Research Article
Volume 2 Issue 4 - 2018
SLUG & YKL-40 Immunohistochemical Expression in Invasive Ductal Carcinoma of the Breast (IDC); Clinicopathological Values
1Lecturer of Pathology, Faculty of Medicine, Zagazig University, Zagazig, Egypt
2Lecturer of General Surgery, Faculty of Medicine, Zagazig, Egypt
2Lecturer of General Surgery, Faculty of Medicine, Zagazig, Egypt
*Corresponding Author: Mouhamed A Fouad, Lecturer of Pathology, Faculty of Medicine, Zagazig University, Zagazig, Egypt.
Received: July 04, 2018; Published: July 13, 2018
Abstract
Background: Invasive ductal carcinoma is a common and fatal breast cancer. It is important to detect recent prognostic biomarkers and therapeutic targets for better risk stratification and management of such cancer type. SLUG, is a transcription factor and a Snail family member, which plays a vital role in Epithelial-mesenchymal transition [EMT] activation in cancer cells. YKL-40 is also named chitinase-3-like-1 and its aberrant expression was detected in a variety of cancers. Results about clinicopathological and prognostic roles of combined SLUG & YKL-40 expression in invasive ductal carcinoma of the breast still needs clarifications.
Aim of the work: to assess SLUG & YKL-40 expression in invasive ductal carcinoma of the breast tissue, and to correlate their combined expression with clinicopathological parameters of the tumor.
Methods: SLUG& YKL-40 expression was evaluated in sections from sixty paraffin blocks that were previously diagnosed as invasive ductal carcinoma of the breast of different grades, stages and molecular subtypes using immunohistochemistry. Then associations between their expressions levels, clinic-pathological parameters of our cases were analyzed.
Results: SLUG high expression was associated with older age of the patients (p=0.002), higher grade (p=0.003), presence of lymph node metastases (p=0.009), advanced stage (p= < 0.001) presence of distant metastases (p=0.011), negative ER (p=0.004), & PR (p=0.005) hormonal receptors positivity, aggressive molecular subtype, (p= < 0.001). YKL-40 high expression was associated with older age of the patients (p = 0.008), higher grade (p=0.007), presence of lymph node metastases (p=0.03), advanced stage (p= < 0.001), presence of distant metastases (p=0.021), negative ER (p=0.004), & PR (p = 0.007) hormonal receptors positivity, aggressive molecular subtype, (p=0.004).
We found a significant positive association between SLUG and YKL-40 tissue protein expression in IDC of the breast. (Spearman’s r= +0.849), (p < 0.001).
Conclusion: SLUG & YKL-40 are markers of poor prognosis of breast carcinoma patients.
Keyword: Invasive ductal carcinoma of the breast; SLUG YKL-40; immunohistochemistry; Clinicopathological values
Introduction
Carcinoma of the breast is the commonest and most fatal female cancer ranking the 2nd among all cancer types worldwide [Wang., et al. 2017]. Invasive ductal carcinoma (IDC) is the commonest subtype of carcinoma of the breast [Ghoncheh., et al. 2016]. IDC is still has high incidence, seriousness and high fatality rate despite improvement in its management modalities e.g. surgery, chemotherapy, radiotherapy, hormonal and molecular targeted therapies [Siegel., et al. 2015]. Most of the management modalities of IDC are based on the novel molecular classification and hormonal receptors positivity [Yawen Guo., et al. 2017]. But not all patients get the best value from these recent therapeutic strategies, because of lack of suitable recent biomarkers which helps early detection, prediction of high incidence of invasion, progression and metastases of such cancer type [Azim., et al. 2016]. There is an urgent need for studying the detailed pathogenesis of IDC of the breast, identify novel factors that are responsible for its initiation, progression and invasion aiming at detection of novel biomarkers and targeted therapies to improve patients prognosis [Wan., et al. 2017]. Classic prognostic clinicopathological parameters for IDC of the breast are grade, stage, lymph node and hormonal receptors status [Coates., et al. 2015]. But roles of these parameters are not clear enough for better risk stratification of patients; additionally novel biomarkers are needed to be correlated with these prognostic clinicopathological parameters aiming to reach novel therapeutic targets for such serious cancer. As the major problem in progression of IDC of the breast and other cancers is the process of invasion, lymph node and metastases most of recent studies focused on the detailed mechanisms that are responsible for such process. The most commonly studied process is the Epithelial-mesenchymal transition (EMT) process which is highly incriminate in the process of cancer invasion and metastases. In EMT malignant epithelial cells lose their adhesive properties, acquire mesenchymal highly mobile and invasive properties that give them the power of invasion and distant metastases [Lamouille., et al. 2014]. So many interacting signaling pathways, transcription factors and proteins are responsible for induction of EMT in cancer; Slug, which is a transcription factor of C2H2-type zinc-finger subtype is a Snail family member named Snail2. SLUG has been found to play many roles in induction of EMT process and progression of various cancer types. It has many roles in repression of E-cadherin in cancer cells [Bai., et al. 2017], and regulation stemness in cancer stem cells [Lee., et al. 2017]. A recent studied cancer biomarker is YKL-40 which is known as chitinase-3-like-1 and is a member of a mammalian proteins family which has an amino acid sequence similar to the bacterial chitinases group of glycosyl hydrolase type [Rehli., et al. 1997& Fusetti., et al. 2003]. YKL-40 is involved in proliferation of chondrocytes, fibroblasts and macrophage, induction of inflammation and remodeling of extracellular matrix [Prakash., et al. 2013]. Aberrant YKL- 40 has been studied in cancer of many organs [Johansen., et al. 2009]. Roles of SLUG1, YKL- 40 and their roles in cancer progression have been extensively studied in various organs but their detailed role in IDC of the breast is
not studied yet.
