Review Article
Volume 1 Issue 2 - 2017
Medical Properties of Herbal Treated Textile Materials – Some Insights on Recent Research Trends
Department of Textile Technology, Park College of Engineering and Technology, Coimbatore, Tamil Nadu, India
*Corresponding Author: N Gokarneshan, Department of Textile Technology, Park College of Engineering and Technology, Coimbatore, Tamil Nadu, India.
Received: November 14, 2017; Published: December 14, 2017
Abstract
The article critically reviews the medical properties of herbal treated fabrics, based on recent research. In order to combat a broad spectrum of microorganisms that cause infections in wounds, new and innovative herbal extracts have been applied onto fabrics with encouraging results.
One such attempt is development of wound dressing with neem leaves and turmeric. The qualitative and quantitative analysis of the major bioactive constitutions of methanol extracts of Terminalia Chebulahas been studied, which prove their potential in resisting a wide range of bacteria. Eco friendly natural antifungal finish has been prepared from plant extract and applied on textile material. Antibacterial fabric has been developed using. Murraya koengii (curry leave) and Zingiber officinale (ginger) oil. It is also intended to produce eco-friendly antimicrobial cotton fabric and to protect the consumer from microorganism’s contamination. Certain plant extracts with good antimicrobial properties have been applied on cotton, bamboo, and soya bean fabrics and assessed for antimicrobial properties.
Keywords: Antimicrobial properties; Herbal extract; Textile material; Wound dressing; Agar diffusion
Introduction [T1]
The healing of a wound involves a number of stages. There are two phases involved- formation of glycosaminoglycan and formation of granulation tissue. Owing to its active ingredient which directly involves in the wound healing process, neem oil can be used as a wound healing agent. Fatty acids present in the neem plays an important role in adding moisture and a soft texture to the skin during the healing process. It also helps in the re-structuring of the skin during the wound healing process. Another commonly used herb readily available in all Indian household is turmeric. Turmeric contains a powerful active chemical compound called curcumin. This compound is not only responsible for its vibrant yellow-orange color and its distinctive zing, but is also the spice’s most powerful medical constituent bestowing it with powerful health benefits. Both laboratory and animal studies have provided evidence of the spice’s powerful anti-inflammatory activity. Textile substrates are widely used as wound dressing materials.
A wound dressing material treated with chitosan, which is effective against bacteria, will be an ideal material for wound care applications without losing their inherent textile characteristics. Textile substrates used as wound closing materials should act as reservoir of antimicrobial agents and should release them gradually at the affected site for a prolonged period of time [1]. Recently there are lot of attraction towards natural based herbs as antimicrobial agent because of their ecofriendly and health hazardless nature [2-9].
Bamboo fibre is a natural textile material that has acquired prominence during the past few years owing to its quality as well as its environmental friendliness. Cotton is the natural vegetable fiber of great economic importance as a raw material for cloth [10]. The use of textile in medicine has a long tradition. An important field of application is wound care and prevention of chronic wounds, in particular pressure sores. Among the long list of textile materials bandages and wound dressing gained great popularity.
Textiles and clothing are in permanent contact with microorganisms from the environment and the human skin [11]. Textile materials are good carriers of various types of microorganisms and can cause health related problems to the wearer. These micro-organisms create problems in textile, including discolouration, stains and fibre damage, unpleasant odour and a slick, slimy feel [12]. Textiles are rapidly developing into interdisciplinary high tech products. Textiles were considered primarily for economical and functional point of view to some end uses in particular for safety and for health [13]. Surveys indicate that functionality in the apparel product is necessary [14]. Antimicrobial property of fabric is an important parameter for applications such as health and hygiene [15].
Influence of neem extract loaded with curcumin
Neem leaf extract and seed have established antimicrobial effect and protects the wound from infection caused due to bacteria, viruses, parasites and fungi [17]. Medical trials reveal that neem also functions in wound healing by inhibiting inflammation and it can replace cortisone acetate [18]. Potency of turmeric’s anti-inflammatory and anti-arthritic effects have been equated with that of the popular pharmaceutical anti-inflammatory drugs such as motrin and hydrocortisone-without the potential side effects and toxicity of this manufacture drugs. Previous researchers [21] found that a daily dosage of curcumin was even more effective in easing post-surgical inflammation as the regular anti-inflammatory prescriptions.
Neem leaf extract and seed have established antimicrobial effect and protects the wound from infection caused due to bacteria, viruses, parasites and fungi [17]. Medical trials reveal that neem also functions in wound healing by inhibiting inflammation and it can replace cortisone acetate [18]. Potency of turmeric’s anti-inflammatory and anti-arthritic effects have been equated with that of the popular pharmaceutical anti-inflammatory drugs such as motrin and hydrocortisone-without the potential side effects and toxicity of this manufacture drugs. Previous researchers [21] found that a daily dosage of curcumin was even more effective in easing post-surgical inflammation as the regular anti-inflammatory prescriptions.
Recent research attributes turmeric’s anti-inflammatory mechanism to this powerful capacity to inhibit the activity of enzymes COX-2 and lipoxygenase [19]. It eases the inflammation caused by the body’ allergic reaction to histamines, as well as trauma, injury and the stiffness of over/or under–inactivity [17]. Turmeric is naturally anti-septic and anti-biotic that has historically been used as an herbal to treat everything from minor cuts and scrapes to scabies, skin and even leprosy. Turmeric’s essential oil is a powerful topical antibiotic that helps prevent infections and sepsis in wound [22]. Two types of wound dressing namely gauze-based dressings and paste bandages are normally used for wound dressing. The development in wound dressing takes place on account of moisture management and antibacterial finishing.
The hydrogel technology has been used in the treatment of major wound in the skin by means of development of new artificial wound coverings [16]. The wound colonization due to microorganisms and infections has caused delay in wound healing [17]. With burn wounds, frequent bacterial infections can cause the accumulation of dead tissues, compromised immune system and blood supply [18]. But in chronic wounds the presence of bacteria persist in an adhesive matrix biofilm form causes more resistant to antimicrobial therapy [19]. Acute and chronic wound infections lead to multidrug-resistant organisms thus compromising the chance of therapeutic options [20]. New approaches for the treatment of bio film-associated infections were developed by overcoming the intrinsic resistance of bacterial cell within biofilm. A research work using silver nano particles has revealed that the use of silver preparations leads to a new antimicrobial activity. It has been found that silver interferes with multiple components of bacterial cell structure and very much effective in wound dressing process [21].
Investigation on wound healing found that the property expected for good wound dressing material is to maintain a moist environment, contamination prevention, and oxygen permeation, absorb excess exudates, non-adherent to the wound and easily removable after treatment. For speedy wound healing wound dressings with hydrocolloids can be used [22].
Technical details
The wound dressing comprises of primary and wound contact layer which are joined by adhesives and then covered by releasable label. Primary layer is Spunbond-meltblown–spunbond (SMS) nonwoven white fabric primarily composed of polypropylene filaments. The filtration ability of the SMS fabric is considered to be good. Wound Contact layer is a spun lace nonwoven fabric composed of viscose and polyester. This material is placed on to the primary layer which helps to adhere in the wound [23].
The wound dressing comprises of primary and wound contact layer which are joined by adhesives and then covered by releasable label. Primary layer is Spunbond-meltblown–spunbond (SMS) nonwoven white fabric primarily composed of polypropylene filaments. The filtration ability of the SMS fabric is considered to be good. Wound Contact layer is a spun lace nonwoven fabric composed of viscose and polyester. This material is placed on to the primary layer which helps to adhere in the wound [23].
