Review Article
Volume 1 Issue 1 - 2017
Hermaphroditism in the Common Orthopaedic Practice: A Review
Department of Orthopaedic Surgery, “G. Gennimatas” Hospital, 54635 Thessaloniki, Greece
*Corresponding Author: N. K. Sferopoulos, Department of Orthopaedic Surgery, “G. Gennimatas” Hospital, 54635 Thessaloniki, Greece.
Received: February 06, 2017; Published: February 13, 2017
Abstract
Disorders of sex development result in problems concerning the sex assignment of the child. The evaluation and management of patients with ambiguous genitalia requires a multidisciplinary team including neonatologist, pediatrician, urologist, endocrinologist, pediatric surgeon, geneticist, gynecologist and psychiatrist or psychologist depending on the age of the treated patient. Although the orthopaedic surgeon is not involved in the primary treatment of these patients, he may be helpful towards making an early referral indicating a child’s undiagnosed clinical sign or may occasionally be involved in the treatment of coexisting traumatic lesions or disorders in adult patients.
In humans, male or female phenotype develops through a cascade of processes which initiate with sex determination and follow with sex differentiation. The karyotype (46, XY or 46, XX) of the embryo (genetic sex) determines whether bipotential primordial gonads differentiate into a testis or an ovary, respectively (gonadal differentiation). This is a complex developmental process involving various genes and hormones. The sex-determining region of the Y chromosome (SRY gene) produces a protein that activates a gene network, which directs the gonads to develop as testes; when it is absent the gonads develop as ovaries. Similarly, internal and external genital organs develop from an indeterminate (undifferentiated) stage from the complex differentiation of the two primitive ducts: the Wolffian and Müllerian ducts. Once the gonad begins to develop as a testis, the two support cells in the testis differentiate: the Leydig cells produce testosterone and the Sertoli cells produce Müllerian inhibiting substance also known as anti-Müllerian hormone.
Differentiation of the Wolffian ducts, urogenital sinus, and external genitalia is androgen dependent. Female sex differentiation appears to be a more passive process that is independent of estrogen. However, several genes have been shown to be necessary to initiate ovarian development, and to actively repress the gene network that promotes testis development. Hormonal production of differentiated gonads is relevant for differentiation of the internal and external genitalia during fetal life, and for the development of secondary sex characteristics at puberty [1,2].
There are four main types of genital anomalies: true hermaphroditism, male or female pseudohermaphroditism and gonadal dysgenesis [3,4].
In biology, a hermaphrodite is an organism that has reproductive organs of both male and female sexes. The term derives from ancient Greek: hermaphroditos (ἑρμαφρόδιτος), the son of Hermes and Aphrodite in Greek mythology. True hermaphroditism, also known as ovotesticular disorder of sex development, is reserved for the very rare cases where both ovarian and testicular tissues are present [5]. The term pseudohermaphroditism was created by Klebs in 1876. It is the condition in which an organism is born with primary sex characteristics of one sex but develops the secondary sex characteristics that are different from what would be expected on the basis of the gonadal tissue (ovary or testis). The diagnosis of pseudohermaphroditism can be made in utero by comparing the karyotype obtained by amniocentesis with the external genitalia of the fetus during a prenatal ultrasound [6].
Male pseudohermaphroditism is a condition in which individuals with a XY karyotype and testes appear with a complete or partial female phenotype, and female pseudohermaphroditism is the presence of complete or partial male phenotypes in individuals with a XX karyotype and ovaries. The term "intersexuality" was introduced by Goldschmidt in 1923 to replace pseudohermaphroditism, but it has also been challenged and replaced by a nomenclature system based on disorders of sex development (DSD), which covers "congenital conditions in which development of chromosomal, gonadal, or anatomical sex is atypical" [7]. The diagnostic investigation of a patient with a genital malformation or phenotype that does not match the chromosomal sex requires additional phenotypic information using metabolic and endocrine testing, imaging studies and genetic analysis.
Genetic technologies have resulted in the discovery of many genes involved in sex determination. Genes involved in sex determination have been isolated by positional cloning using patient samples in which microscopically visible chromosomal alterations were identified using standard cytogenetic techniques [8-10]. The use of linkage analysis is restricted due to the small number of patients and the lack of large number of individuals involved in families. New genomic technologies may be used as a first-stage diagnostic technique because they allow for early identification of a genetic cause that may be critical for patient management. Recent advances of chromosomal microarray and exome sequencing technologies are allowing for higher rates of diagnostic success [11-13].