Aim of the work: To assess SLUG & YKL-40 expression in invasive ductal carcinoma of the breast tissue, and to correlate their combined expression with clinicopathological parameters of the tumor.
Patients and Methods
This is a retrospective study done on sixty archival paraffin blocks of IDC of the breast of various grades and stages that were previously operated in General Surgery Department, Faculty of Medicine, Zagazig University, by modified radical mastectomy & axillary clearance in the period from March 2014 to March 2018. Samples are sent to, processed in Pathology department, Faculty of Medicine, Zagazig University, slides of all the sixty blocks are reevaluated, graded using Nottingham (Elston–Ellis) modification of Scarff. Bloom Richardson grading system [Elston, Ellis IO, 2002]. and staged using American Joint Committee on Cancer staging system classification (8th edition) [Giuliano., et al. 2017], then we have cut sections on positively charged slides for immunohistochemistry for SLUG & YKL-40, ER, PR, HER2 neu & KI labeling index are made for all cases, clinical data of the cases are taken by retrospective examination of patients files.
The technique of immunohistochemical staining:-
The method used for immunohistochemistry is streptavidine-biotin technique [Hsu., et al. 1981]. We have incubated sections of the sixty paraffin blocks that were put on positively charged slides with primary mouse anti- SLUG (ab180714) antibody and primary mouse monoclonal anti-YKL-40 (ab86428) antibody (1:100 dilution), at 4°C overnight. We have counterstained stained sections with hematoxylin, dehydrated, and cover slipped. We have used sections from adenocarcinoma of the colon as positive control for SLUG and ovarian carcinoma tissue as positive control for YKL-40, negative control by omission of the primary antibodies and replacing them with normal saline.
The method used for immunohistochemistry is streptavidine-biotin technique [Hsu., et al. 1981]. We have incubated sections of the sixty paraffin blocks that were put on positively charged slides with primary mouse anti- SLUG (ab180714) antibody and primary mouse monoclonal anti-YKL-40 (ab86428) antibody (1:100 dilution), at 4°C overnight. We have counterstained stained sections with hematoxylin, dehydrated, and cover slipped. We have used sections from adenocarcinoma of the colon as positive control for SLUG and ovarian carcinoma tissue as positive control for YKL-40, negative control by omission of the primary antibodies and replacing them with normal saline.
Interpretation of SLUG & YKL-40 immunohistochemical expression
We have considered cells which have brown granules in their nuclei and in their cytoplasm as positive for SLUG expression and YKL-40 expression respectively. We have scored results of both markers expression semi-quantitatively by calculating the extent and intensity of stained tumor cells. We gave the extent of stain scores from 0-3 (0 = < 5%, one = 5–25%, two = 26–50%, thee > 50%). We gave the intensity of stain scores from 0-3 (0 = negative expression, 1 = weak expression, 2 = moderate expression, 3 = strong expression,). Then we have multiplied both scores to yield the final score from1 to 9 and the value 4 is taken as a cut point where results below it were considered low and results above it are considered high expression [Han Hee Lee1., et al. 2017].
We have considered cells which have brown granules in their nuclei and in their cytoplasm as positive for SLUG expression and YKL-40 expression respectively. We have scored results of both markers expression semi-quantitatively by calculating the extent and intensity of stained tumor cells. We gave the extent of stain scores from 0-3 (0 = < 5%, one = 5–25%, two = 26–50%, thee > 50%). We gave the intensity of stain scores from 0-3 (0 = negative expression, 1 = weak expression, 2 = moderate expression, 3 = strong expression,). Then we have multiplied both scores to yield the final score from1 to 9 and the value 4 is taken as a cut point where results below it were considered low and results above it are considered high expression [Han Hee Lee1., et al. 2017].
Statistical Analysis
All statistics were performed using SPSS 22.0 for windows and MedCalc windows. The categorical variables were expressed as a number and Continuous variables were expressed as the mean ± SD & median. Continuous variables are checked using Mann Whitney U test to compare between two groups. Percent of categorical variables were compared using Pearson’s Chi-square test. A p-value <0.05 was considered significant.
All statistics were performed using SPSS 22.0 for windows and MedCalc windows. The categorical variables were expressed as a number and Continuous variables were expressed as the mean ± SD & median. Continuous variables are checked using Mann Whitney U test to compare between two groups. Percent of categorical variables were compared using Pearson’s Chi-square test. A p-value <0.05 was considered significant.
Results
Clinicopathological and demographic results of our patients were presented in (Table 1)
We have included sixty female patients with invasive duct carcinoma of no special type (IDC), with their ages ranged from (43–77) years. Immunohistochemical results
We have included sixty female patients with invasive duct carcinoma of no special type (IDC), with their ages ranged from (43–77) years. Immunohistochemical results
| Characteristics | Number | Percent | Characteristics | Number | Percent |
| Age (years) | T | ||||
| Mean ± SD | 66.38 | ±8.99 | T1 | 13 | 25% |
| Median Range | 67 | (43-77) | T2 | 25 | 38.3% |
| ≤ 55 years | 20 | 40% | T3 | 13 | 25% |
| > 55 years | 40 | 60% | T4 | 9 | 11.7% |
| Grade | Lymph node | ||||
| Grade I | 15 | 25% | Negative | 22 | 31.7% |
| Grade II | 25 | 35% | Positive | 38 | 68.3% |
| Grade III | 20 | 30% | N | ||
| ER | N0 | 22 | 28.7% | ||
| Negative | 20 | 30% | N1 | 10 | 17.3% |
| Positive | 40 | 70% | N2 | 16 | 26.7% |
| N3 | 12 | 18.3% | |||
| PR | M | ||||
| Negative | 25 | 35% | M0 | 46 | 78.3% |
| Positive | 35 | 65% | M1 | 14 | 21.7% |
| HER2/neu | AJCC Stage group | ||||
| Negative | 40 | 70% | Stage I | 10 | 17.6% |
| Positive | 20 | 30% | Stage II | 12 | 20% |
| Ki-67 | Stage III | 24 | 30% | ||
| low | 20 | 30% | Stage IV | 14 | 21.7% |
| high | 40 | 70% | |||
| ER/PR | SLUG | ||||
| Positive/Positive | 30 | 50% | Low | 22 | 46.7% |
| Positive/Negative | 4 | 6.7% | High | 38 | 53.3% |
| Negative/Positive | 6 | 10% | YKL | ||
| Negative/Negative | 20 | 33.3% | low | 30 | 50% |
| High | 30 | 50% | |||
| Molecular type | SLUG/YKL-40 | ||||
| Luminal A | 30 | 50% | Low/Low | 22 | 43.3% |
| Luminal B | 10 | 16.7% | Low/ High | 8 | 3.3% |
| HER2 amplified | 10 | 16.7% | High/Negative | 8 | 6.7% |
| Triple -ve | 10 | 16.7% | High/ High | 30 | 50% |
Table 1: Demographic, clinicopathological and immunohistochemical data of our cases.
