Research Article
Volume 5 Issue 1 - 2020
Effect of Aqueous Extract of Triclisia Dictyophylla on Induced Depression in Mice
1Animal Physiology Laboratory, Department of Biological Sciences, Higher Teacher’s Training College, University of Yaoundé I, Cameroon
2Organic Chemistry Laboratory, Department of Chemistry, Higher Teacher’s Training College, University of Yaoundé I, Cameroon
3Animal Physiology Laboratory, Department of Animal Biology, University of Yaoundé I, Cameroon
4Animal Physiology Laboratory, Department of Biological Sciences, Faculty of Sciences, University of Ngaoundéré, Cameroon
*Corresponding Author: Ayissi Mbomo Rigobert Espoir, Senior Lecturer, Higher Teacher Training College, University of Yaoundé I, PoBox 47 Yaounde (Cameroon).
Abstract
Depression affect between 2 and 5% of world’s population, the conventional medicine provides a wide variety of antidepressants not safe for patients. We assessed antidepressant properties of Triclisia dictyophylla using animal models of depression including Forced Swimming Test (FST), Tail Suspension Test (TST) and anhedonia test. Five groups of six animals each were fed-up with distilled water, imipramine and doses 50, 100, 150 mg/kg. We then considered within FST and TST, duration of immobility (TI) and time of the immobility occurrence. During the anhedonia test, we measured the variation of sugar water consumption and body mass variation. Four doses of the plant The 50, 100, 150 and 300 mg/kg were used to assess the acute toxicity. In the FST, dose 150 mg/kg induced a significant (p˂0.05) decrease of the immobility time up to 89.1 s and significantly (p ˂0.05) increased up to 117.83 s the delay of immobility onset. As in the FST, it was observed in TST a significant (P ˂ 0.01) increase of immobility occurrence of almost 442%, and a reduction of 48.64% of immobility time at dose 150 mg/kg. The anhedonia test presented a significant dose-dependent increase (p ˂ 0.05) up 19.2 ml in the consumption of sugar water at dose 50 mg/kg. These observed effects on FST, TST and anhedonia test suggest the presence in the extract of T. dictyophylla of bioactive compounds against depression. In the acute toxicity study, T. dictyophylla induced half deaths at dose 300 mg/kg. Dose 150mg/kg could be the good therapeutic.
Keywords: Depression; Triclisia dictyophylla; anhedonia; Forced Swimming Test; Tail Suspension Test
Abbreviations: Triclisia dictyophylla: T. dictyophylla; Forced Swimming Test: FST; Tail Suspension Test: TST; Duration of immobility: TI
Introduction
Now a days characterized with high stress, there is an urgent need for neuro-protective and neuro-pharmacological agents. Usually, complex biochemical, neuronal and immunological mechanisms are involved in this stress. The stress plays a key role in the genesis and spread of a variety of pathological states ranging from endocrine disorders including diabetes mellitus, male impotence, immune-suppression, cardiovascular diseases (hypertension), cognitive dysfunctions, to psychiatric disorders such as anxiety and depression [1].
The Oxford dictionary defines depression as a state of lack of energy or vitality. Clinically, depression, also known as major depressive disorder, is a neuropsychiatric syndrome, sometimes inherited and characterized by relatively subtle cellular and molecular alterations distributed in the circuits of neural substrates. According to recent epidemiological studies, severe forms of depression affect between 2 and 5% of the world's population, while up to 20% suffer from mild forms. According to some authors, depression causes the greatest health decline compared to chronic diseases such as diabetes and arthritis, and in 2020, it will be the second leading cause of disability in the world, making it a real public health problem [2]. Moreover, depression is present in many psychiatric disorders, but also in acute intoxications by drugs (alcohol, cannabis, cocaine, caffeine ...), and sometimes associated with many organic diseases (asthma, hypoglycemia ...). To treat depression, the conventional medicine provides a wide variety of antidepressant drugs, but these currently prescribed treatments are still not safe for patients. Economically, these drugs are generally very expensive and therefore not readily available to people living in poor countries, especially in rural areas. It also emerges report of the World Bank and the World Health Organization (WHO) published on December 13, 2017 that, at least half of the world's population does not have access to essential health services [3].
In the frantic search for new treatments for neurological diseases (especially antidepressants), most of the population especially in rural Africa has turned to alternative medicine. It is as well as in developed and developing countries, alternative medicine including acupuncture, naturopathy, phytotherapy, and homeopathy is booming, in the disadvantage of conventional medicine. The use of medicinal plants (phytotherapy) has prompted several authors to invest in ethnobotanical, pharmacological, photochemical and technological researches, reason why the plant of interest is Triclisia dictyophylla (T. dictyophylla) of Tracheophyta Phylum, belonging to the Class of Magnoliopsida, Order of Ranunculales, Family of Menispermacea, Genus of Triclisia and Specie Triclisia dictyophylla. This plant is used in the traditional pharmacopoeia all over Africa. T. dictyophylla helps in conditions such as joint pain, edema, venereal disease, anemia, fever, malaria, diarrhea, gastric disorders [4].