Two herbal components have been identified based on the fact that these components aid in the process of wound healing by providing an anti-microbial and anti-bacterial effect. The herbal components are extracted from neem and Curcumin as the above herbal components have been identified as the complementary constituents in promoting accelerated wound healing. All these components are in powder form, which are taken in known weights and are mixed thoroughly in lukewarm distilled water. There different solutions were prepared initially:
- 50%: 50% Neem: Curcumin
- 70%: 30% Neem: Curcumin
- 30%: 70% Neem: Curcumin
Various types of textile substrates primarily comprising of nonwoven fabric free from lint and hypoallergenic to skin have been considered. The wound contact layer reinforced on to the textile substrate is a liquid repellent nonwoven fabric. The contact layer is incorporated with desired composition of neem and curcumin extracts, the composition and method of application is carried out using padding mangle method. However several percentage of composition of neem and curcumin would be evaluated for its performance. The primary layer is given an adhesive coating on the side which adheres on to the skin. The wound contact side of the dressing is protected using release label. The wound dressing is a flexible adhesive based dressing which can be applied on to various contours of body. The wound contact layer of each dressing is protected from external environment with adhesive release paper. The dressing is proposed to be sealed and packed in a transparent pouch which allows the user to examine the dressing prior to application.
Testing of dressing layers
Very good results have been reported regarding the water repellency and spray impact test and moisture vapor permeability of the primary layer (Polypropylene produced by SMS technique) which could be attributed to polypropylene that has low moisture absorption. But the alcohol repellency was failure.
Very good results have been reported regarding the water repellency and spray impact test and moisture vapor permeability of the primary layer (Polypropylene produced by SMS technique) which could be attributed to polypropylene that has low moisture absorption. But the alcohol repellency was failure.
Wound dressing test
Rabbit has been used as the test specimen with three different concentration of wound dressing (figure). All three concentration samples exhibit good healing but the sample 1 gives better healing property [23].
Rabbit has been used as the test specimen with three different concentration of wound dressing (figure). All three concentration samples exhibit good healing but the sample 1 gives better healing property [23].
Antimicrobial activity test (Broth Dilution Test)
The technique has been used to determine the minimum concentration of extract necessary for inhibition of the growth of microorganisms [23]. Tests have been carried out for the samples of three different neem and curcumin concentrations. All the three concentrations have been tested on two organisms [Staphylococcus aureus and equilie]. All the concentrations studied showed better result because neem and curcumin has high anti-microbial activity.
The technique has been used to determine the minimum concentration of extract necessary for inhibition of the growth of microorganisms [23]. Tests have been carried out for the samples of three different neem and curcumin concentrations. All the three concentrations have been tested on two organisms [Staphylococcus aureus and equilie]. All the concentrations studied showed better result because neem and curcumin has high anti-microbial activity.
Assessment of terminalia chebula extract
The role of medicinal plants in the treatment of diseases has been recognized by the traditional Indian systems of Ayurveda and Siddha medicines [24]. During the new millennium, about 170 herbal drugs have been officially recognized in the U.S.P and N.F [25]. The Director of WHO Traditional Medicine reported in 1993 that 80% of the world population rely chiefly on traditional medicine, mainly plant based, especially for their primary health care needs [26]. In India 70% of populations is reported using traditional medicine for primary health care [27].
The role of medicinal plants in the treatment of diseases has been recognized by the traditional Indian systems of Ayurveda and Siddha medicines [24]. During the new millennium, about 170 herbal drugs have been officially recognized in the U.S.P and N.F [25]. The Director of WHO Traditional Medicine reported in 1993 that 80% of the world population rely chiefly on traditional medicine, mainly plant based, especially for their primary health care needs [26]. In India 70% of populations is reported using traditional medicine for primary health care [27].
The present annual turnover of herbal medicinal products manufactured by large companies is estimated to be approximately US $300 million, compared to a turnover of approximately US $2.5 billion for modern drugs [28]. Terminalia chebulais an important medicinal plant in Indian traditional medicine and it is most frequently used herb in Ayurveda. Terminalia chebulais a medium- to large-sized tree distributed throughout tropical and sub-tropical Asia, including China and Tibet [28]. This tree is found in the forests of northern India, Uttar Pradesh and Bengal, and is common in Tamil Nadu, Karnataka and southern Maharastra. Terminalia chebulais commonly known as black myroblans in English and harad in Hindi. The Terminaliaconsists of 250 species and widely distributed in tropical areas of the world [29]. The fruit of Terminalia chebulais considered as the "king of medicines" by Tibetans and second-to- none by ayurvedic apothecaries, and also held in high regard by other folk medicinal practitioners [30].
For the treatment of various diseases like fever, cough, diarrhea, gastroenteritis, skin diseases, candidiasis, urinary tract infection and wound infections Terminalia chebula is routinely used as traditional medicine in the name of ‘Kadukkaai’ by villages of Tamil Nadu in India [31]. Extracts from different parts of diverse species of plants like root, flower, leaves, seeds, that exhibit antibacterial properties were applied on cotton material for wound, healthcare care application [32,33]. It is a well-known fact that the demand for the herbal drug treatment of various ailments is increasing and plant drugs from the Ayurvedic system are being explored more, not only in India but also globally. As a result, in this study an attempt has been made to analyse the phyto-chemical properties of Terminalia chebulaby both quantitatively and qualitatively. The HPLC studies performed confirm the presence of active components objectively.
The in-vitro antibacterial studies of finished textile material were performed against wide spectrum of wound isolate pathogens and confirmed the potentiality of herb against them. The minimum inhibitory concentration studies were also conducted to identify the minimum concentration requirement of the drug.
Technical details
Terminalia chebulafruits have been shade dried and powdered and some specified quantity of powder is dissolved in methanol separately for 24 hours to obtain 20% concentrated solution, resulting in active substances being dissolved. The extract were filtered and used for phytochemical analysis and antimicrobial finishing. A specified concentration has been maintained. Qualitative phytochemical analysis of the ethanol extract has been done. The phytoconstituents have been identified by characteristic colour changes using standard procedure [34,35]. Quantitative determination of alkaloids, flavonoids, saponins, tannins were performed [36,37]. High Performance Liquid Chromatography (HPLC) fingerprints have been prepared using waters. Solvents were pre-filtered and analysis was performed. The methanol extracts of Terminalia chebula were injected in HPLC system.
Terminalia chebulafruits have been shade dried and powdered and some specified quantity of powder is dissolved in methanol separately for 24 hours to obtain 20% concentrated solution, resulting in active substances being dissolved. The extract were filtered and used for phytochemical analysis and antimicrobial finishing. A specified concentration has been maintained. Qualitative phytochemical analysis of the ethanol extract has been done. The phytoconstituents have been identified by characteristic colour changes using standard procedure [34,35]. Quantitative determination of alkaloids, flavonoids, saponins, tannins were performed [36,37]. High Performance Liquid Chromatography (HPLC) fingerprints have been prepared using waters. Solvents were pre-filtered and analysis was performed. The methanol extracts of Terminalia chebula were injected in HPLC system.
Plain knitted Cotton fabric was desired, scoured and bleached prior to the application of the antimicrobial finish. The methanol extract was applied to the cotton fabric by dipping in the bath with material to liquor ratio of 1:10 and then Pad-dried. Finally the fabric samples were tested for antimicrobial activity as per the AATCC test standards.
Bacterial cultures used in the investigations have been obtained from Microbial Type Culture Collection (MTCC). The bacterial strains Staphylococcus aureus, Escherichia coli Klebsiella pneumoniae, Proteus vulgaris, Salmonella typhi, B. licheniformis, M. luteus and Pseudomonas Sp., Corynebacterium Sphave been used. The treated and untreated fabric samples were placed in the AATCC bacteriostatic agar, which has been previously inoculated (Mat culture) with a test organism. After incubation, a clear area of uninterrupted growth underneath and along the side of the test material indicates the antibacterial effectiveness of the fabric. The area of the inhibition zone is a measure of antibacterial effectiveness of the material [38].
Macro broth dilution assay technique has been used to determine the minimal inhibitory concentrations (MICs) of the extracts of Terminalia chebulaagainst all the test strains. Two-fold serial dilutions of all the extracts (6.25 to 200μg/ml) were prepared in tubes with Nytrient Broth (Hi-media, Mumbai, India) as diluents. Each dilution was seeded with 40 μl of test micro-organisms to the standard concentration. Two-fold serial dilution of Tetracycline (6.25–200 μg/ml) was used as experimental positive control. The tubes were incubated at 37C for 24h. The least concentration of the extract or standard drug showing no visible growth was taken as the MIC [39,40].