Categorical variables were expressed as number (percentage).
Continuous variables were expressed as mean ± SD & median (range).
Categorical variables were expressed as number (percentage).
Continuous variables were expressed as mean ± SD & median (range).
Interpretation of SLUG expression, as correlated to Clinicopathological and demographic findings of our patients
| Characteristics | All | SLUG | p-value | ||||
| Low (N=22) | High (N=38) | ||||||
| (N=60) | |||||||
| No. | (%) | No. | (%) | No. | (%) | ||
| Age (years) | |||||||
| Mean ± SD | 66.38 | ±8.99 | 51.60 | ±9.01 | 60.50 | ±11.01 | 0.002 |
| Median (Range) | 67 | (43-77) | 50 | (40-76) | 60 | (39-87) | |
| ≤ 55 years | 20 | 40% | 10 | (75%) | 10 | (25%) | 0.002‡ |
| > 55 years | 40 | 60% | 12 | (27.8%) | 28 | (72.2%) | |
| Grade | |||||||
| Grade I | 15 | 25% | 9 | (75%) | 6 | (25%) | 0.004§ |
| Grade II | 25 | 35% | 10 | (76.5%) | 15 | (23.5%) | |
| Grade III | 20 | 30% | 3 | (22.2%) | 17 | (77.8%) | |
| ER | |||||||
| Negative | 20 | 30% | 2 | (4.2%) | 18 | (95.8%) | 0.003‡ |
| Positive | 40 | 70% | 20 | (75%) | 20 | (25%) | |
| PR | |||||||
| Negative | 25 | 35% | 2 | (4.2%) | 23 | (95.8%) | 0.005‡ |
| Positive | 35 | 65% | 20 | (75%) | 15 | (25%) | |
| ER/PR | |||||||
| Positive/Positive | 30 | 50% | 20 | (84.4%) | 10 | (15.6%) | 0.003§ |
| Positive/Negative | 4 | 6.7% | 0 | (0%) | 4 | (100%) | |
| Negative/Positive | 6 | 10% | 0 | (0%) | 6 | (100%) | |
| Negative/Negative | 20 | 33.3% | 2 | (5%) | 18 | (95%) | |
| HER2/neu | |||||||
| Negative | 40 | 70% | 20 | (77.1%) | 20 | (22.9%) | <0.001‡ |
| Positive | 20 | 30% | 2 | (4%) | 18 | (96%) | |
| Ki-67 | |||||||
| low | 20 | 30% | 18 | (87%) | 2 | (13%) | <0.001‡ |
| high | 40 | 70% | 4 | (21.6%) | 36 | (78.4%) | |
| Molecular type | |||||||
| Luminal A | 30 | 50% | 20 | (660%) | 10 | (33%) | <0.001‡ |
| Luminal B | 10 | 16.7% | 0 | (0%) | 10 | (100%) | |
| HER2 amplified | 10 | 16.7% | 0 | (0%) | 10 | (100%) | |
| Triple -ve | 10 | 16.7% | 2 | (20%) | 8 | (80%) | |
| T | |||||||
| T1 | 13 | 25% | 9 | (60%) | 4 | (40%) | 0.002§ |
| T2 | 25 | 38.3% | 12 | (65.2%) | 13 | (34.8%) | |
| T3 | 13 | 25% | 1 | (26.7%) | 12 | (73.3%) | |
| T4 | 9 | 11.7% | 0 | (0%) | 9 | (100%) | |
| N | |||||||
| N0 | 22 | 28.7% | 18 | (84.2%) | 4 | (15.8%) | 0.02§ |
| N1 | 10 | 17.3% | 2 | (54.5%) | 8 | (45.5%) | |
| N2 | 16 | 26.7% | 2 | (31.6%) | 14 | (68.4%) | |
| N3 | 12 | 18.3% | 0 | (0%) | 12 | (100%) | |
| Lymph node | |||||||
| Negative | 22 | 31.7% | 10 | (84.2%) | 12 | (15.8%) | 0.009‡ |
| Positive | 38 | 68.3% | 12 | (29.3%) | 26 | (70.7%) | |
| M | |||||||
| M0 | 46 | 78.3% | 20 | (55.3%) | 26 | (44.7%) | 0.011‡ |
| M1 | 14 | 21.7% | 2 | (15.4%) | 12 | (48.6%) | |
| AJCC Stage group | |||||||
| Stage I | 10 | 17.6% | 6 | (75%) | 4 | (25%) | <0.001§ |
| Stage II | 12 | 20% | 10 | (76.5%) | 2 | (23.5%) | |
| Stage III | 24 | 30% | 4 | (22.2%) | 20 | (77.8%) | |
| Stage IV | 14 | 21.7% | 2 | (15.4%) | 12 | (84.6%) | |
| YKL40 | |||||||
| Low | 30 | 50% | 20 | (86.7%) | 10 | (13.3%) | <0.001‡ |
| High | 30 | 50% | 2 | (6.7%) | 28 | (93.3%) | |
Table 2: Correlations between clinicopathological data and SLUG immunohistochemical expression in our cases
Mann Whitney U test; ‡ Chi-square test; § Chi-square test for trend; p < 0.05 is significant.