As with palpitations, pain reliever for the mentally ill during epileptic and respiratory attacks, abortifacient (substance that causes abortion) and emmenagogue (substance that regulates the menstrual cycle), furthermore as an ingredient in arrow poisons [5]. With this broad therapeutic potential in traditional medicine, the main objective of this work being to highlight the antidepressant properties of this plant already used in traditional medicine to treat several conditions of the nervous system. More specifically, it is a question of evaluating the effect of T. dictyophylla on:
- - Immobility occurrence and immobility time on the Forced Swimming Test (FST) and the Tail Suspension Test (TST);
- - The consumption of sugar water and variation in body mass;
- - Acute toxicity.
Materials and Methods
Materials
Animal material
The experiments were carried out on white mice Mus musculus swiss of both sexes. They were eight weeks old, and the body weight varied between 20 g and 30 g. The animals were bred at the animal room of the Higher Teacher’s Training College (HTTC) of Yaoundé (Cameroon), under the following conditions: a relative humidity of 50 ± 10%; an average temperature of 23°C; a normal cycle of 12 hours of light and 12 hours of darkness. The breeding was done in plastic cages of which the chip constituted the litter and the animals had free access to food and tap water (supplied by plastic bottles). Animals’ handling was done in accordance with the National Ethics Guide (FWA-IRB00001954).
Plant material
The plant of interest is Triclisia dictyophylla. The bark of T. dictyophylla vines was harvested in December 2016 in the village Sok-Elle by Pouma (district of Messondo in the center region Cameroon), then its subsequent identification carried out by Mr. Nana Victor, botanist at the national herbarium of Cameroon (HNC) where the reference sample was deposited. The bark of T. dictyophylla were cut and then exposed to dry for several days on sunlight. 500 g of T. dictyophylla powder have thus been obtained after grinding the dried bark fragments in a crushing mill, and then used to prepare the aqueous extract.
Methods
Preparation of solutions
Preparation of the aqueous extract of the plant
The extract of the plant was obtained by maceration method as previously described by Pierre and Lis in 2007. To realize maceration of T. dictyophylla, 500 g of powder were immersed in 10 L of cold water and the mixture left for maceration during 48 hours. After filtration using Whatman paper N° 3, 470.14 g of solid residue was separated from the solution. This filtrate obtained (5.8 L), subsequently underwent concentration on a rotary evaporator. After complete separation with the solvent, 29.86 g of crude extract was thus obtained, representing extraction yield of 5.97%.
Preparation of solutions to be administered to animals
The different concentrations of the extract of T. dictyophylla used were obtained by a progressive dilution of the stock solution of concentration 30 mg/mL. By diluting the main solution to 1/2, 1/3 and 1/6, the different concentrations (15, 10 and 5 mg/mL) required were obtained. All the substances were administered ip at a volume of 10 mL/kg, therefore the different doses corresponding to these dilutions were 150, 100 and 50 mg/kg respectively.
Imipramine (imipramine hydrochloride supplied by Amdipharm limited, Ireland)
The principle of preparation of the solution of the reference chemical drug was identical to that described for the preparation of the plant. Therefore, on the day of handling, 20 mg of imipramine powder was introduced into a 10 mL vial. After adding distilled water to the mark, a solution of imipramine at a concentration of 2 mg/mL was obtained. The reference drug was also administered ip at the volume of 10 mL/kg. The dose of imipramine of 20 mg/kg was thus obtained.
Glucose solution
A solution of glucose 3% was obtained by introducing 30 g of glucose powder into a 1 L flask. Once the volume has been completed with distilled water until with the gauge line, the homogenization was done using a stirrer.
Distribution of animals
The experimental protocol implemented during this work consisted of five groups of six animals each. The different groups were homogeneous in weight and sex, consisted of mice of both sexes weighing between 20 and 30 g. Each group was subjected to a specific treatment, and substances administered ip, 30 minutes before the beginning of the test. One group, treated with distilled water was considered to be the negative control. Three other groups, each treated with one dose of T. dictyophylla extract, represented the test groups and a fifth group having received the reference chemical was the positive control. 24 hours before handling, the mice were transferred from the animal house to the laboratory for acclimatization. Each test day, the first activity focused on measuring animals’ body weight.
Behavioral tests
To study the antidepression effects of T. dictyophylla in mice, three behavioral tests were performed as follows.
Forced Swimming Test (FST)
The FST, a recognized and reliable test for the activity of antidepressants, is widely used in antidepressant research laboratories. It was developed in 1977 by Porsolt and his Collaborators [6]. The test consists to submit the mouse in a situation where it must fight to survive, with no possibility to escape. We then measure the motivation shown by the mouse to escape. The FST was carried out for 6 minutes, so from the 2nd minute the following parameters were noted (in seconds): the time of onset of the first immobility (s) and the total immobility (s).