Male pseudohermaphroditism is due to hypoandrogenism in XY individuals [14,15]. Deficit in the presence and in the action of androgens is due to different conditions that can be divided into three main categories:
1) androgen resistance: it is the main cause of male pseudohermaphroditism and it leads to the androgen insensitivity (testicular feminization) syndrome, 2) deficit in the production of testosterone and 3) deficit in the production of dihydrotestosterone [16].
The androgen insensitivity syndrome was defined by Morris in 1953 [17,18]. It is a rare inherited disorder that leads to partial or complete lack of response to androgens caused by a receptor dysfunction. The condition of androgen insensitivity is due in the great majority of cases to point mutations or micro deletions in the androgen receptor gene [19-22].
Androgen insensitivity syndrome can be divided into three categories based on the genital phenotype:
1) Complete androgen insensitivity syndrome (CAIS) is a condition of complete inability of the cell to respond to androgens. The resistance of cell toward testosterone and its more potent androgen metabolite dihydrotestosterone prevents the complete masculinization of male genitalia in the developing fetus and the development of male secondary sexual characteristics with absence of pubic or axillary hair at puberty. Individuals with complete androgen insensitivity, as well as individuals with severe deficit in testosterone production are born with a complete female phenotype, despite having a 46, XY karyotype [23,24].
The typical presentation is either primary amenorrhoea in adolescence, or inguinal swellings in an infant. Therefore, prepubertal girls with inguinal hernia should be carefully examined to exclude the syndrome [25]. Ultrasonography and magnetic resonance imaging are used to locate non-palpable testis [26].
2) Partial or incomplete androgen insensitivity syndrome (PAIS) was described in 1963 [27]. It consists in the partial inability of the cell to respond to androgens. There is a wide spectrum of clinical phenotypes in which the external genitalia are predominantly male, ambiguous or predominantly female. Patients who present predominantly male phenotypes may present small penis, cryptorchidism, bifid scrotum and hypospadias.
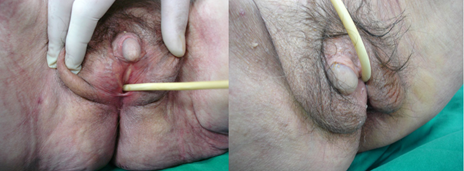
Patients with predominantly female phenotypes may present normal female genital phenotype with pubic and/or axillary hair at puberty or essentially female phenotype with separate urethral and vaginal orifices, mild clitoromegaly or labial fusion. Patients with ambiguous phenotype (Figure 1) may present with a phallic structure intermediate between penis and clitoris, urogenital sinus with perineal orifice and labioscrotal folds and severely limited masculinization [28-30].
3) Mild androgen insensitivity syndrome (MAIS) is a condition of mild inability of cells to respond to androgens. Individuals with mild androgen insensitivity syndrome are born phenotypically male, according to their XY karyotype. They have fully masculinized genitalia and their problems are mostly related to the condition of infertility (oligospermia or azoospermia), decreased secondary terminal hair, and high pitch of voice. The external male genitalia (penis, scrotum, and urethra) are normal, as well as internal genitalia, including Wolffian structures and prostate. However testicular volume can be diminished due to the condition of infertility [31-34].
Figure 1: An 80-year-old patient, with ambiguous genitalia since birth, was treated for a hip fracture. Bilateral orchiectomy was reported at 13 years of age. The chromosome studies showed a 46, XY normal male pattern. The patient was diagnosed with partial androgen insensitivity syndrome (formerly known as testicular feminization syndrome). Diagnosis was based on the small penis (closed external urethral orifice), testes, a double blind ended vagina and poorly developed labia, in association with normal male chromosomes.
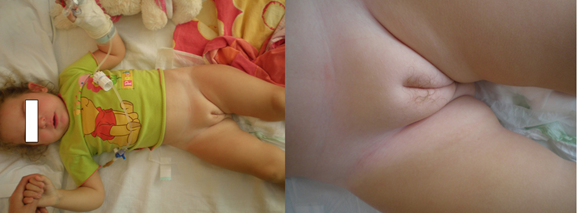
Female pseudohermaphroditism may be due to: 1) Hyperandrogenism that is caused by excess of androgens of extragonadal origin (Figure 2). The main cause of accumulation of androgens is congenital virilizing adrenal hyperplasia, and 2) Deficit in the synthesis of estrogens that eventually results in a similar accumulation of androgens.