Identification and Quantitative analysis of phytochemical components
The biological activities found in certain plants can be attributed to secondary metabolites that are chemical substances responsible for such activities. Major secondary metabolites like saponins, tannins, steroids, flavonoids, and so on, have been found during the phytochemical screening of the extracts of terminalia chebulla fruits.
The biological activities found in certain plants can be attributed to secondary metabolites that are chemical substances responsible for such activities. Major secondary metabolites like saponins, tannins, steroids, flavonoids, and so on, have been found during the phytochemical screening of the extracts of terminalia chebulla fruits.
Phytochemical studies have proved to be very beneficial for the assessment of certain active biological components of plants [40]. The qualitative and quantitative analysis of Terminalia chebula have been determined and represents the phytochemical components in methanol extract of Terminalia chebula. Important medicinal phytochemicals such as Tannin, saponins, flavonoids, phenol and glocosides were identified as major components of the Terminalia chebula. Quantitative evaluation of extracts showed that tannin content was highest at 10.90%, saponins at 3.32%, flavonoids at 0.68% while10.10% was obtained for phenols.
The HPLC spectrum of crude extract of Terminalia chebula has been obtained. Four significant peaks have been observed with the retention time of 3.06, 3.32, 3.79 and 4.3. Comparison has been made with the standards for three out of four peaks. The results show that the peaks at this retention times 3.32, 3.79 and 4.3 minutes were identified as Saponin, ascorbic acid and Gallic acid [41]. It has also documented the shigellocidal properties of Indian medicinal plants and identified the standard peak for Saponin as 3.31 min. which confirms the presence of Saponin content in the Terminalia chebula extract [42]. The presence of gallic acid confirms the phenolic compound is presence objectively.
In-vitroantimicrobial Studies
Depending on the presence of different human pathogenic bacterial strains in human skin as well as their presence in different kinds of wounds, in vitro antibacterial investigations have been conducted. A number of research investigations have been directed towards the isolates of wound pus and infection [43-46]. The National nosocomial infections surveillance report represents that, the Staphylococcus aureus was recorded as the highest isolation rate of 41% while E. coli recorded the least with 20% presence in the human wound [47]. The study by obi., et al. identified that, Klebsiella spp (25.4%) was the most prevalent organism in the wound samples, followed by Staphylococcus aureus with (24.1%); Pseudomonas spp was next, (18.2%), then Escherichia coli, with 1(18.0%) and finally Streptococcus pyogenes with the least percentage (14.1%) [48].
Depending on the presence of different human pathogenic bacterial strains in human skin as well as their presence in different kinds of wounds, in vitro antibacterial investigations have been conducted. A number of research investigations have been directed towards the isolates of wound pus and infection [43-46]. The National nosocomial infections surveillance report represents that, the Staphylococcus aureus was recorded as the highest isolation rate of 41% while E. coli recorded the least with 20% presence in the human wound [47]. The study by obi., et al. identified that, Klebsiella spp (25.4%) was the most prevalent organism in the wound samples, followed by Staphylococcus aureus with (24.1%); Pseudomonas spp was next, (18.2%), then Escherichia coli, with 1(18.0%) and finally Streptococcus pyogenes with the least percentage (14.1%) [48].
In a similar study elsewhere, Lucinda J Bessa., et al. [49] isolated 28 species from 217 infected wounds The most common bacterial species detected were Staphylococcus aureus (37%), followed by Pseudomonasaeruginosa (17%), Proteusmirabilis (10%), Escherichia coli (6%) and Coryne bacterium spp. (5%). It is possible that the type of environment and the state of the wound at any particular time influence the type and prevalent of organisms isolated from a given wound sample. In general, most of the wound isolates having Pseudomonas aeruginosa followed by Staphylococcus aureus, Klebsiella spp, E. coli, and Proteus sp., [50,51].
But, Staphylococcus aureus, Escherichia coli, Klebsiella pneumonia, Proteus vulgaris, Salmonella typhi, Pseudomonas Sp, Bacillus sp, Micrococcus sp and Corynebacterium sppare the abundant bacteria in wounds. Depending on this survey, to analyze the wound healing ability of the Terminalia chebulaextracts, the in-vitro antibacterial investigation has been conducted for the extract finished textile material by agar diffusion test as mentioned by SN 195920. The finding indicates the potential bacterial resistance by the extract of Terminalia chebula. The agar diffusion test zone of inhibition results have been determined.
The in-vitro antibacterial analysis of herbal extract finished textile indicates the strength on the wound healing ability of the selected herb. It could be attributed to the presence of the phytochemical compounds in the extract. The strong inhibition of treated cotton textile material against all selected microorganism has been observed. The colour change in the agar medium is due to the migration of extract material through the agar. The zone of inhibition should not be expected if the antimicrobial agent is firmly attached to the textile (e.g. covalently) which prevents its diffusion into the agar (in that case the antibacterial activity can be seen only below the fabric sample). If the antimicrobial agent can diffuse into the agar (in case not bonded), a zone of inhibition becomes apparent and its size provides some indication of the potency of the antimicrobial activity or the release rate of the active agent [52].
Among the phytochemical copounds especially alkaloids, saponins, tannins, phenol and flavonoids are known to have curative activity against several pathogens [53] Tannins have been reported to the inhibition of cell protein synthesis as well as production of typical tanning effect which is important in treating inflamed or ulcerated tissues, burns, wounds, pneumonia, and dysentery. Philips [54] reported that tannins and alkaloids are natural products that have medicinal like curing burn wounds to heal injury and cuts to stop bleeding. Moreover, it stop infections on the skin surface, internally tannins continue to heal the wound. In the case of third degree burns using strong tannins sources will not only prevent septicemia, but also helps to save life [55]. Flavonoids have been referred to as nature’s biological response modifiers, because of their inherent ability to modify the body’s reaction to allergies and virus and they showed their anti-allergic, anti-inflammatory, anti- microbial and anti-cancer activities [56].
The favonoids are potent antioxidants and exhibit various physiological activities including anti-infammatory, antiallergic, anticarcinogenic, antihypertensive, antiarthritic and antimicrobial activities [57] Steroids and triterpenoids showed the analgesic properties. Phenolic compounds are one of the most important groups of compounds occurring in plants [58].
Such compounds have been found to show anticarcinogenic, anti-inflammatory, antiatherogenic, antithrombotic, immune modulating and analgesic activities, among others and exert these functions as antioxidants [59].
Saponins are known to have antifungal properties. Saponins have been ascribed a number of pharmacological actions [60,61]. Saponins are believed to react with the cholesterol rich membranes of cancer cells, thereby limiting their growth and viability [62]. Mudi and Salisu also reported that tannins and saponins exhibit similar antibacterial activities [63]. Therefore, antibacterial activity showed in this present work may be due to tannins, flavonoids and saponins. The wound healing ability of the extract could therefore be attributed to saponins, flavonoids, alkaloids and tannins.
Investigations have been carried out on the FTIR spectrum of the Terminalia chebula treated and untreated samples. From the spectrum it is identified that the Terminalia chebula treated cotton fabric contain more carboxyl group [C (=O)-OH] due to the presence of active substance like gallic and ascorbic acid in their extract. The absorption in the region 3200 cm-1 to 3600 cm-1 and 1200 cm-1 to 1700 cm-1 of treated sample confirms the presence of -OH group stretching [40]. The presence of –(C=O)- group stretching also confirms the absorption, in the region of 1600 cm-1 to 1900 cm-1 in the treated sample. This stretching in 1760 cm-1 to 1670 cm-1 confirms the presence of ester group. The Gallic acid could react with cellulosic -OH group which resulted in the ester formation in the treated fabric. The presence of [C (=O)-OH] carboxyl group and – (OH) group confirms the presence of carboxylic acids (ascorbic and Gallic acid) and the presence of ester group proves the deposits of glycosides (Saponin) in the fabric.