Tail Suspension Test (TST)
This test measures immobilization in rodents, with the principle of observing for six minutes the alternating periods of agitation and immobility of a mouse suspended by the tail. It was developed by Stéru and colleagues in 1985 [7]. During the four last minutes of this test, we note the time of onset of immobility (s) and the total immobility time (s).
Anhedonia test
The anhedonia test is based on the evaluation of the rodent's preference for two possibilities. It is particularly carried out in depression models. The experimental device used was adapted from studies previously carried out by Krishnan and his collaborators in 2007, then the subsequent ones by Robison and his collaborators in 2014, and Serchov and his collaborators in 2015 [8]. The test took place in two phases:
Stress induction
To do this, the animals' legs were first placed on a hot plate for 3 minutes at 60°C within 30 seconds per trial; later these same animals were subjected to forced swimming in cold water (5° C). These actions were repeated during the first four days of the test. After forced swimming, to prevent the mice from dying from hypothermia, they were quickly removed from the water and wrapped in a towel, then warmed for 1 hour in a special cage. After restoration of normal temperature, each animal was reintroduced into its experimental cage.
Test
Two identical, labeled bottles were filled with 50 mL tap water for one and 50 mL glucose solution for the other. From day 2 to day 5, the parameters noted were the amount of sweet water consumed by each animal (in mL), as well as the body mass (mg) of each animal. To avoid habituating animals to the content of a single bottle, at regular intervals of 5 hours the positions of these bottles were reversed.
Acute toxicity
To assess the acute toxicity of the plant, a total of 30 albino mice of the Mus musculus swiss strain, male and female, weighing between 20 and 30 g were used. The mice were shared into 5 groups of 6 animals each (3 males and 3 females), and were deprived of food and water for 12 hours before the beginning of the test. The doses of 50, 100, 150 and 300 mg/kg of the extract of T. dictyophylla were administered to the mice ip. Once the various treatments have been carried out, the general behavior of the subjects in each group was observed at specific time intervals (1h, 2h and 24h) and mortality in each group observed.
Data analysis
Data were expressed as mean (n=6) ± SEM. The analysis was done using the ANOVA (analysis of variances) one way followed by a post-hoc (Dunnet) and the probabilities of less than 5% were selected as significant.
Results
Effects of T. dictyophylla on tail suspension test parameters
Delay of appearance of immobility
The mice in the negative control group spent an average 9.5 s of agitation in the suspension box before stopping for the first time. No significant difference in the time of occurrence of the first immobilization was observed with the smallest dose of T. dictyophylla (50 mg/kg). However, the treatment of animals with the two largest doses of the aqueous extracts of T. dictyophylla (100 and 150 mg/kg) results in an increase in the time of appearance of the first immobility (respectively to 35.4 s and 51.5 s). Indeed, the treatment of animals with dose 150 mg/kg of the aqueous extract of T. dictyophylla resulted in a very significant increase (P ˂ 0.01) of almost 442% of the result obtained with the negative control group. After treatment with the reference antidepressant, imipramine at dose 20 mg/kg, there was also a significant increase (p ˂ 0.05) in the time of occurrence of the first immobility at 41 s, i.e. an increase of approximately 331% compared to the negative control.
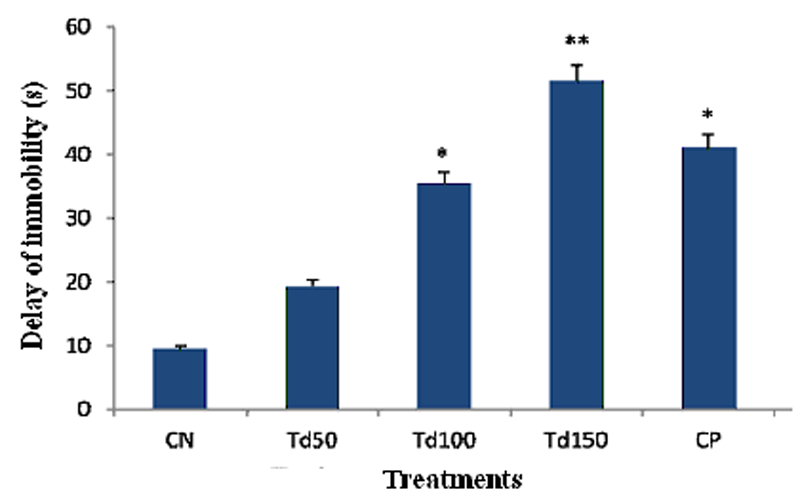
Figure 1: Effect of the aqueous extract of T. dictyophylla (ip) on the time to onset of immobility during the TST.
Each bar of the histogram represents the average time of occurrence of immobility (s) + SEM; with n = 6. * p ˂ 0.05, ** p ˂ 0.01, significant difference compared to the negative control group treated with distilled water. With CN: negative control; Td50, Td100 and Td150: aqueous extract of T. dictyophylla at doses 50, 100, 150 mg/kg respectively; CP positive control (treated with imipramine 20 mg/kg).