Figure 2: A 22-month-old girl that presented with a painful hip. The diagnosis of premature adrenarche was based on the clinical appearance of pubic hair (pubarche), 3 months ago, and biochemically on the elevated adrenal androgen concentrations.
The presence of testosterone and other androgens during fetal live of female fetuses with a XX karyotype, obviously leads to genital ambiguity due to partial masculinization of external genitalia. The high levels of testosterone result in clitoromegaly [28]. In addition, the vaginal opening can be closed, as well as female urethra, while a male urethra can appear inside the phallus. The most severely affected female infants can even appear as a real male [35,36].
Gonadal dysgenesis includes the Turner syndrome, the pure gonadal dysgenesis (Swyer syndrome), the asymmetrical gonadal differentiation, and the gonadal dysgenesis of some forms of trisomy. It may be associated with sensorineural deafness in females, and deafness in affected males (Perrault syndrome) [37-39].
The diagnostic process in cases with ambiguous genitalia requires evaluation by a skilled multidisciplinary team including clinical, imaging, hormonal, genetic and molecular examination with an apparent shift, recently, towards molecular genetic testing to reach a correct diagnosis [40,41]. Several cases without etiologic diagnosis or with syndromic features need advanced investigation [42-45]. Physical examination is the key to diagnosis and the search for gonads with palpation and imaging is the first element [46].
The diagnosis of female pseudohermaphroditism seems advisable when gonads are absent. A diagnosis of male pseudohermaphroditism is more appropriate whenever they are palpated. Karyotyping may indicate the presence of a Y chromosome, while the gene for the determination of maleness in the sex determining region on the short arm of the Y chromosome may be identified. Hormonal investigation of 17-OH- progesterone will confirm the diagnosis of congenital adrenal hyperplasia due to deficiency in 21-hydroxylase. Testicular stimulation with human chorionic gonadotropin will determine the functional value of testicular tissue. Whenever testosterone rises normally androgen resistance is indicated, but if it does not rise after the test testicular dysgenesis or disturbance in testosterone biosynthesis may be responsible.
In cases of female pseudohermaphroditism, the newborn should always be declared to be of female sex, while in cases of male pseudohermaphroditism, great care should be taken in the declaration of male sex [47]. Prompt evaluation of the newborn with ambiguous genitalia will permit the detection of life-threatening conditions (salt-losing crisis due to congenital adrenal hyperplasia or Wilm’s tumour) [48]. Hormonal therapy forms part of the treatment of every intersex condition [49]. Judicious hormonal supplementation based upon type of the disorder and gender assigned can provide a psychological and cosmetic benefit to patients [50].
Surgical treatment is a source of major concern regarding timing, choice of the individual and irreversibility of surgical procedures [51,52]. The diagnosis of disorders of sex differentiation raises concerns of tumor risk and treatment as well as future fertility preservation. The management of such patients is complex and evaluation of tumor risk is aided by advances in genotyping for Y-chromosomal material not evident in traditional karyotyping. The necessity of prophylactic gonadectomy in all patients with Y chromosome is stressed because of a close association with the arising of tumors in the dysgenetic gonads. Future studies utilizing more advanced histologic examination of gonads will improve our understanding of the true incidences of malignancy in this diverse population [53-55].
Conflict of interest statement: The author certifies that he has no commercial associations (such as consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article. The author received no financial support for this study.
References
- Sinisi AA., et al. “Sexual differentiation”. Journal of Endocrinological Investigation 26(3 Suppl) (2003): 23-28.
- Warne GL and Kanumakala S. “Molecular endocrinology of sex differentiation”. Seminars in Reproductive Medicine 20.3 (2002): 169-180.
- Zaparackaite I and Barauskas V. “Congenital genital anomalies. Aspects of diagnostics and treatment”. Medicina (Kaunas, Lithuania) 39.2 (2003): 105-113.
- El-Sherbiny M. “Disorders of sexual differentiation: I. Genetics and pathology”. Arab Journal of Urology 11.1 (2013): 19-26.
- Jones HW Jr., et al. “Pathologic and cytogenetic findings in true hermaphroditism; Report of 6 cases and review of 23 cases from the literature”. Obstetrics & Gynecology 25.4 (1965): 435-447.
- Chitayat D and Glanc P. “Diagnostic approach in prenatally detected genital abnormalities”. Ultrasound in Obstetrics & Gynecology 35.6 (2010): 637–646.
- Lee PA., et al. “Consensus statement on management of intersex disorders. International Consensus Conference on Intersex”. Pediatrics 118.2 (2006): e488-500.