Minimum inhibitory concentration (MIC) analysis
In in-vitro testing by disk diffusion test mentions the inhibition as size of the growth-free zone. The MIC number is the lowest concentration (in μg/mL) that inhibits the growth of a given strain of bacteria. The Minimum inhibitory concentration (MIC) studies were performed for the methanol extract of Terminalia chebulaagainst all wound isolate test strains.
In in-vitro testing by disk diffusion test mentions the inhibition as size of the growth-free zone. The MIC number is the lowest concentration (in μg/mL) that inhibits the growth of a given strain of bacteria. The Minimum inhibitory concentration (MIC) studies were performed for the methanol extract of Terminalia chebulaagainst all wound isolate test strains.
The chosen herb has been found to have the potential effectiveness against all bacterial strains. The findings show that minimum of 200 μg/ml of Terminalia chebulais extract is necessary for the inhibition of Corynebacteriumsp. [40]. In such manner, the findings show that growth of all the test organism have been inhibited by the chosen extract. Among the tested bacterial wound isolate pathogens, gram-positive bacterial strains have been found to be more susceptible than gram-negative bacterial strains. This may be attributed to the fact that the cell wall in gram-positive bacteria consists of a single layer, whereas, the gram-negative cell wall is a multilayered structure bounded by an outer cell membrane. Thus our findings support the traditional use of Terminalia chebulafruits against infections and treated fabric application for wound dressing.
Evaluation of anti-fungal activity
During the past few years bamboo fabric has gained prominence in terms of its quality and its environmental friendliness. Cotton which is a natural textile material has very good economic significance as a raw material for fabric. The use of textile in medicine has a long tradition. An important field of application is wound care and prevention of chronic wounds, in particular pressure sores [64]. Among the long list of textile materials bandages and wound dressing gained great popularity.
During the past few years bamboo fabric has gained prominence in terms of its quality and its environmental friendliness. Cotton which is a natural textile material has very good economic significance as a raw material for fabric. The use of textile in medicine has a long tradition. An important field of application is wound care and prevention of chronic wounds, in particular pressure sores [64]. Among the long list of textile materials bandages and wound dressing gained great popularity.
Nature has blessed mankind with medicinal plants that can cure limitless several diseases of human beings [65]. The abundance of plants on the earth surface has resulted in an increasing interest in the study of various extracts derived from the traditional medicinal plants as potential sources of new antimicrobial agents.
Some selective species of plants were identified and screened for their antifungal activities and applied on bamboo/cotton fabrics. As the fabric is subjected to washing, the wash durability of fungal finish are evaluated.
Technical details
The fabric used as the textile substrates is combed bamboo/cotton blended woven fabrics. The material is treated with soap at 50°C for 20 minutes to remove the dirt on the untreated fabric with water. The soap solution is added into water in suitable proportion. Then the material is given hot wash and cold wash [66]. The plant chosen for the study is michelia X Alba. The extract was tested for its antifungal activity by Aspergillus nigermethod. The fresh leaves have been collected from the suitable soil condition and then the leaves were dried under room temperature. Then the dried leaves were grinded in a powder form. For extraction, few gms of dry powder (michelia X Alba) was taken and mixed into Methanol. The container was closed and kept for overnight. The extract was finished on the fabric by pad dry cure method and tested for its antifungal activity.
The fabric used as the textile substrates is combed bamboo/cotton blended woven fabrics. The material is treated with soap at 50°C for 20 minutes to remove the dirt on the untreated fabric with water. The soap solution is added into water in suitable proportion. Then the material is given hot wash and cold wash [66]. The plant chosen for the study is michelia X Alba. The extract was tested for its antifungal activity by Aspergillus nigermethod. The fresh leaves have been collected from the suitable soil condition and then the leaves were dried under room temperature. Then the dried leaves were grinded in a powder form. For extraction, few gms of dry powder (michelia X Alba) was taken and mixed into Methanol. The container was closed and kept for overnight. The extract was finished on the fabric by pad dry cure method and tested for its antifungal activity.
After testing the sample by qualitative method and the result for Aspergillus niger zonewas analyzed. The herbal extract finished fabric shows good result.
The extraction was finished on the bamboo/cotton fabric using padding mangle. The fabric has been run through the three roll padding machine for few minutes. After padding, the sample has been dried and cured Antifungal activity of finished fabric has been done based on qualitative analysis of Herbal extract. The herbal extract finished sample shows better result in wash durability using Qualitative test method.
Evaluation of antifungal activity
The Antifungal test for Qualitative method was applied on herbal based method. The properties for Antifungal were tested by Aspergillus niger [66].It has been found that the bamboo/cotton sample showed good result.
The Antifungal test for Qualitative method was applied on herbal based method. The properties for Antifungal were tested by Aspergillus niger [66].It has been found that the bamboo/cotton sample showed good result.
Wash durability test
As can be seen from figure below the antifungal activity after laundering is found to be well improved.
As can be seen from figure below the antifungal activity after laundering is found to be well improved.
Herbally treated cotton fabric
Antibacterial agents can be applied on textile materials (fabrics) so as to protect the wearer from infection. Antimicrobial textiles with improved functionality finds a variety of applications such as health and hygiene products, specially the garments worn close to the skin and several medical applications, such as infection control and barrier material [67]. Herbal antimicrobial finish is one of the special finishes which can be applied to the textile material to protect the skin of the wearer and the textile substrate itself [68].
Antibacterial agents can be applied on textile materials (fabrics) so as to protect the wearer from infection. Antimicrobial textiles with improved functionality finds a variety of applications such as health and hygiene products, specially the garments worn close to the skin and several medical applications, such as infection control and barrier material [67]. Herbal antimicrobial finish is one of the special finishes which can be applied to the textile material to protect the skin of the wearer and the textile substrate itself [68].
There is a vast resource of natural antimicrobial agent/antimicrobial finish which can be used for imparting antimicrobial property to textile substrates [69,70]. Some of mostly used natural antimicrobial agents are clove, cardamom, curry leaves, neem, tulsi stem, leave, aloevera, etc. [71]. But most of them are carcinogenic. Several studies have been done on antimicrobial activity of cotton fabric treated with aloe gel extract, effect of laundering on herbal finishes, antibacterial treatment on cotton fabric from neem oil, aloe vera &tulsi etc. [72]. Based on the literature review, it was decided to apply the Murraya Koengii (curry leave) and Zingiber Officinale (ginger) oil on cotton fabric by pad-dry-cure method in two concentrations for assessment of antibacterial and antifungal activity.
Ginger is a natural antioxidant and anti-carcinogenic compound with antimicrobial properties whereas curry leaves are also naturally occurring antifungal agents which are non-carcinogenic. Some ginger compounds such as α-pinene, borneol, camphene and linalool are responsible for its antimicrobial activities [73]. Curry tree is a tropical tree with 2-4 cm long aromatic leaves. These leaves are often used in making dishes. The chemical components of curry leaves include linalool (32.83%), elemol (7.44%), geranylacetate (6.18%), mycrene (6.12%), allloocimene (5.02%), α-terpiene (4.9%), β-ocimene (3.68%) and nerylacetate (3.45%).
Technical details
Pure plain cotton fabric has been used for antimicrobial finishing. To improve the exhaustion rate of oil, acid desizing and scouring on grey cotton fabric was performed. The fabric was padded with acid solution at specified temperature and time duration followed by hot water washing to remove maximum amount of starch. Conventional scouring was carried out by using caustic soda, sodium carbonate, and Non-ionic emulsifier at defined temperature and time duration [74].
Pure plain cotton fabric has been used for antimicrobial finishing. To improve the exhaustion rate of oil, acid desizing and scouring on grey cotton fabric was performed. The fabric was padded with acid solution at specified temperature and time duration followed by hot water washing to remove maximum amount of starch. Conventional scouring was carried out by using caustic soda, sodium carbonate, and Non-ionic emulsifier at defined temperature and time duration [74].