Duration of immobility
The mean duration of immobility was 173.75 s in mice of the negative control group given intraperitoneal administration of distilled water. As expected, imipramine decreased the duration of immobility to 143.9 s, a reduction of about 17%, although it was not statistically significant. The results obtained from this study also revealed a dose-dependent reduction in the duration of immobility after treatment of the animals with the three doses of the aqueous extract of T. dictyophylla. Indeed, the duration of immobility went from a significant value (p˂0.05) of 119.66 s in mice treated with the dose 50 mg/kg of T. dictyophylla to 89.1 s with dose 150 mg/kg of T. dictyophylla, a reduction of 48.64% compared to the negative control group.
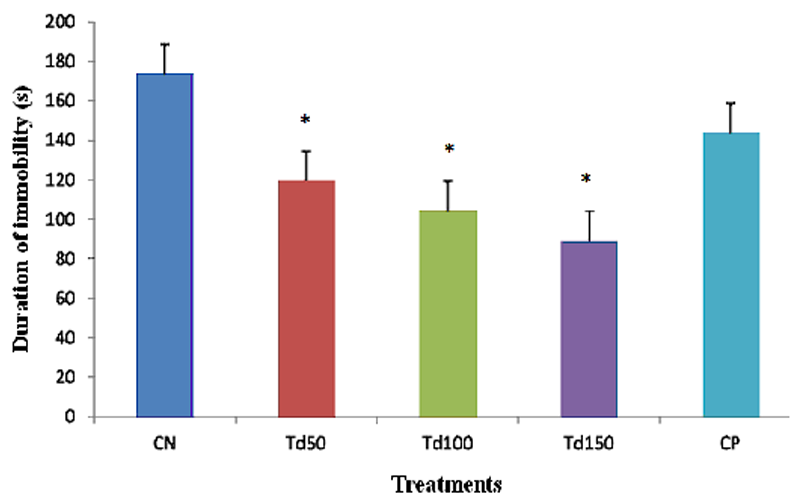
Figure 2: Effect of the aqueous extract of T. dictyophylla (ip) on the total immobility time during the TST.
Each bar of the histogram represents the average of the duration of the immobility (s) + SEM; with n = 6. * p ˂ 0.05, significant difference compared to the negative control group treated with distilled water. With CN: negative control; Td50, Td100 and Td150: aqueous extract of T. dictyophylla at doses 50, 100, 150 mg/kg respectively; CP positive control (treated with imipramine 20 mg/kg).
Effects of T. dictyophylla on parameters of forced swimming test
Delay of appearance of immobility
In the FST, the mean time of immobility occurrence was 51.75 s in the negative control group. ip administration of the aqueous extract of T. dictyophylla at the three doses of 50, 100 and 150 mg/kg resulted in an increase in the time of immobility appearance. This increase appeared to be dose dependent for the two largest doses 100 and 150 mg/kg. At dose 150 mg/kg, the extract increased the time of immobility onset to a significant value (p˂0.05) of 117.83 s, an increase of approximately 127 % in comparison with the negative control group. Fluoxetine also induced an increased time of immobilization occurrence to 71.25 s, but unfortunately this value was not statistically significant.
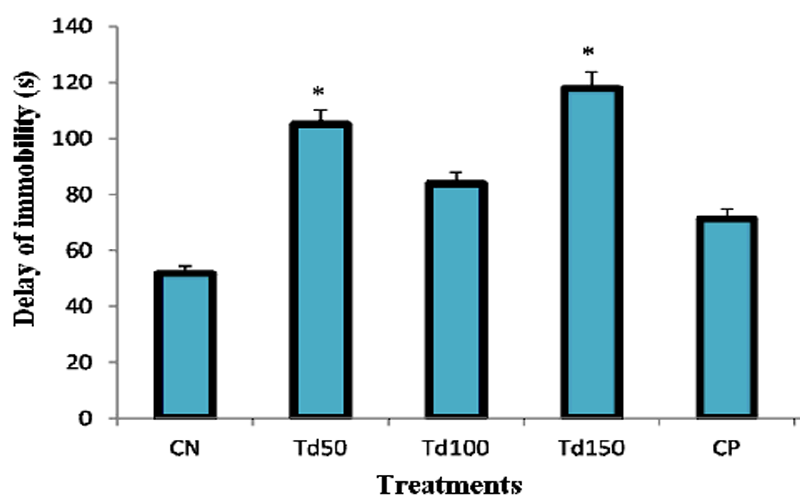
Figure 3: Effect of the aqueous extract of T. dictyophylla (ip) on the time to onset of immobility during the FST.
Each bar of the histogram represents the average time of the appearance of the first immobility (s) + SEM; with n = 6. * p ˂ 0.05, significant difference compared to the negative control group treated with distilled water. With CN: negative control; Td50, Td100 and Td150: aqueous extract of T. dictyophylla at doses 50, 100, 150 mg/kg respectively; CP positive control (treated with imipramine 20 mg/kg).