Mann Whitney U test; ‡ Chi-square test; § Chi-square test for trend; p < 0.05 is significant.
| Characteristics | All | YKL40 | p-value | ||||
| Low (N = 30) | High (N = 30) | ||||||
| (N = 60) | |||||||
| No. | (%) | No. | (%) | No. | (%) | ||
| Age (years) | |||||||
| Mean ± SD | 66.38 | ±8.99 | 61.60 | ±8.01 | 59.50 | ±10.01 | 0.002 |
| Median (Range) | 67 | (43-77) | 54 | (42-75) | 55 | (49-77) | |
| ≤ 55 years | 20 | 40% | 15 | (75%) | 5 | (25%) | 0.008‡ |
| > 55 years | 40 | 60% | 15 | (27.8%) | 25 | (72.2%) | |
| Grade | |||||||
| Grade I | 15 | 25% | 12 | (75%) | 3 | (25%) | 0.007§ |
| Grade II | 25 | 35% | 10 | (76.5%) | 15 | (23.5%) | |
| Grade III | 20 | 30% | 8 | (22.2%) | 12 | (77.8%) | |
| ER | |||||||
| Negative | 20 | 30% | 3 | (4.2%) | 17 | (95.8%) | 0.004‡ |
| Positive | 40 | 70% | 27 | (75%) | 13 | (25%) | |
| PR | |||||||
| Negative | 25 | 35% | 5 | (4.2%) | 20 | (95.8%) | 0.007‡ |
| Positive | 35 | 65% | 25 | (75%) | 10 | (25%) | |
| ER/PR | |||||||
| Positive/Positive | 30 | 50% | 26 | (84.4%) | 4 | (15.6%) | 0.006§ |
| Positive/Negative | 4 | 6.7% | 0 | (0%) | 4 | (100%) | |
| Negative/Positive | 6 | 10% | 0 | (0%) | 6 | (100%) | |
| Negative/Negative | 20 | 33.3% | 4 | (5%) | 16 | (95%) | |
| HER2/neu | |||||||
| Negative | 40 | 70% | 27 | (77.1%) | 8 | (22.9%) | 0.002‡ |
| Positive | 20 | 30% | 1 | (4%) | 24 | (96%) | |
| Ki-67 | |||||||
| Negative | 20 | 30% | 18 | (87%) | 2 | (13%) | 0.003‡ |
| Positive | 40 | 70% | 12 | (21.6%) | 28 | (78.4%) | |
| Molecular type | |||||||
| Luminal A | 30 | 50% | 27 | (100%) | 3 | (0%) | 0.004‡ |
| Luminal B | 10 | 16.7% | 1 | (10%) | 9 | (90%) | |
| HER2 amplified | 18 | 28% | 0 | (0%) | 18 | (100%) | |
| Triple -ve | 10 | 17.7% | 2 | (20%) | 8 | (80%) | |
| T | |||||||
| T1 | 13 | 25% | 9 | (60%) | 4 | (40%) | 0.002§ |
| T2 | 25 | 38.3% | 15 | (65.2%) | 10 | (34.8%) | |
| T3 | 13 | 25% | 6 | (26.7%) | 7 | (73.3%) | |
| T4 | 9 | 11.7% | 0 | (0%) | 9 | (100%) | |
| N | |||||||
| N0 | 22 | 28.7% | 15 | (84.2%) | 7 | (15.8%) | 0.02§ |
| N1 | 10 | 17.3% | 7 | (54.5%) | 3 | (45.5%) | |
| N2 | 16 | 26.7% | 8 | (31.6%) | 8 | (68.4%) | |
| N3 | 12 | 18.3% | 0 | (0%) | 12 | (100%) | |
| Lymph node | |||||||
| Negative | 22 | 31.7% | 16 | (84.2%) | 6 | (15.8%) | 0.03‡ |
| Positive | 38 | 68.3% | 14 | (29.3%) | 24 | (70.7%) | |
| M | |||||||
| M0 | 46 | 78.3% | 26 | (55.3%) | 20 | (44.7%) | 0.021‡ |
| M1 | 14 | 21.7% | 4 | (15.4%) | 10 | (48.6%) | |
| AJCC Stage group | |||||||
| Stage I | 10 | 17.6% | 9 | (75%) | 1 | (25%) | <0.001§ |
| Stage II | 12 | 20% | 8 | (76.5%) | 4 | (23.5%) | |
| Stage III | 24 | 30% | 10 | (22.2%) | 14 | (77.8%) | |
| Stage IV | 14 | 21.7% | 3 | (15.4%) | 11 | (84.6%) | |
| SLUG | |||||||
| Low | 22 | 46.7% | 20 | (86.7%) | 2 | (13.3%) | <0.001‡ |
| High | 38 | 53.3% | 10 | (6.7%) | 28 | (93.3%) | |
Table 3: Correlations between clinicopathological data and YKL40 immunohistochemical expression in our cases.