Herbal antimicrobial finish was applied on fabric by pad-dry-cure method. Ginger and curry leave oil were treated with carrier oil to form solution of required concentrations. Four solutions were prepared. Padding treatment was given.
Most of the antibacterial agents work under two main principles: inhibition of the growth of the cells (biostatic) and killing of the cell (biocidal). This is a Qualitative test used to detect bacteriostatic activity on textile materials. The test Method determines antibacterial activity of diffusible antimicrobial agents on treated textile materials.
Evaluation of anti-bacterial activity of the Finished Fabric
The finished fabric samples with the diameter of 4.8 + 0.1 cm were taken for the analysis. Both the sides of samples were presterilized under steam flow for 15minutes. Sterile bacteriostasis agar was dispensed in sterile petridishes. Broth cultures (24 hours) of the test organisms were used as inoculums [74]. Using sterile cotton swab the test organisms (Klebsiella pneumoniae ATCC 4352 Staphylococcus aureus ATCC 6538) were swabbed over the surface of the agar plate. Presterilized samples were placed over the pre-swabbed agar surface by using sterile spatula. After placing the samples all the plates were incubated at 37ºC for 24 to 48 hours. After incubation the plates were examined.
The finished fabric samples with the diameter of 4.8 + 0.1 cm were taken for the analysis. Both the sides of samples were presterilized under steam flow for 15minutes. Sterile bacteriostasis agar was dispensed in sterile petridishes. Broth cultures (24 hours) of the test organisms were used as inoculums [74]. Using sterile cotton swab the test organisms (Klebsiella pneumoniae ATCC 4352 Staphylococcus aureus ATCC 6538) were swabbed over the surface of the agar plate. Presterilized samples were placed over the pre-swabbed agar surface by using sterile spatula. After placing the samples all the plates were incubated at 37ºC for 24 to 48 hours. After incubation the plates were examined.
Evaluation of anti-fungal property of the finished fabric
The antifungal activity of finished Fabric is analysed by qualitative method AATCC. The two purposes of this test method are to determine the susceptibility of textile materials to mildew, rot and to evaluate the efficacy of fungicides on textile materials [74].
The antifungal activity of finished Fabric is analysed by qualitative method AATCC. The two purposes of this test method are to determine the susceptibility of textile materials to mildew, rot and to evaluate the efficacy of fungicides on textile materials [74].
The fungus, Aspergillus niger, is grown on solid medium and a spore suspension is created after appropriate growth time. An inoculum of 1.0 ml was evenly distributed over the surface of the agar.
The specimens were incubated at a temperature of 28ºC for seven days. A constant condition with respect to temperature was maintained throughout the length of the experiment procedure. The samples were evaluated visually after 7 days.
Measured properties
Wicking property
Prior to testing, the samples were conditioned in a standard atmosphere of 20 ± 2ºC and 65 ± 2% relative humidity for 24 hours. Sample of size 2.5cm x 20cm each were cut from the conditioned sample. The samples were then mounted on the pinned frame such that 3 cm of the sample length was kept in immersed condition in reservoir containing distilled water [74]. A ruler was placed parallel to the sample strip to enhance the accuracy of the measurement. The wicking height of the advancing liquid front as a function of time was recorded by visual observation after 5 minutes. At least 10 samples were tested and the average value was taken.
Wicking property
Prior to testing, the samples were conditioned in a standard atmosphere of 20 ± 2ºC and 65 ± 2% relative humidity for 24 hours. Sample of size 2.5cm x 20cm each were cut from the conditioned sample. The samples were then mounted on the pinned frame such that 3 cm of the sample length was kept in immersed condition in reservoir containing distilled water [74]. A ruler was placed parallel to the sample strip to enhance the accuracy of the measurement. The wicking height of the advancing liquid front as a function of time was recorded by visual observation after 5 minutes. At least 10 samples were tested and the average value was taken.
Stiffness Test
A widely used method for determination of stiffness of fabric is cantilever test method. In this test, fabric specimen is allowed to bend under its own weight as the length of overhanging portion of specimen, is gradually increased [74]. The free length which bends under its own weight is sufficient to make its leading edge intersect with a plane of 41.5◦ inclination and is taken as the measure of stiffness. By this value of length, bending modulus and flexural rigidity was calculated.
A widely used method for determination of stiffness of fabric is cantilever test method. In this test, fabric specimen is allowed to bend under its own weight as the length of overhanging portion of specimen, is gradually increased [74]. The free length which bends under its own weight is sufficient to make its leading edge intersect with a plane of 41.5◦ inclination and is taken as the measure of stiffness. By this value of length, bending modulus and flexural rigidity was calculated.
Tear Strength Test
Elmendorf tear tester was used to test tear strength of finished and unfinished fabric. A pendulum is used to measure the force required to propagate an existing slit a fixed distance to the edge of the test sample [74].
Elmendorf tear tester was used to test tear strength of finished and unfinished fabric. A pendulum is used to measure the force required to propagate an existing slit a fixed distance to the edge of the test sample [74].
Thermal Conductivity Testing
Thermal conductivity is total heat transmitted through fabric per unit time with unit temperature difference. It is influenced by fabric/fibre properties, surface treatment, temperature and other factors [74]. Thermal properties were Guarded plate method was used to measure thermal conductivity.
Thermal conductivity is total heat transmitted through fabric per unit time with unit temperature difference. It is influenced by fabric/fibre properties, surface treatment, temperature and other factors [74]. Thermal properties were Guarded plate method was used to measure thermal conductivity.
Vertical wicking test
It has been found that the samples with oil finish do have much better wicking property as compared to plain fabric i.e water molecules are showing better adhesion to the coated yarns than plain ones [74]. It means fabric can easily hold water in the interstices of cloth and hence more absorbent spaces are formed. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between water molecules. Reason of this phenomenon which has occurred in our testing is dependent on capillary action as well as on sticky nature of water molecules. As the surface tension of oil coated yarns is more, it attracts the water molecules to adhere to its surface which causes the meniscus to flow upward. Every liquid aspires to reach at Max. Surface tension to stabilize, similarly water gets more attracted towards the coated yarn than plain one and hence wicking property of oil coated samples is more.
It has been found that the samples with oil finish do have much better wicking property as compared to plain fabric i.e water molecules are showing better adhesion to the coated yarns than plain ones [74]. It means fabric can easily hold water in the interstices of cloth and hence more absorbent spaces are formed. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between water molecules. Reason of this phenomenon which has occurred in our testing is dependent on capillary action as well as on sticky nature of water molecules. As the surface tension of oil coated yarns is more, it attracts the water molecules to adhere to its surface which causes the meniscus to flow upward. Every liquid aspires to reach at Max. Surface tension to stabilize, similarly water gets more attracted towards the coated yarn than plain one and hence wicking property of oil coated samples is more.
Tear strength test
There is no significant variation in stiffness of fabric. Although, results show that stiffness of fabric is increased with a minimal amount but it is not significant to affect the handle of fabric when wore on human skin. After analyzing results, it has been found that stiffness of fabric doesn’t very much in finished and unfinished fabric. Flexural rigidity and bending modulus of unfinished fabric is approximately similar to that of finished fabric [74].
There is no significant variation in stiffness of fabric. Although, results show that stiffness of fabric is increased with a minimal amount but it is not significant to affect the handle of fabric when wore on human skin. After analyzing results, it has been found that stiffness of fabric doesn’t very much in finished and unfinished fabric. Flexural rigidity and bending modulus of unfinished fabric is approximately similar to that of finished fabric [74].
The result in chart 3 shows tear strength of fabric increases after finishing. It took more force to tear the fabric samples in case of finished fabric than unfinished fabric. Tear strength of fabric increases as we increase the percent concentration of oil in fabric because, yarns slip over each other due to the presence of the oil on their surface and the force required to tear the fabric will be more.
Thermal conductivity
Thermal conductivity is an important factor in determining the fabric comfort properties [74]. The thermal conductivity of finished fabric is less as compared to unfinished fabric. During finishing the presence of oil in the fabric hinders the path for the free ions to move forward and hence thermal conductivity decreases which is a positive change for fabric as textile fabric is required to be an insulator.