Duration of immobility
This study shows that the aqueous extract of T. dictyophylla caused a decrease in the duration of immobility. In fact, in the negative control group treated with distilled water, the average duration of immobility was 141.25 s. Although the smallest doses of the extract 50 and 100 mg/kg exhibited the non-significant decreases, dose 150 mg/kg induced a significant reduction in the duration of immobility at 72.5 s.
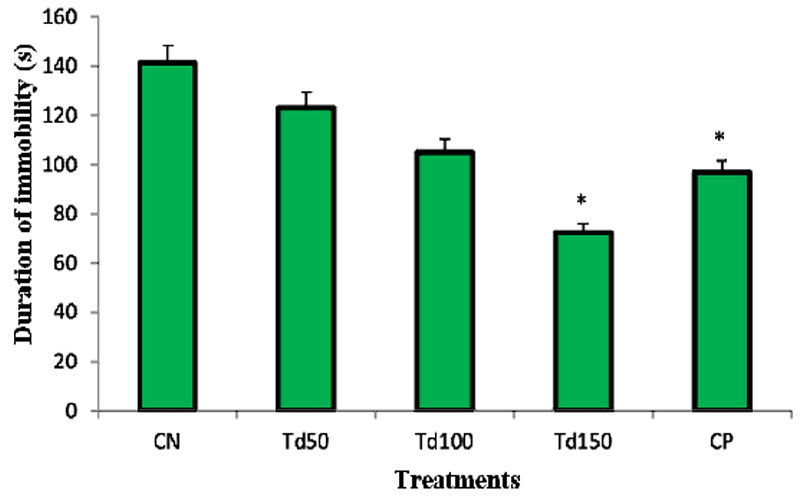
Figure 4: Effect of the aqueous extract of T. dictyophylla (ip) on the total immobility time during the FST.
Each bar of the histogram represents the average of the duration of the immobility (s) + SEM; with n = 6. * p ˂ 0.05, significant difference compared to the negative control group treated with distilled water. With CN: negative control; Td50, Td100 and Td150: aqueous extract of T. dictyophylla at doses 50, 100, 150 mg/kg respectively; CP positive control (treated with imipramine 20 mg/kg).
Anhedonia test
Effect of T. dictyophylla on the consumption of sugar water
The results present the volume of total sugar solution consumed by the animals of the different groups during the 5 days of the anhedonia test. It appears that the amount of sugar solution consumed by the animals in the negative control group was approximately 5 ml. The reference drug imipramine at dose 20 mg/kg induced a significant increase of 295% (p ˂ 0.05) in the consumption of sugar water when compared to the negative control. All the doses of the extract resulted in a significant increase (p ˂ 0.05) in the consumption of sugar water, with the maximum value of 19.2 ml observed in the animals of the group treated with the extract of T. dictyophylla at dose of 50 mg/kg.
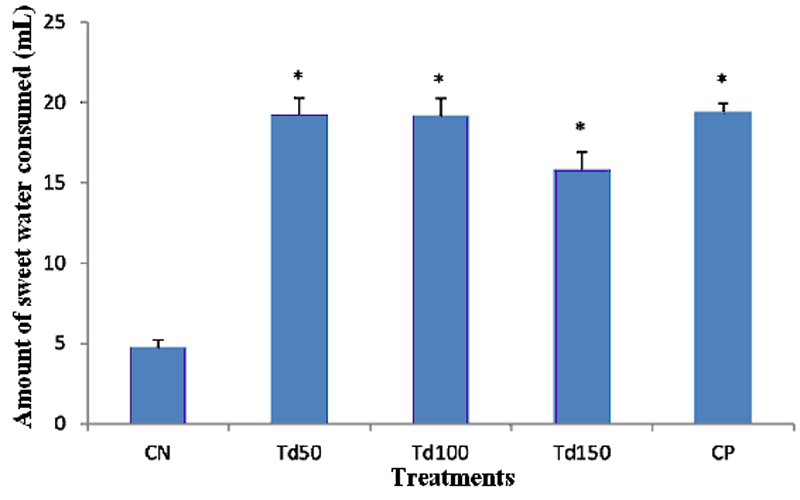
Figure 5: Effect of the aqueous extract of T. dictyophylla (ip) on the amount of sugar water consumed (mL) during the anhedonia test.
Each bar of the histogram represents the average of the volume of sugar water consumed (mL) + SEM; with n = 6. * p ˂ 0.05, significant difference compared to the negative control group treated with distilled water. With CN: negative control; Td50, Td100 and Td150: aqueous extract of T. dictyophylla at doses 50, 100, 150 mg/kg respectively; CP positive control (treated with imipramine 20 mg/kg).