Mann Whitney U test; ‡ Chi-square test; § Chi-square test for trend
Mann Whitney U test; ‡ Chi-square test; § Chi-square test for trend
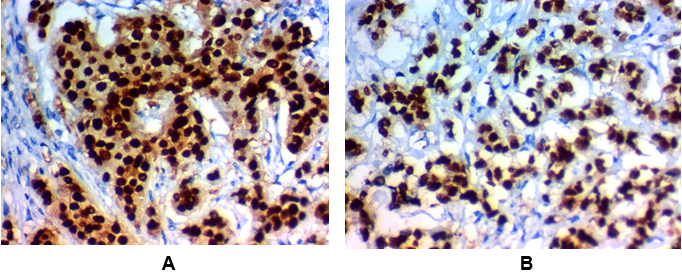
Figure 1: High SLUG expression in invasive carcinoma of the breast (IDC):
(A) High nuclear SLUG expression in grade III invasive duct carcinoma of the breast (NOS) stage IV x400.
(B) High nuclear SLUG expression in grade II invasive duct carcinoma of the breast (NOS) stage III x400.
(A) High nuclear SLUG expression in grade III invasive duct carcinoma of the breast (NOS) stage IV x400.
(B) High nuclear SLUG expression in grade II invasive duct carcinoma of the breast (NOS) stage III x400.
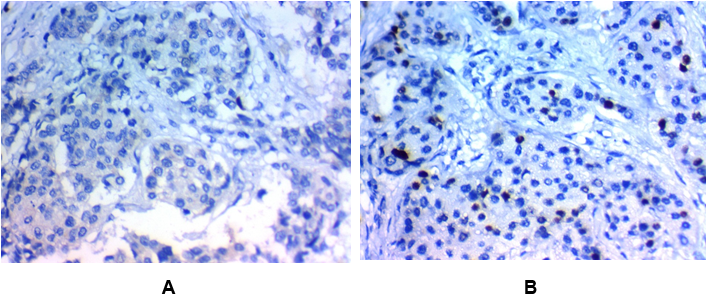
Figure 2: Low SLUG expression in invasive carcinoma of the breast (IDC):
(A) Low nuclear SLUG expression in grade II invasive duct carcinoma of the breast (NOS) stage IIx400
(B) Low nuclear SLUG expression in grade I invasive duct carcinoma of the breast (NOS) stage Ix400
(A) Low nuclear SLUG expression in grade II invasive duct carcinoma of the breast (NOS) stage IIx400
(B) Low nuclear SLUG expression in grade I invasive duct carcinoma of the breast (NOS) stage Ix400
SLUG high expression was found in 38 (53.3%) of IDC of the breast and its high tissue protein expression was associated with older age of the patients (p = 0.002), higher grade (p = 0.003), presence of lymph node metastases(p=0.009), advanced stage (p= < 0.001), presence of distant metastases (p = 0.011), negative ER (p = 0.004), & PR (p = 0.005) hormonal receptors positivity, aggressive molecular subtype, (p = < 0.001).
Interpretation of YKL-40 expression, as correlated to Clinicopathological and demographic findings of our patients
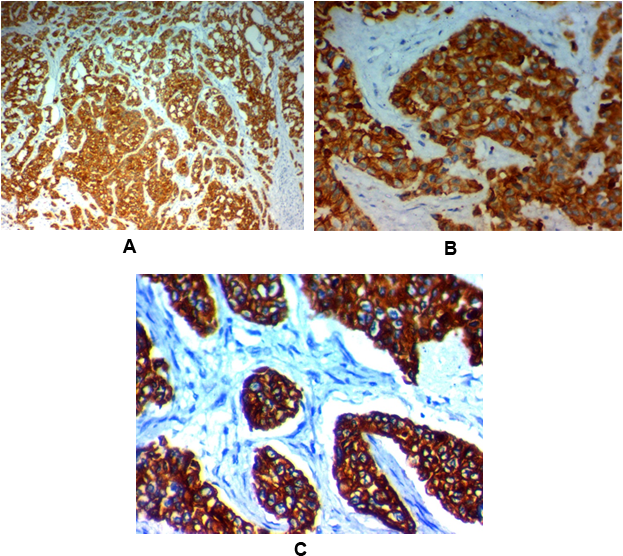
Figure 3: High YKL-40 expression in invasive carcinoma of the breast (IDC):
(A) High cytoplasmic YKL-40 expression in grade III invasive duct carcinoma of the breast (NOS) stage IV x100.
(B) High cytoplasmic YKL-40 expression in grade III invasive duct carcinoma of the breast (NOS) stage IV x400.
(C) High cytoplasmic YKL-40 expression in grade II invasive duct carcinoma of the breast (NOS) stage III x400.
(A) High cytoplasmic YKL-40 expression in grade III invasive duct carcinoma of the breast (NOS) stage IV x100.
(B) High cytoplasmic YKL-40 expression in grade III invasive duct carcinoma of the breast (NOS) stage IV x400.
(C) High cytoplasmic YKL-40 expression in grade II invasive duct carcinoma of the breast (NOS) stage III x400.
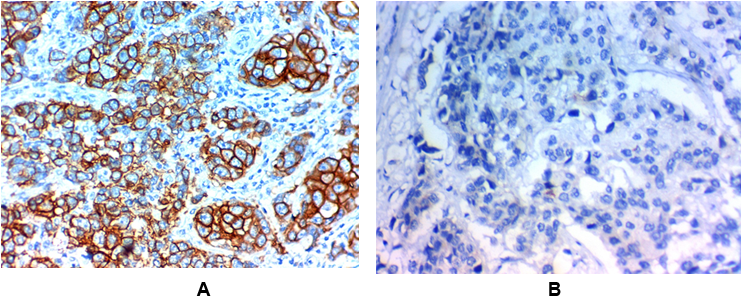
Figure 4: Low YKL-40 expression in invasive carcinoma of the breast (IDC).