Thermal conductivity is an important factor in determining the fabric comfort properties [74]. The thermal conductivity of finished fabric is less as compared to unfinished fabric. During finishing the presence of oil in the fabric hinders the path for the free ions to move forward and hence thermal conductivity decreases which is a positive change for fabric as textile fabric is required to be an insulator.
Test Results of Antimicrobial Activity
The growth of S Aureus and K Pneumonia has been compared on untreated and treated sample with different concentration of the ginger oil and curry leave oil [74]. It has been found that the growth of K pneumonia bacteria is more in all the samples as compared to S aureus. It is attributed that bacterial inhibition is due to the slow release of active substances from the fabric surface. Reduction in growth of bacteria can be seen in treated samples as compared to untreated sample 5. The fabric exhibited high antimicrobial property at 80 gpl concentration due to attachment of anti-microbial agent to the substrate through bond formation on the surface. Better results can be observed in sample treated with 100% curry leave oil.
The growth of S Aureus and K Pneumonia has been compared on untreated and treated sample with different concentration of the ginger oil and curry leave oil [74]. It has been found that the growth of K pneumonia bacteria is more in all the samples as compared to S aureus. It is attributed that bacterial inhibition is due to the slow release of active substances from the fabric surface. Reduction in growth of bacteria can be seen in treated samples as compared to untreated sample 5. The fabric exhibited high antimicrobial property at 80 gpl concentration due to attachment of anti-microbial agent to the substrate through bond formation on the surface. Better results can be observed in sample treated with 100% curry leave oil.
Herbally treated fabrics from cotton, bamboo and soya bean
In order to satisfy the needs of consumer textile products synthetic antimicrobial agents like triclosan, organometallics, phenols and quaternary ammonium compounds have been developed and they are also in the market. Even though synthetic antimicrobial agents are effective they have side effects. Hence, there is a need to develop antimicrobial textiles based on ecofriendly agents. Chitosan, a natural biopolymer is of great use in this area of research5. India a known biodiversity center of the world has more than 15,000 to 20,000 plants having medicinal value6. Extracts from different parts of these plants exhibit antimicrobial properties. Few of them act as bactericides and few act as bacteriostatic [75]. The present study aims to develop an ecofriendly natural antimicrobial finish from extracts of plants for application in textiles. Few selective species of plants were identified. The extracts were applied to Cotton, Bamboo and Soyabean fabrics and tested to assess their antimicrobial effectiveness by standard test methods (AATCC–147, 2004).
In order to satisfy the needs of consumer textile products synthetic antimicrobial agents like triclosan, organometallics, phenols and quaternary ammonium compounds have been developed and they are also in the market. Even though synthetic antimicrobial agents are effective they have side effects. Hence, there is a need to develop antimicrobial textiles based on ecofriendly agents. Chitosan, a natural biopolymer is of great use in this area of research5. India a known biodiversity center of the world has more than 15,000 to 20,000 plants having medicinal value6. Extracts from different parts of these plants exhibit antimicrobial properties. Few of them act as bactericides and few act as bacteriostatic [75]. The present study aims to develop an ecofriendly natural antimicrobial finish from extracts of plants for application in textiles. Few selective species of plants were identified. The extracts were applied to Cotton, Bamboo and Soyabean fabrics and tested to assess their antimicrobial effectiveness by standard test methods (AATCC–147, 2004).
Six types of herbs have been used in the finishing of Cotton, Bamboo and Soya bean fabrics and have been found to show significant antimicrobial properties. The Asteraceae treated Cotton and Bamboo fabrics have zero efficacy. However it has appreciable efficacy with respect to Soya bean fabric which may be due natural protein origin [76]. The present investigation highlighted that Lemon Grass Oil finished Cotton fabric shows highest efficacy in terms of both gram positive and gram negative bacteria indicating that this technique can be used in the textile industry as antimicrobial finishing of medical textiles for both the gram positive and gram negative bacteria as value added products.
Technical details
The yarn for the fabric is produced using ring spinning system with a blend combination of cotton and bamboo fibre (80/20 ratio), the fabric is produced in loom using the cotton–bamboo yarn as warp and weft [77]. The details are given blow The fabric samples were scoured with a solution of Sodium hydroxide 2%, Sodium carbonate 1%, Turkey red oil 1%, MLR ratio 1:30, at the boiling temperature for about 2 hours with a pH 10 -11. After that the fabrics were rinsed thoroughly and air dried at room temperature.
The yarn for the fabric is produced using ring spinning system with a blend combination of cotton and bamboo fibre (80/20 ratio), the fabric is produced in loom using the cotton–bamboo yarn as warp and weft [77]. The details are given blow The fabric samples were scoured with a solution of Sodium hydroxide 2%, Sodium carbonate 1%, Turkey red oil 1%, MLR ratio 1:30, at the boiling temperature for about 2 hours with a pH 10 -11. After that the fabrics were rinsed thoroughly and air dried at room temperature.
Herbs
The following natural herbs available in large quantity in Tamil Nadu region of India were selected for this study the names given in parenthesis are local names.
Asteraceae (Vettukaya Thalai),
Terminalia chebula (Kadukai),
Coleus aromaticos (Karpuravalli),
Aloe barbadensis (Aloe Vera),
Ocimum tenuiflorum (Tulsi)
Cymbopogon flexuosus (Lemon Grass Oil)
The following natural herbs available in large quantity in Tamil Nadu region of India were selected for this study the names given in parenthesis are local names.
Asteraceae (Vettukaya Thalai),
Terminalia chebula (Kadukai),
Coleus aromaticos (Karpuravalli),
Aloe barbadensis (Aloe Vera),
Ocimum tenuiflorum (Tulsi)
Cymbopogon flexuosus (Lemon Grass Oil)
Extraction of Medicinal Herbs
For extraction, 5 grams of dry powder from the herbs were taken separately and mixed into 120 ml of 80% Methanol 15. The container was closed and kept for overnight at room temperature. After overnight incubation, the extract was filtered through filter paper and evaporated to concentrate the extract.
For extraction, 5 grams of dry powder from the herbs were taken separately and mixed into 120 ml of 80% Methanol 15. The container was closed and kept for overnight at room temperature. After overnight incubation, the extract was filtered through filter paper and evaporated to concentrate the extract.
Finishing of Fabrics with Herbal Extracts
Methanol extracts of herbs were applied directly on 100% Cotton, Bamboo and Soya fabrics by pad-dry-cure method. The fabrics were padded with herbal extracts for 10min at room temperature with a liquor ratio set at 20:1 with a pressure of 20kgf/cm2 at a speed of 15 m/min. The padded fabrics were air-dried and then cured at 120.C for 3 min.
Methanol extracts of herbs were applied directly on 100% Cotton, Bamboo and Soya fabrics by pad-dry-cure method. The fabrics were padded with herbal extracts for 10min at room temperature with a liquor ratio set at 20:1 with a pressure of 20kgf/cm2 at a speed of 15 m/min. The padded fabrics were air-dried and then cured at 120.C for 3 min.
Assessment of Antimicrobial Activity of Finished Fabrics
Antimicrobial activity of 3 fabrics finished with 6 herbal extracts were evaluated by qualitative (AATCC 147) against S. aereus, a Gram-positive organism and Escherichia coli, a Gram-negative organism using nutrient agar15. The fabric samples cut into rectangular shape with 25* 50 mm was taken for the analysis [77]. Sterile bacteriostasis agar was dispensed in to Petri dishes. Broth cultures (24 hours) of the test organisms were used as inoculum. Using sterile inoculation loop, the test organisms (Escherichia coli & Staphylococcus aureus) were streaked, 5 lines with 4 mm width over the surface of the agar plate. Presterilized samples were placed over the culture inoculated agar surface by using sterile forceps. After placing the samples, all the plates were incubated at 37ºC for 18 to 24 hours. After incubation, the plates were examined for the zone of bacterial inhibition around the fabric sample. The size of the clear zone was used to evaluate the inhibitory effect of the sample.