- Tanoue A., et al. “Sex-determining region Y (SRY) in a patient with 46,XX true hermaphroditism”. The Japanese journal of human genetics 37.4 (1992): 311-320.
- Braun A., et al. “True hermaphroditism in a 46, XY individual, caused by a postzygotic somatic point mutation in the male gonadal sex-determining locus (SRY): molecular genetics and histological findings in a sporadic case”. American Journal of Human Genetics 52.3 (1993): 578-585.
- Miyado M., et al. “The p.R92W variant of NR5A1/Nr5a1 induces testicular development of 46, XX gonads in humans, but not in mice: phenotypic comparison of human patients and mutation-induced mice”. Biology of Sex Differences 2016; 8.7 (2016): 56. eCollection 2016.
- Baxter RM and Vilain E. “Translational genetics for diagnosis of human disorders of sex development”. Annual Review of Genomics and Human Genetics 14 (2013): 371-392.
- Barseghyan H., et al. “New genomic technologies: an aid for diagnosis of disorders of sex development”. Hormone and Metabolic Research 47.5 (2015): 312-320.
- Baetens D., et al. “Non-coding variation in Disorders of Sex Development”. Clinical Genetics 91.2 (2017): 163-172.
- Jacobs PA., et al. “Chromosomal sex in the syndrome of testicular feminization”. Lancet 17.2(7103) (1959): 591-592.
- Mauvais-Jarvis P., et al. “Studies on testosterone metabolism in subjects with testicular feminization syndrome”. The Journal of Clinical Investigation 49.1 (1970): 31-40.
- Balducci R., et al. “clinician looks at androgen resistance”. Steroids 61.4 (1996): 205-211.
- Morris JM. “The syndrome of testicular feminization in male pseudohermaphrodites”. American Journal of Obstetrics & Gynecology 65.6 (1953): 1192-1211.
- Glenn JF. “Testicular feminization syndrome: current clinical considerations”. Urology 1976; 7.6 (1976): 569-577.
- Jeffcoate SL., et al. “Secretion of androgens and oestrogens in testicular feminization: studies in vivo and in vitro in two cases”. TheBMJ 27.1(5586) (1968): 208-210.
- Lobaccaro JM., et al. “Molecular analysis of the androgen receptor gene in 52 patients with complete or partial androgen insensitivity syndrome: a collaborative study”. Hormone Research in Paediatrics 37.1-2 (1992): 54-59.
- Brinkmann AO. “Molecular basis of androgen insensitivity”. Molecular and Cellular Endocrinology 179.1-2 (2001): 105-109.
- Chen MJ., et al. “Androgen Insensitivity Syndrome: Management Considerations from Infancy to Adulthood”. Pediatric Endocrinology Reviews 12.4 (2015): 373-387.
- Zourlas PA and Jones HW Jr. “Clinical, histologic, and cytogenetic findings in male hermaphroditism. II. Male hermaphrodites with feminine external genitalia (Testicular feminization)”. Obstetrics & Gynecology 25.6 (1965): 768-778.
- Castelazo-Ayala L., et al. “Steroid production by gonadal tumors in male pseudo-hermaphroditism with isolated clitoromegaly. Biochemical studies in vivo”. Steroidologia 2.3 (1971): 138-142.
- Konar S., et al. “Chromosomal Study is Must for Prepubertal Girl with Inguinal Hernia: Opportunity to Diagnose Complete Androgen Insensitivity Syndrome”. Journal of Clinical and Diagnostic Research 9.4 (2015): GD01- GD03.
- Kanemoto K., et al. “Accuracy of ultrasonography and magnetic resonance imaging in the diagnosis of non-palpable testis”. International Journal of Urology 12.7 (2005): 668-672.
- Morris JM and Mahesh VB. “Further observations on the syndrome, "Testicular feminization"”. American Journal of Obstetrics & Gynecology 15.87 (1963): 731-748.
- Copcu E., et al. “Idiopathic isolated clitoromegaly: A report of two cases”. Reproductive Health 1.1 (2004): 4.
- Galani A., et al. “Androgen insensitivity syndrome: clinical features and molecular defects”. Hormones (Athens, Greece) 7.3 (2008): 217-229.
- Bhangoo A., et al. “Isolated micropenis reveals partial androgen insensitivity syndrome confirmed by molecular analysis”. Asian Journal of Andrology 12.4 (2010): 561-566.
- Savage MO and Grant DB. “The incomplete male”. Archives of Disease 53.9 (1978): 701-703.