(A) Low cytoplasmic YKL-40 expression in grade II invasive duct carcinoma of the breast (NOS) stage IIx400
(B) Low cytoplasmic YKL-40 expression in grade I invasive duct carcinoma of the breast (NOS) stage Ix400
(A) Low cytoplasmic YKL-40 expression in grade II invasive duct carcinoma of the breast (NOS) stage IIx400
(B) Low cytoplasmic YKL-40 expression in grade I invasive duct carcinoma of the breast (NOS) stage Ix400
YKL-40 high expression was found in 30 (60%) of IDC of the breast and its high tissue protein expression was associated with older age of the patients (p=0.008), higher grade(p=0.007), presence of lymph node metastases(p=0.03), advanced stage (p= < 0.001), presence of distant metastases(p=0.021), negative ER(p=0.004), & PR (p=0.007) hormonal receptors positivity, aggressive molecular subtype, (p=0.004).
Correlation between tissue protein expression of SLUG and YKL-40 in our cases
We found a significant positive association between SLUG and YKL-40 tissue protein expression in IDC of the breast. (Spearman’s r= +0.849), (p < 0.001)
We found a significant positive association between SLUG and YKL-40 tissue protein expression in IDC of the breast. (Spearman’s r= +0.849), (p < 0.001)
| SLUG | SLUG % | YKL-40 | YKL-40 % | |||||
| r | p-value | r | p-value | r | p-value | r | p-value | |
| Age (years) | +0.536 | 0.002 | +0.761 | <0.001 | +0.813 | 0.005 | +0.726 | <0.001 |
| Size | +0.625 | 0.005 | +0.629 | <0.001 | +0.670 | 0.003 | +0.747 | <0.001 |
| Grade | +0.544 | 0.003 | +0.755 | <0.001 | +0.650 | 0.004 | +0.728 | <0.001 |
| T | +0.634 | 0.004 | +0.657 | <0.001 | +0.760 | 0.008 | +0.795 | 0.005 |
| N | +0.732 | 0.008 | +0.682 | <0.001 | +0.743 | <0.001 | +0.833 | 0.003 |
| Stage | +0.890 | <0.001 | +0.763 | <0.001 | +0.768 | <0.001 | +0.862 | 0.004 |
| SLUG | --- | --- | --- | --- | +0.813 | <0.001 | +0.806 | 0.008 |
| SLUG (%) | --- | --- | --- | --- | +0.737 | <0.001 | +0.849 | 0.005 |
| YKL-40 | +0.737 | <0.001 | +0.849 | <0.001 | --- | --- | --- | --- |
| YKL-40 (%) | +0.726 | <0.001 | +0.849 | <0.001 | --- | --- | --- | --- |
Table 4: Correlation between SLUG, YKL41 expression with each other and clinicopathological parameters in our cases.
r -correlation coefficient
r -correlation coefficient
Discussion
Due to the seriousness of cancer breast it is essential to find novel prognostic markers for prediction of its prognosis and improving its management strategies', the first step in detection of recent therapeutic targets is studying molecular pathogenesis of such cancer type. The most frequently studied issue is EMT process that is responsible for invasion and metastases of various cancer types and it is controlled by plethora of transcription factors. We have assessed the expression of SLUG which was found to be important factor that is incriminated in induction of EMT.
We have found that SLUG high expression in IDC of the breast was associated with older age of the patients, larger tumor size higher grade, presence of lymph node metastases advanced stage, presence of distant metastases, negative ER& PR hormonal receptors, aggressive molecular subtype.
Similarly, results of previous studies Matysiak., et al. 2017, GRZEGRZOLKA., et al. 2015, El-Seaidy, 2015, Phillips& Kuperwasser, 2014 and Liu., et al. 2013, that found positive associations between poor clinco-pathological parameters of IDC of the breast patients in addition to presence of inverse correlations between SLUG and ER/PR hormonal receptors levels, significant correlations between SLUG and presence of axillary lymph nodes metastasis tumor stage, poor survival, higher incidence of tumor recurrence and distant metastasis.
Similar to our results in IDC of the breast many previous studies found similar results in tumors of other organs e.g. in lung cancer Bai., et al. 2017 demonstrated the association between SLUG expression and poor prognosis, in gastric cancer Lee., et al. 2017, demonstrated that Slug is a bas prognostic factor for high incidence of lymph node metastasis in patients even. There are many explanations of our results as Slug is found to act by suppression of the epithelial phonotype of cancer cells by inhibition and down regulation of E-cadherin through binding to E-box DNA sequence which leads to loss of cellular attachments, initiate EMT and leads to malignant cells invasion and metastases [Lee., et al. 2017]. Additionally, SLUG could be able to regulate levels of many other EMT transcription factors, like ZEB1.
And there are several signaling pathways which are associated with SLUG expression in IDC of the breast, such as WNT and NOTCH [Gonzalez DM, Medici D (2014)]. Moreover SLUG could be able to promote cancer initiation and progression by increasing resistance to apoptosis, by antagonizing the anti-apoptotic p53 protein activity [Liu., et al. 2013]. Interestingly, SLUG could be able to bind directly to the estrogen receptor α and inhibit their expression. Which leads to targeted hormonal therapy resistance [Gonzalez DM, Medici D, 2014], so anti SLUG targeted therapy might increase the sensitivity to hormonal therapy in hormonal receptor positive breast cancer cases.
A part from its role in EMT other studies clarified novel functions of SLUG which is totally different from EMT-TFs, as it is responsible for control of stemness in cancer stem cells (CSCs) [Luanpitpong., et al. 2016]. Luanpitpong., et al. 2016 observed the association between high levels of SLUG and aggressive lung cancer and they showed that SLUG knockdown could inhibit CSCs, that was in line with the previous report pointing to their role in cancer breast initiation and progression [Guo., et al. 2017], as results of Yao., et al. 2016 demonstrated that the association between SLUG expression and cancer progression mainly is due to activation of CSCs not only by activation of EMT, so Slug participates in cancer progression.