Antimicrobial activity of 3 fabrics finished with 6 herbal extracts were evaluated by qualitative (AATCC 147) against S. aereus, a Gram-positive organism and Escherichia coli, a Gram-negative organism using nutrient agar15. The fabric samples cut into rectangular shape with 25* 50 mm was taken for the analysis [77]. Sterile bacteriostasis agar was dispensed in to Petri dishes. Broth cultures (24 hours) of the test organisms were used as inoculum. Using sterile inoculation loop, the test organisms (Escherichia coli & Staphylococcus aureus) were streaked, 5 lines with 4 mm width over the surface of the agar plate. Presterilized samples were placed over the culture inoculated agar surface by using sterile forceps. After placing the samples, all the plates were incubated at 37ºC for 18 to 24 hours. After incubation, the plates were examined for the zone of bacterial inhibition around the fabric sample. The size of the clear zone was used to evaluate the inhibitory effect of the sample.
Evaluation
The inoculated plates were examined for the interruption of growth along the streaks of the inoculums beneath the fabrics and for a clear zone of inhibition beyond the fabric edge. The average width of the zone of inhibition around the test specimen was calculated in mm using Eq.1.
Zone of inhibition MM) = (T-I)/2
Where:
T–Width of zone of inhibition, I–width of specimen
The inoculated plates were examined for the interruption of growth along the streaks of the inoculums beneath the fabrics and for a clear zone of inhibition beyond the fabric edge. The average width of the zone of inhibition around the test specimen was calculated in mm using Eq.1.
Zone of inhibition MM) = (T-I)/2
Where:
T–Width of zone of inhibition, I–width of specimen
The Antimicrobial Activity of Cotton Fabric
Antimicrobial activity of Cotton fabric treated with Asteraceae(Vettukaya Thalai), Terminalia chebula(Kadukai), Coleus aromatics (Karpuravalli), Aloe barbadensis(Aloe Vera), Ocimum tenuiflorum(Tulsi) and Cymbopogon flexuosus(Lemon Grass Oil) were determined using qualitative methods (AATCC 147) [77].
Antimicrobial activity of Cotton fabric treated with Asteraceae(Vettukaya Thalai), Terminalia chebula(Kadukai), Coleus aromatics (Karpuravalli), Aloe barbadensis(Aloe Vera), Ocimum tenuiflorum(Tulsi) and Cymbopogon flexuosus(Lemon Grass Oil) were determined using qualitative methods (AATCC 147) [77].
The results of agar diffusion method show that except the cotton fabric treated with Asteraceae (Vettukaya Thalai), all the other herbal treated cotton fabrics are having very good antimicrobial properties to both gram positive and gram negative micro-organisms. They reveal a zone of inhibition ranging from 25 mm to 60 mm for both gram positive and gram negative bacteria. Figure 1 indicates that there is a bacterial growth in the untreated cotton fabric.
The Antimicrobial Activity of Bamboo Fabric
It has been found that except the bamboo fabric treated with Asteraceae (Vettukaya Thalai), all the other herbal treated bamboo fabrics are having very good antimicrobial properties to both gram positive and gram negative micro-organisms [77]. They reveal a zone of inhibition ranging from 25 mm to 45 mm for both gram positive and gram negative bacteria. Figure 2 also indicates that there is a bacterial growth in the untreated bamboo fabric.
It has been found that except the bamboo fabric treated with Asteraceae (Vettukaya Thalai), all the other herbal treated bamboo fabrics are having very good antimicrobial properties to both gram positive and gram negative micro-organisms [77]. They reveal a zone of inhibition ranging from 25 mm to 45 mm for both gram positive and gram negative bacteria. Figure 2 also indicates that there is a bacterial growth in the untreated bamboo fabric.
The Antimicrobial Activity of Soya fabric
From the Figure 3 it is observed that except the soya fabric treated with Asteraceae (Vettukaya Thalai), all the other herbal treated bamboo fabrics are having good antimicrobial properties to both gram positive and gram negative micro-organisms. They reveal a zone of inhibition ranging from 25 mm to 30 mm for both gram positive and gram negative bacteria. Figure 3 also indicates that there is a bacterial growth in the untreated bamboo fabric [77].
From the Figure 3 it is observed that except the soya fabric treated with Asteraceae (Vettukaya Thalai), all the other herbal treated bamboo fabrics are having good antimicrobial properties to both gram positive and gram negative micro-organisms. They reveal a zone of inhibition ranging from 25 mm to 30 mm for both gram positive and gram negative bacteria. Figure 3 also indicates that there is a bacterial growth in the untreated bamboo fabric [77].
Conclusion
There is an increasing global awareness about hygiene products as dictated by modern life style. This provides scope for the growth of the textile market by satisfying the requirement through textile products finished with antimicrobial properties. The herbal textile wound dressing with neem and curcumin extract has a good scope of promoting the wound healing process since these possess anti-bacterial, anti-septic values. The dressings with neem and curcumin extract can serve as a primary first aid to minor wounds preventing from further infection. Fabric finished with ginger and curry leaf extracted essential oil shows very good antibacterial activity as compared to unfinished fabric. As the concentration of extract increased, its effectiveness against bacterial growth also increased. No negative effect was observed on physical properties of fabric after finish application. The effect of finish on antifungal activity was minimal, and other methods of finish application with different concentrations can be used for better results. There is a vast resource of natural antimicrobial agent, which can be used for imparting useful antimicrobial property to textile substrates.
The finishing of Cotton, Bamboo and Soya bean fabrics with six different herbs were found to exhibit appreciable antimicrobial properties. The Asteraceae treated Cotton and Bamboo fabrics have zero efficacy. However it has appreciable efficacy with respect to Soya bean fabric which may be due natural protein origin. The present investigation highlighted that Lemon Grass Oil finished Cotton fabric shows highest efficacy in terms of both gram positive and gram negative bacteria indicating that this technique can be used in the textile industry as antimicrobial finishing of medical textiles for both the gram positive and gram negative bacteria as value added products. Textile material treated with Terminalia Chebulaextract shows the potential inhibition against the most common wound pathogens in terms of agar diffusion technique. The herb treated textile material shows inhibition up to 37mm of maximum.
The Phytochemical analysis shows that, the presence of secondary metabolites viz., saponins, tannins, steroids, flavonoids and etc. These components are the major responsible factor for their antibacterial effect. The Minimum inhibition concentration studies were also performed against all test pathogens to measure the effectiveness of the selected herb. This may help in developing an effective alternative antimicrobial agent from plant origin in near future. Further in-vivo studies are needed to uncover the real time relevance of this plant material. michelia ×Albaherbal extracts treated fabrics are found to be eco-friendly, bio-degradable and non-toxic to the skin. The treated fabrics were found to be very hygienic with less fungus. These types of herbs can be used for medical textile also.
References
- Shanmugasundaram OL., et al. “Drug release and antimicrobial studies on chitosan-coated Cotton yarns”. Indian Journal of Fibre & Textile Research 31.4 (2006): 543-547.
- Kannan P., et al. “Antibacterial activity of Terminalia chebula fruit extract”. African Journal of Microbiology Research 3.4 (2009): 180-184.
- Mostafa GM., et al. “Antimicobial Activity of Terminalia Chebula”. International Journal of Medicinal Plants 1.2 (2011): 175-179.
- Sato Y., et al. “Extraction and purification of effective antimicrobial constituents of Terminalia chebulaRETS. Against methicillin-resistant Staphylococcus aureus”. Bio & Pharm Bulletin 20.4 (1997): 401-4.
- Iqbal Ahmad., et al. “Screening of some Indian medicinal plants for their antimicrobial properties”. Journal of Ethnopharmacology 2.2 (1998): 183-193.
- Malekzadeha F., et al. “Antibacterial activity of black myrobalan (Terminalia chebula Retz) against Helicobacter pylori”. International Journal of Antimicrobial Agents 18.1 (2001): 85-88.
- Jagtap AG and Karkera SG. “Potential of the aqueous extract of Terminalia chebulaas an anticaries agent”. Journal of Ethnopharmacology 1.3 (1999): 299-306.