- Gooren L and Cohen-Kettenis PT. “Development of male gender identity/role and a sexual orientation towards women in a 46,XY subject with an incomplete form of the androgen insensitivity syndrome”. Archives of Sexual Behavior 20.5 (1991): 459-470.
- Brinkmann A., et al. “Molecular basis of androgen insensitivity”. Steroids 61.4 (1996): 172-175.
- Hughes IA., et al. “Androgen insensitivity syndrome”. Lancet 380.9851 (2012): 1419-1428.
- Kamijo H and Narita O. “Female pseudohermaphroditism”. Nihon rinsho. Japanese journal of clinical medicine 55.11 (1997): 2925-2929.
- Dacou-Voutetakis C., et al. “Encyclopedia of Endocrine Diseases”. Elsevier Inc (2004): 99-105.
- Böhm W and Göretzlehner G. “Morphology of the genitals in gonadal dysgenesis”. Zentralbl Gynakol 105.24 (1983): 1553-1560.
- Meyers CM., et al. “Gonadal (ovarian) dysgenesis in 46,XX individuals: frequency of the autosomal recessive form”. American Journal of Medical Genetics 63.4 (1996): 518-524.
- Newman WG., et al. “Perrault Syndrome”. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle (2014): 1993-2017.
- Torres L., et al. “Molecular analysis in true hermaphrodites with different karyotypes and similar phenotypes”. American Journal of Medical Genetics 63.2 (1996): 348-355.
- Lambert SM., et al. “A practical approach to ambiguous genitalia in the newborn period”. The Urologic clinics of North America 2010; 37.2 (2010): 195-205.
- Cox K., et al. “Novel associations in disorders of sex development: findings from the I-DSD Registry”. The Journal of Clinical Endocrinology & Metabolism 99.2 (2014): E348-355.
- Jaruratanasirikul S and Engchaun V. “Management of children with disorders of sex development: 20-year experience in southern Thailand”. World Journal of Pediatrics 10.2 (2014): 168-174.
- Kyriakou A., et al. “Current models of care for disorders of sex development - results from an International survey of specialist centres”. Orphanet Journal of Rare Diseases 11.1 (2016): 155.
- De Paula GB., et al. “408 Cases of Genital Ambiguity Followed by Single Multidisciplinary Team during 23 Years: Etiologic Diagnosis and Sex of Rearing”. International Journal of Endocrinology 4963574 (2016): 1-9.
- Alaniz VI., et al. “Utility of Ultrasound and Magnetic Resonance Imaging in Patients with Disorders of Sex Development Who Undergo Prophylactic Gonadectomy”. Journal of Pediatric & Adolescent Gynecology 29.6 (2016): 577-581.
- Sultan C., et al. “Galifer RB. Ambiguous genitalia in the newborn”. Seminars in Reproductive Medicine 20.3 (2002): 181-188.
- Zdravković D., et al. “Causes of ambiguous external genitalia in neonates”. Srpski arhiv za celokupno lekarstvo 129.3-4 (2001): 57-60.
- Warne GL., et al. “Hormonal therapies for individuals with intersex conditions: protocol for use”. Treatments in endocrinology 4.1 (2005): 19-29.
- Sharma S and Gupta DK. “Gender assignment and hormonal treatment for disorders of sexual differentiation”. Pediatric Surgery International 24.10 (2008): 1131-1135.
- Krstić Z., et al. “Surgical treatment of intersex disorders”. Journal of Pediatric Surgery 30.9 (1995): 1273-1281.
- Mouriquand PD., et al. “Surgery in disorders of sex development (DSD) with a gender issue: If (why), when, and how?” Journal of Pediatric Urology 12.3 (2016): 139-149.
- Troche V and Hernandez E. “Neoplasia arising in dysgenetic gonads”. Obstetrical & Gynecological Survey 41.2 (1986): 74-79.
- Kathrins M and Kolon TF. “Malignancy in disorders of sex development”. Translational Andrology and Urology 5.5 (2016): 794-798.
- Jiang JF., et al. “Gonadal malignancy in 202 female patients with disorders of sex development containing Y-chromosome material”. Gynecological Endocrinology 2016; 32.4 (2016): 338-341.
Citation:
N. K. Sferopoulos. “Hermaphroditism in the Common Orthopaedic Practice: A Review”. Gynaecology and Perinatology 1.1 (2017): 25-30.
Copyright: © 2017 N. K. Sferopoulos. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.