Consistent with results of previous studies, basal-type breast cancer which has elevated SLUG expression over-express CSCs genes e.g. CD133, BMI1, CD44 and CD24, but it is still unsure if SLUG provides cells with CSCs properties, or converts them into stem cells [Phillips1., et al. 2014].
We have explored the association between expression SLUG expression and another novel cancer biomarker that is YKL-40 which have been previously studied in breast cancer patients but the results are inconclusive. We have found that YKL-40 high tissue protein expression was associated with older age of the patients, larger tumor size higher grade, presence of lymph node metastases advanced stage, presence of distant metastases, negative ER& PR hormonal receptors, aggressive molecular subtype, (p < 0.001), similarly, results of Kim., et al. 2007 proved the association between YKL-40 overexpression and breast cancer patients dismal prognosis, but Roslind., et al. 2008 haven't prove any association between YKL-40 overexpression and patients prognosis.
Wan., et al. 2017 have assessed YKL-40 tissue expression in cancer breast and also they have performed a meta-analysis regarding its expression in other cancer types and they have found a significant positive association between YKL-40 high expression and dismal outcome of cancer patients. Moreover, Shao., et al. 2011 stated that YKL-40 over-expression levels are related to increased vascular invasion of breast cancer cells and Jefri., et al. 2015 stated that YKL-40 over-expression levels are related to dismal outcome of lung cancer patients.
Adding to our results Hao., et al. 2017 Özdemir., et al. 2012 have found that prostate cancer cells with high YKL-1 expression levels are more mobile and invasive than cells with low expression levels. Qin., et al. 2016 found the same results about the association of YKL-40 and poor prognosis in glioblastoma.
While many previous studies have found results similar to ours that high YKL-40 expression is associated with poor prognosis and might be a useful therapeutic targets for cancer patients, Roslind., et al. 2008 and Kim., et al. 2007 have found that high YKL-40 expression was associated with good prognosis as it is associated with low grade breast cancer, ER and PR positive expression and these are considered the standard good prognostic factors which point to a good prognosis. These variable results are due to different clones of primary antibodies used, different number of patients, and variable assessement method of YKL-40 expression used in these studies. There are any explanations for YKL-40 role in cancer initiation, progression and cancer cells proliferation [Low., et al. 2015].
First YKL-40 could promote angiogenesis in malignant cells by increasing vascular endothelial growth factor (VEGF) expression, second YKL-40 interact with syndecan-1 that is located on endothelial cells, third YKL-40 stimulates cancer cells invasion and metastasis by increasing production of pro-invasive substances like MMP-9 [Francescone., et al. 2011, Libreros and Iragavarapu-Charyulu 2015]. So, YKL-40 is considered a novel prognostic biomarker for IDC of the breast and might be considered an attractive targeted therapy [Kzhyshkowska., et al. 2 016].
We have found a positive correlation between SLUG& YKL-40 in IDC of the breast cancer and both markers are associated with poor clinicopathological parameters of the patients and these results may be explained by the role of both markers in induction of EMT.
Jefri., et al. 2015& Hao., et al. 2017 explained YKL-40 role of EMT induction that the key role in cancer progression malignant cells invasion and metastasis which leads to dismal prognosis.
Hao., et al. 2017 stated that YKL-40 leads to induction of EMT by increasing the expression of N-cadherin and Vimentin which are mesenchymal markers, Snail and Twist which are EMT inducers and it decreases the expression of E-cadherin which is epithelial markers like. Also, YKL-40 has an essential role in phosphatidylinositol 3 kinase (PI3K)/AKT/ mTOR cascade regulation, that is a central feature of EMT process [Hao., et al. 2017].
Summery and Conclusions
We have found high tissue protein expression of both SLUG and YKL-40 in IDC of the breast, and their high expression is related to poor clinicopathological parameters as higher grade and advanced stage. We have clarified our results by their roles in EMT initiation, malignant cells invasion and metastases which proved that therapeutic targets against both markers could be used as therapeutic targets for such fatal and common cancer.
Recommendations
We recommended performing future studies on large number of cancer patients and followed them to detect the prognostic roles of combined SLUG&YKL-40 expression in IDC of the breast.
We recommended performing future studies on large number of cancer patients and followed them to detect the prognostic roles of combined SLUG&YKL-40 expression in IDC of the breast.
References
- Azim HA., et al. “Analysis of PI3K/mTOR pathway biomarkers and their prognostic value in women with hormone receptor-positive, HER2-negative early breast cancer”. Translational Oncology 9 (2016): 114-123.
- Bai., et al. “The zinc-finger transcriptional factor Slug transcriptionally down regulates ERα by recruiting lysine-specific demethylase 1 in human breast cancer.” Oncogenesis 6 (2017): e330.
- Coates AS., et al. “Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015”. Annals of Oncology 26 (2015): 1533-1546.
- El-Seaidy AZ., et al. “Prognostic value of epithelial mesenchymal transition (EMT&Slug) markers in ductal carcinoma of the breast”. The Medical Journal of Cairo University 83 (2015): 1-11.
- Elston CW and Ellis IO. “Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up”. Histopathology 19 (2002): 403-410.
- Francescone RA., et al. “Role of YKL- 40 in the angiogenesis, radioresistance, and progression of glioblastoma”. The Journal of Biological Chemistry 286 (2011): 15332-15343.
- Fusetti F., et al. “Crystal structure and carbohydrate-binding properties of the human cartilage glycoprotein-39”. The Journal of Biological Chemistry 278 (2003): 37753-37760.