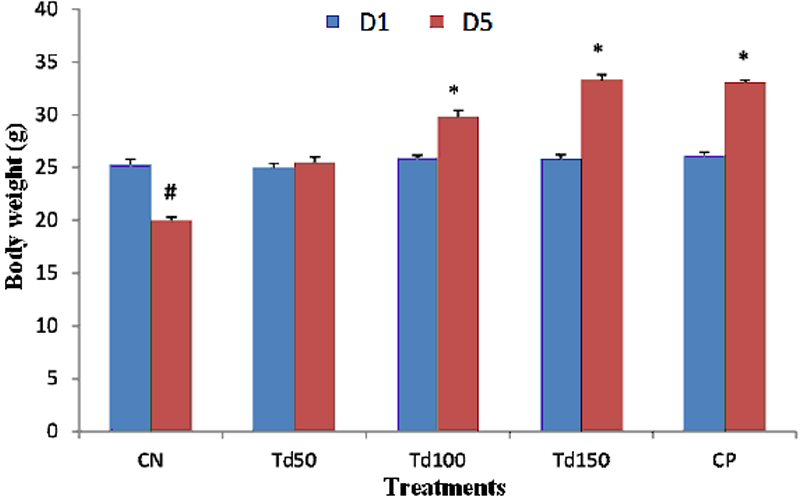
Effect of T. dictyophylla on body mass
In all groups, at day1 of the test, the average mass of the mice was 25.64 g. between day1 and day5, a variation of animals mass was observed in the different test groups. While it was note in the negative control group, a significant decrease (p˂0.05) in the body mass of 5.3 g. However on day5, a dose-dependent increase in body mass was observed in animals treated with the reference substance and the different doses of the plant. The mass therefore increased from the average value of 20 mg in the negative control group to a significant value (p˂0.05) of approximately 33 g in the group treated with the aqueous extract of T. dictyophylla at dose 150 mg/kg.
Each bar of the histogram represents the average of the mass (g) + SEM; with n = 6. * p˂ 0.05, significant difference compared to the negative control group having received the distilled water, # p˂0.05, significant difference compared to day 1 in the CN group. With CN: negative control; Td50, Td100 and Td150: aqueous extract of T. dictyophylla at doses 50, 100, 150 mg/kg respectively; CP positive control (treated with imipramine 20 mg/kg).
Acute toxicity and determination of LD50
A few minutes (05) after administration of the treatment, the mice of the negative control group having received distilled water showed normal locomotor activity. No death was recorded in group treated with dose 50, 100 and 150 mg/kg of upon 24 hours observation. Whereas 30 minutes later, three deaths were recorded in mice given dose 300 mg/Kg, making them the LD50.
Discussion
In this work, we evaluated the effects of the aqueous extract of T. dictyophylla on desperation behavior and anhedonia (loss of interest in things formerly pleasant) using some animal models of depression. Still called Porsolt's swimming test, the FST was originally developed in rats. Nowadays very widespread in other rodents and mainly mice, the FST is commonly used to assess the effectiveness of antidepressant drugs and the effects of different behavioral and neurological manipulations in the basic and preclinical research [9]. Predictive test, reliable of antidepressants activity, and very widespread in antidepressants research laboratories, it involves submitting the mouse in a situation where it has to fight for survive, but with no possibility to escape. During the FST, it was observed a dose-dependent and even significant increase (p <0.05) in the delay of immobility occurrence, starting from dose 100 mg/kg.
A significant (p <0.05) and dose-related decrease in immobility time was also observed with mice treated with doses 50, 100 and 150 mg/kg of the extract of T. dictyophylla, when compared to the animals of the negative control group. According to the preliminary work of Porsolt and his colleagues in 1977, followed by those of several other research teams, it appears that a relatively low immobility time in comparison to the negative control, as well as relatively higher delay of immobility occurrence compared to the negative control are indicators of antidepressant activity [10] which could testify to the effectiveness of our plant against the depression induced by the swimming in the FST. The antidepressant components of the extract of T. dictyophylla could interact with some neurotransmitter systems as earlier studies have demonstrated the reduction of the immobility time after treatment with catecholamines, serotonin and neurosteroids in the Porsolt swimming test [11].
Originally developed by Porsolt and his collaborators in 1977, the FST influenced and preceded the TST developed later by Stéru and his collaborators [7]. These two tests are similar and are based on the same principles (measurement of immobility). Like the FST, the TST is a mean of screening for antidepressant substances with good reliability and predictive validity [12]. However, the TST is better appreciated for evaluating of antidepressant efficacy, because unlike the FST, there is no hypothermia risk after submersion in water [13]. There is a notorious difference between the TST and the FST in terms of experimental animals’ performance and sensitivity; therefore, the results obtained with the FST not need necessarily be reproduced in the TST [14].
