinical studies that used Dydrogesterone in the first trimester have indicated the same. Realistically, It is a commonly used drug by women in almost 100 countries around the world to regulate their periods or to support their luteal phase with a high chance of incidental use in early pregnancies, apart from the miscarriage indications. Nevertheless, a recent report estimated that over the past 50 years (1960 - 2014) >20 million pregnancies were exposed to dydrogesterone without increased fetal malformations. One poorly designed study [10] tried to link it with higher incidence of congenital heart disease but more likely this will turn like the hypospadias accusation that started in 1999 and was refuted by a strong evidence in the study of Kallen in 2010 [11].
Conclusion
More than 130 peer-reviewed publications have convinced me as well as certain International societies around the globe that dydrogesterone is superior to any other medical treatments in reducing the rate of miscarriages by up to 50%. Unfortunately, this drug is not included in the American or the British formulary, however, this published dramatic increase in live births among victims of miscarriage has led the American Society of Reproductive Medicine and the Royal College of Obstetricians and Gynecologist to hint in their recent reports that some progestogens may have a potential benefit in the management of miscarriage of unknown etiology [12].
Recommended dosage of Oral Dydrogesterone for cases of threatened miscarriage and those with a history of recurrent miscarriages is displayed in table 1.
| Threatened Miscarriage | Recurrent Miscarriage |
| Option 1: 40 mg loading, then 10 mg BD Option 2: 20 mg BD till bleeding stop, then 10 mg BD till 16 weeks |
Oral Dydrogesterone 10 mg BD Treatment to be started after confirmation of pregnancy and continued until 12 week, up to 20 weeks. |
Table 1: Recommended Dosage of Oral Dydrogesterone.
Declaration of interest
The author reports no conflicts of interest.
The author reports no conflicts of interest.
References
- Indonesian Obstetric and Gynecological Society (HIFERI-POGI). Methodological guidelines for recurrent miscarriage. Jakarta; 2011.
- Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG). Progesterone support of the luteal phase and the first trimester. 2013.
- Saudi Society of Obstetrics and Gynecology Guidelines (SOGS). National Guidelines for prevention and treatment of miscarriages. Saudi Journal of Obstetrics and Gynecology 15 (2014): 40-63.
- Schindler AE., et al. “European Progestin Club Guidelines for prevention and treatment of threatened or recurrent (habitual) miscarriage with progestogens”. Gynecological Endocrinology (2015): 1-3.
- Whitcomb BW., et al. “Circulating chemokine levels and miscarriage”. American Journal of Epidemiology166.3 (2007): 323-231.
- Raghupathy R. et al. “Modulation of cytokine production by dydrogesterone in lymphocytes from women with recurrent miscarriage”. BJOG: An International Journal of Obstetrics & Gynaecology 112.8 (2005): 1096-1101.
- Kalinka J., et al. “The Impact of dydrogesterone supplementation on hormonal profileand PIBF concentrations in womenwiththreatendabortion”. American Journal of Reproductive Immunology 53.4 (2005): 166-171.
- Ganesh A., et al.“Comparisonof oral dydrogesterone with progesterone gel and micronized progesterone”. Fertility and Sterility95.6 (2011): 1961-1965.
- Carp H. “A systematic review of dydrogesterone for the treatment of recurrent miscarriage”. Journal of Gynecological Endocrinology 31.6 (2015): 422-430.
- Zaqout M. “The Impact of Oral Intake of Dydrogesterone on Fetal Heart Development During Early Pregnancy”. Pediatric Cardiology 36.7 (2015): 1483-1488.
- Kallen., et al. “Congenital malforma1ons in infants born acer in vitro fer1liza1on in Sweden. Birth Defects Research”. Clinical and Molecular Teratology 2010.
- Practice Committee of the American Society for Reproductive Medicine. “Evaluation and treatment of recurrent pregnancy loss: a committee opinion”. Fertility and Sterility 98.5 (2012): 1103-1111.
Citation:
Hisham Arab. “An Insight into Early Fetal Loss”. Gynaecology and Perinatology 1.1 (2017): 67-69.
Copyright: © 2017 Hisham Arab. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.