- Ghoncheh M., et al. “Incidence and Mortality and Epidemiology of Breast Cancer in the World.” Asian Pacific Journal of Cancer Prevention 17(2016): 43-46.
- Giuliano AE., et al. “Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual”. CA: A Cancer Journal for Clinicians 67.4 (2017): 290-303.
- Gonzalez DM and Medici D. “Signaling mechanisms of the epithelial-mesenchymal transition”. Science Signaling 7 (2014): 8.
- GRZEGRZOLKA J., et al. “Expression of EMT Markers SLUG and TWIST in Breast Cancer.” Anticancer Research 35: 3961-3968 (2015).
- Guo Y., et al. “Overexpression of enhancer of zeste homolog 2 (EZH2) and focal adhesion kinase (FAK) is associated with cancer metastasis and poor prognosis in breast cancer”. International Journal of Clinical and Experimental Medicine 10.2 (2017): 2672-2683.
- Hao H., et al., “YKL-40 promotes the migration and invasion of prostate cancer cells by regulating epithelial mesenchymal transition”. American Journal of Translational Research 9.8 (2017): 3749-3757.
- Hsu SM., et al. “Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures”. Journal of Histochemistry & Cytochemistry 29 (1981): 577-580.
- Jefri M., et al. “YKL-40 regulated epithelial-mesenchymal transition and migration/invasion enhancement in non-small cell lung cancer. BMC Cancer 15 (2015).
- Johansen JS., et al. “Plasma YKL-40: a potential new cancer biomarker?”. Future Oncology 5 (2009): 1065-1082.
- Kim S H., et al. “Prognostic implications of immunohistochemically detected YKL-40 expression in breast cancer”. World Journal of Surgical Oncology5 (2007): 17.
- Kzhyshkowska J., et al. “Role of chitinase-like proteins in cancer”. The Journal of Biological Chemistry 397 (2016): 231-247.
- Lamouille S., et al. “Molecular mechanisms of epithelialmesenchymal transition”. Nature Reviews Molecular Cell Biology 15.3 (2014): 178-196.
- Lee HH., et al. “Evaluation of Slug expression is useful for predicting lymph node metastasis and survival in patients with gastric cancer.” BMC Cancer 17 (2017): 670.
- Libreros S and Iragavarapu-Charyulu V. “YKL-40/CHI3L1 drives inflammation on the road of tumor progression”. Journal of Leukocyte Biology 98 (2015): 931-936.
- Liu T., et al. “Dysregulated expression of Slug, vimentin, and E-cadherin correlates with poor clinical outcome in patients with basal like breast cancer”. Journal of Surgical Oncology 107.2 (2013): 188-194.
- Low D., et al. “Chitinase 3-like 1 induces survival and proliferation of intestinal epithelial cells during chronic inflammation and colitis-associated cancer by regulating S100A9”. Oncotarget 6 (2015): 36535-36550.
- Luanpitpong S., et al. “SLUG is required for SOX9 stabilization and functions to promote cancer stem cells and metastasis in human lung carcinoma.” Oncogene 35.22 (2016): 2824–2833.
- Matysiak M., et al.“EMT promoting transcription factors as prognostic markers in human breast cancer”. Archives of Gynecology and Obstetrics 295 (2017): 817-825.
- Ozdemir E., et al. “Association of serum YKL-40 level with tumor burden and metastatic stage of prostate cancer”. Urology Journal 9 (2012): 568-573.
- Phillips S and Kuperwasser. “SLUG: Critical regulator of epithelial cell identity in breast development and cancer”. Cell Adhesion & Migration 8.6 (2014): 578-587.
- Prakash M., et al. “Diverse pathological implications of YKL-40: answers may lie in ‘outside-in’ signaling”. Cell Signaling Technology 25 (2013): 1567-1573.
- Qin G., et al. “Prognostic Value of YKL-40 in Patients with Glioblastoma: a Systematic Review and Meta-analysis”. Molecular Neurobiology (2016).
- Rehli M., et al. “Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation”. Genomics 43 (1997): 221-225.
- Roslind A., et al. “YKL-40 protein expression is not a prognostic marker in patients with primary breast cancer”. Breast Cancer Research and Treatment 112 (2008): 275-285.
- Shao R., et al. “Breast cancer expression of YKL-40 correlates with tumour grade, poor differentiation, and other cancer markers”. British Journal of Cancer 105 (2011): 1203-1209.
- Siegel RL., et al. “Cancer statistics”. CA: A Cancer Journal for Clinicians 65 (2015): 5-29.
- Uchikado Y., et al. “Slug Expression in the E-cadherin preserved tumors is related to prognosis in patients with esophageal squamous cell carcinoma”. Clinical Cancer Research 11.3 (2005): 1174-1180.
- Wan G., et al. “Elevated YKL-40 expression is associated with a poor prognosis in breast cancer patients”. Oncotarget 8.3 (2017): 5382-5391.
- Wang J., et al. “FOXC1 is associated with estrogen receptor alpha and affects sensitivity of tamoxifen treatment in breast cancer.” Cancer Medicine 6.1 (2017): 275-287.
- Yao C., et al. “IGF/STAT3/NANOG/Slug Signaling Axis Simultaneously Controls Epithelial-Mesenchymal Transition and Stemness Maintenance in Colorectal Cancer”. Stem Cells 34.3 (2016): 820-831.
Citation:
Mouhamed A Fouad., et al. “SLUG & YKL-40 Immunohistochemical Expression in Invasive Ductal Carcinoma of the Breast
(IDC); Clinicopathological Values”. Chronicle of Medicine and Surgery 2.4 (2018): 206-218.
Copyright: © 2018 Mouhamed A Fouad., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.