In the TST, animals face uncomfortable and unavoidable situations (suspension) but moderately stressful. Lack of behavior related to escape is considered immobility. So when the animal is suspended by the tail, he is immediately engages in several agitations. Generally, this behavior is followed by immobility access and reversed by antidepressants [9, 15]. As in the FST, it was observed in TST a dose-dependent reduction in immobility time of mice. Dose 150 mg/kg induced a significant (p˂0.05) decrease of the immobility time compared to the negative control group. On the other hand, the delay of immobility occurrence of treated animals has significantly increased (p ˂ 0.05) for doses 50 and 150 mg/kg. The results of this test noticed that the immobility time of mice treated with various doses of T. dictyophylla is smaller than those of negative control group and their delay of immobility occurrence is higher than those of negative control group, thus corroborating findings of several researchers engaged in screening for antidepressant substances [7,16].
This result confirms the hypothesis made with the FST, suggesting that the extract of T. dictyophylla contain antidepressant components [17, 18]. Anhedonia is a loss of interest in things that used to be pleasurable, and may be assessed by the degree of consumption of sweet foods [8]. Naturally, mice have a preference for sweet foods, with an assurance that this preference is proportional to the pleasure the animal experiences when it consumes. When the opposite situation arises, the animal is considered to be exposed to anhedonia which is one of the classic depression sign [19]. The main parameters recorded on mice treated with aqueous extracts of T. dictyophylla during the test were the consumption of sugar water and the change in body mass. Our results show a significant dose-dependent increase (p˂0.05) in the consumption of sugar water, suggesting the presence in the extract of T. dictyophylla of bioactive compounds against depression, thus supporting previous results of Hoffman in 2016 [20]. The result of this high consumption of sugar water would increase body mass.
This increase of body mass could be the consequence of glucocorticoids and neuropeptides Y2 secreted during the stress induction. Indeed, glucocorticoids would increase the consumption of sugary foods, which by well-known metabolic pathways (synthesis of fatty acids), would transform sugars into fatty acids, and The peptide Y2 would act on the fatty mass by promoting the proliferation of adipocytes. However the induced stress would lead to a reduction in weight given that the animals in the negative control group showed a decrease in body weight. Therefore we might think that T. dictyophylla would act as an inhibitor of weight loss induced by the stress. Phytochemistry is interested in the elucidation of the structure and the characterization of the biological activity of various chemicals produced by plants. Many natural bioactive compounds with pharmacological properties have been isolated from T. dictyophylla. These are bisbenzylisoquinoline alkaloids (the phaeanthin, N, N’-dimethylphaeanthin [21] and tetrandrine), related dioxin alkaloids (trigilletin, trigilletimine) and a morphine alkaloid known as tridyctyophylline [22].
Morphine is widely used in the context palliative care, where the objective becomes improving living comfort, with first place the fight against pain. Beyond the analgesic effect, one can feel a temporary well-being and fullness effect linked to this molecule. Thus, it could be suggested that the tridyctyophylline (morphinan alkaloid) contained in the extract of T. dictyophylla would be responsible for the inhibition of anhedonia observed in the glucose preference test and therefore responsible for its antidepressant effects. Indeed, like morphine, tridictyophylline would act in the same way as endorphins. Its resemblance to endorphin molecules would allow its bounding on opiate receptors Mu (μ) (in the nucleus accumbens in the brain), and Kappa (κ) and Delta (δ) receptors (in the spinal cord) which would inhibit these receptors by making them not receptive to information from the nerves.
The direct consequence of bounding on μ receptors would primarily be the supra-spinal analgesia. The binding of morphine molecules on μ receptors, would excite dopaminergic neurons to release dopamine into the body, thus inducing a feeling of pleasure or euphoria. The work of Elham’s team on the effects of forced swimming stress on morphine awareness and involvement dopaminergic receptors D1/D2 in the nucleus accumbens would confirm this hypothesis [23]. Very recent work has also argued that the beneficial effects of morphine are associated with improved and attenuated dopamine metabolism, in the nucleus caudate and nucleus accumbens respectively. Serotonin metabolism is improved in both regions [24]. In the acute toxicity study, a decoction of T. dictyophylla produced three deaths at dose 300 mg/kg. This would confirm that T. dictyophylla is very toxic. Preliminary research of Kronlund and his collaborators in 1970 showed that the extract aqueous of T. dictyophylla contained pheanthine and N, N-1-dimethylpheanthine. These compounds produce symptoms of toxicity similar to that of curare [21]. The toxicity of these compounds has been proven and, as a result, extracts of T. dictyophylla are used in the Central African Republic to immobilize animals during hunting [25]. Certainly, these compounds would be the cause of the death of the three mice at dose 300 mg/kg, and the reduced mobility following the injection in the less than or equal to 150 mg/Kg.
Conclusions
The result of this work shows a dose-dependent increase in immobility and delay of immobility occurrence; a dose-dependent increase in the consumption of sugar water and consequently a dose dependent increase in body mass; and finally the death of the half of the mice at dose 300 mg/kg. Dose 150 mg/Kg presented a significant difference in all tests. Overall, it should be noted that the aqueous extract of Triclisia dictyophylla has bioactive compounds against depression, with the dose 150 mg/Kg considered as therapeutic dose. These results would explain the use of T. dictyophylla in medicine traditional in the treatment of certain central nervous system disorders.
Acknowledgements
The authors thank the Laboratory of Organic Chemistry of the HTTC of Yaoundé for the extraction and Mr. Nana Victor, botanist at the national herbarium of Cameroon for identification.
Conflict of interest
Financial interest or any conflict of interest: NONE
References
- Dennis TA. “Neurophysiological Markers for Child Emotion Regulation from the Perspective of Emotion-Cognition Integration: Current Directions and Future Challenges”. Developmental Neuropsychology 35.2 (2010): 212-230.
- Moussavi S., et al. “Depression, chronic diseases, and decrements in health: results from the World Health Surveys”. Lancet 370.9590 (2007): 851-858.
- Zhang ZJ. “Therapeutic effects of herbal extracts and constituents in animal models of psychiatric disorders”. Life Sciences 75.14 (2004): 1659-1699.
- Kokwaro JO. “Medicinal plants of East Africa”. 2nd Edition. Kenya Literature Bureau 1993, Nairobi, Kenya. 401pp.
- Neuwinger HD. “African traditional medicine: a dictionary of plant use and applications”. Medpharm Scientific Publishers 2000; 589 pp.
- Cong-Cong Q., et al. “Biological Factors Influencing the Mice Forced Swim Test”. Journal of Neurology & Neuromedicine 1.4 (2016): 21-24
- Muhammad A. “Tail suspension test to evaluate the antidepressant activity of experimental drugs”. Bangladesh Journal of Pharmacology 11(2016): 292-294
- Serchov T., et al. “Increased Signaling via Adenosine A1 Receptors, Sleep Deprivation, Imipramine, and Ketamine Inhibit Depressive-like Behavior via Induction of Homer1a”. Neuron 87.3 (2015): 549- 562.
- Hongkun B., et al. “Griflola frondosa (GF) produces significant antidepressant effects involving AMPA receptor activation in mice”. Pharmaceutical Biology 55.1 (2017): 299-305.
- Rennekamp AJ., et al. “σ1 receptor ligands control a switch between passive and active threat responses”. Nature Chemical Biology 12.7 (2016): 552-558.
- Dhir A and Kulkarni SK. “Involvement of nitric oxide (NO) signaling pathway in the antidepressant action of bupropion, a dopamine reuptake inhibitor”. European Journal of Pharmacology 568.1 (2007): 177-185
- Dhingra D and Valecha R. “Evaluation of the antidepressant-like activity of Convolvulus pluricaulis choisy in the mouse forced swim and tail suspension tests”. Medical Science Monitor 13.7 (2007): 155-161.
- O'Leary OF, Cryan JF. “Mood and Anxiety Related Phenotypes in Mice”. Humana Press 42 (2009): 119-137.
- Robles-Molina E., et al. “Increased antidepressant-like effect of desipramine combined with central stimulants (caffeine and amphetamine) in mice”. Central European Journal of Biology 7.3 (2012): 391-396.
- Can A., et al. “The Tail Suspension Test”. Journal of Visualized Experiments 59 (2012): 3769.
- Crowley JJ., et al. “Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test”. Psychopharmacology 183.2 (2005): 257-264.
- Gould TD., et al. “Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test”. Neuropharmacology 54.3 (2008): 577-587.
- Gwenolé L and Boyer P. “Anhedonia in endogenomorphic depression”. Psychiatry Research 60.1 (1996): 57-65.
- Anika K., et al. “Sugar intake from sweet food and beverages, common mental disorder and depression: prospective findings from the Whitehall II study”. Scientific Reports 7 (2017): 6287.
- Kronlund A., et al. “Occurrence of pheanthine and N,N1-dimethylpheanthine in Triclisia dictyophylla and T. patens. New simple method for estimation of muscle relaxant effect”. Acta Pharmaceutica Suecica 7.3 (1970): 279–284.
- Tiam ER., et al. “Secondary metabolites from Triclisia gilletii (De Wild) Staner (Menispermaceae) with antimycobacterial activity against Mycobacterium tuberculosis”. Natural Product Research 33.5 (2019): 642-650.
- Elham C., et al. “The effect of forced swim stress on morphine sensitization: Involvement of D1/D2-like dopamine receptors within the nucleus accumbens”. Progress in Neuro-Psychopharmacology and Biological Psychiatry 70 (2016): 92-99.
- Darakhshan JH., et al. “Dopamine and serotonin metabolism associated with morphine reward and its inhibition with buspirone: A study in the rat striatum”. Pharmacology Biochemistry Behavior 170 (2018): 71-78.
- Bahuchet S. Les Pygmées Aka et la forêt centrafricaine. Coll " Ethnosciences 1985, Selaf, Paris: 638p.
Citation: Ayissi Mbomo Rigobert Espoir., et al. “Effect of Aqueous Extract of Triclisia Dictyophylla on Induced Depression in Mice”. Current Opinions in Neurological Science 5.1 (2020): 01-11.
Copyright: © 2020 Ayissi Mbomo Rigobert Espoir., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.