- Lonchin Suguna., et al. “Influence of Terminalia chebula on dermal wound healing in rats”. Phytotherapy Research 16.3 (2002): 227-231.
- Phulan Rani and Neeraj Khullar. “Antimicrobial evaluation of some medicinal plants for their anti-enteric potential against multi-drug resistant Salmonella typhi”. Phytotherapy Research 18.8 (2004): 670-673.
- Mandoli KC., et al. “Handbook of cotton in India”. Mumbai First Edition 23 (1999).
- Testing for Antimicrobial Activity in Textiles – Quick Overview
- N Hein., et al. “A Study on the Effect of Antimicrobial Agent from Aloe Vera Gel on Bleached Cotton Fabric”. International Journal of Emerging Technology and Advanced Engineering 4.2 (2013): 7-11.
- Saraf NM and Alast DV. “Processing of Cotton Solution Providers – Sarex”, Colourage 52.11 (2005): 73.
- Gupta S “Smart Textiles – Their Production and Marketing Strategies “. National Institute of Fashion Technology New Delhi (2000).
- Sathianarayanan MP., et al. Indian Journal of Fibre & Textile Research (2010): 35-55.
- Bhuvanesh Gupta., et al. “Textile-based smart wound dressings”. Indian Journal of Fibre & Textile Research 35 (2010): 174- 187.
- Percival S., et al. “The antimicrobial efficacy of a silver alginate dressing against a broad spectrum of clinically relevant wound isolates”. International Wound Journal 8.3 (2011): 237-243.
- Bloemsma GC., et al. “Mortality and causes of death in a burn center”. Burns 34.8 (2008): 1103-1107.
- Abedini F., et al. “Comparison of silver nylon wound dressing and silver sulfadiazine in partial burn wound therapy”. International Wound Journal 10.5 (2013): 573-578.
- Rhoads DD., et al. “Biofilms in wounds: Management strategies”. Journal of Wound Care 17.11 (2008): 502-508.
- Lipsky BA and Hoey C. “Topical antimicrobial therapy for treating chronic wounds”. Clinical Infectious Diseases 49.10 (2009): 1541–1549.
- Bowler PG., et al. “Multidrug- resistant organisms, wounds and topical antimicrobial protection”. International Wound Journal 9 (2012): 387–396.
- Dhinakaran M., et al. “Detailed study on the synergistic effect of Neem extract loaded with curcumin in wound healing using textile substrate”. International Research Journal of Pharmacy 8.7 (2017): 104-109.
- Beusher N., et al. “Antiviral activity of African medicinal plants”. Journal of Ethnopharmacology 42.2 (1994): 101-109.
- Shavez ML., et al. “Herbs and other Dietary Supplements in the United States”. (2000):
- Akerele O. “Nature's Medicinal Bounty: don't throw it away”. World Health Forum 14.4 (1994): 390-395.
- “Regulatory Status of Herbal Medicines- A Worldwide Review”. WHO/TRM/98 I.
- Inamul Haq. “Safety of medicinal plants”. Pakistan Journal of Medical Research 43.4 (2004): 203-210.
- Ammar S., et al. “Inhibition of Cancer Cell Growth by Crude Extract and the Phenolics of Terminalia chebula Fruit”. Journal of Ethnopharmacology 81.3 (2002): 327-336.
- Karel DK., et al. “The Structural and Conformational analyses and antioxidant activities of Chebulinic acid and its thrice-hydrolyzed derivative 2,4-chebuloyl-ß-d-glucopyranoside,isolated from the fruit of Terminalia chebula”. ARKIVOC 7 (2004): 83-105.
- Dash B. “Materia Medica of Ayurveda”. B. Jain Publishers (1991): 170-174.
- Bag A., et al. “Evaluation of antibacterial properties of Chebulic myrobalan (fruit of Terminalia chebula Retz.) Extracts against methicillin resistant Staphylococcus aureus and trimethoprim-sulphametho-xazole resistant uropathogenic Escherichia coli”. African Journal of Plant Science 3.2 (2009): 25-29.
- Jothi D. “Experimental study on antimicrobial activity of cotton fabric treated with aloe gel extract from Aloe Vera plant for controlling the Staphylococcus aureus (bacterium)”. African Journal of Microbiology Research 3.5 (2009): 228-232.
- Harborne JB. “Phytochemical Methods - A guide to modern technique of plant analysis”. Chapman and Hall (1998): 271.
- Sofowara EA. “Phytochemical assays. In: Medicinal plants and traditional medicine in Africa”. Spectrum Book Ltd (1993): 150-163.
- Oloyed OI. “Chemical profile of unripe pulp of Carica pagaya”. Pakistan Journal of Nutrition 4.6 (2005): 379-381.
- Onwuka GI. “Food Analysis and Instrumentation theory and Paractice”. 1st Edn, Naphthali prints, Lagos. (2005): 114-169.
- Thilagavathi G and Kannaian T. “Combined antimicrobial and aroma finishing for cotton, using Microencapsulation of geranium leaves”. Indian Journal of Natural Products and Resources 1.3 (2010): 348-352.
- Anwesa Bag., et al. “Evaluation of antibacterial properties of Chebulic myrobalan (fruit of Terminalia chebula Retz.) Extracts against methicillin resistant Staphylococcus aureus and Trimethoprim-sulphamethoxazole resistant uropathogenic Escherichia coli”. African Journal of Plant Science 3.2 (2009): 25-29.
- Rathinamoorthy R and Thilagavathi G. “Characterisation and in-vitro evaluation of terminalia chebula extract for antibacterial potential”. International Journal of Pharmacy and Pharmaceutical Sciences 6.2 (2014): 932-938.
- Anjana shrama., et al. “Shigellocidal activity of some medicinal plants used in folklore remedies by tribal of Mahakoshal region of Central India”. Nat Prod Radiance 7.5 (2008): 426-436.
- Bayram Y., et al. “Three-year Review of Bacteriological Profile and Antibiogram of Burn Wound Isolates in Van, Turkey”. International Journal of Medical Sciences 10.1 (2013): 19-23.
- Garba I., et al. “Antibiotics Susceptibility Pattern of Pseudomonas aeruginosa Isolated from Wounds in Patients Attending Ahmadu Bello University Teaching Hospital, Zaria, Nigeria”. Nigerian Journal of Basic and Applied Sciences 20.1 (2012): 32-34.
- Sani RA., et al. “Antibiotic Resistance Profile of Gram Positive Bacteria Isolated from Wound Infections in Minna, Bida, Kontagora and Suleja Area of Niger”. State Journal of Health Sciences 2012; 2(l3): 19-22.
- Rajendra Gautam. “Antibiotic susceptibility pattern of bacterial isolates from wound infection in Chitwan Medical College Teaching Hospital, Chitwan, Nepal”. International Journal of Biomedical and Advance Research 4.4 (2013): 248-252.
- CDC. “National nosocomial infections surveillance report (NNISS) data summary”. American Journal of Infection Control 27 (1996): 510-532.
- Robert Kelechi Obi., et al. “Ethanolic extraction and phytochemical screening of two nigerian herbs on pathogens isolated from wound infections”. Pharmacie Globale 10.2 (2011): 1.
- Lucinda J Bessa., et al. “Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: some remarks about wound infection”. International Wound Journal 12.1 (2015): 47-52.
- Agnihotri N., et al. “Aerobic bacterial isolates from burn wound infections and their antibiograms- a five-year study”. Burns 30.3 (2004): 241–243.
- Shittu AO., et al. “A Study of Wound Infections in Two Health Institutions in Ile-Ife, Nigeria”. African Journal of Biomedical Research 5 (2003): 97-102.
- Yuan Gao. “Robin Cranston Recent Advances in Antimicrobial Treatments of Textiles”. Textile Research Journal 78 (2008): 60.
- Usman H., et al. “Qualitative phytochemical screening and in vitro antimicrobial effects of methanol stem bark extract of Ficus thonningii (Moraceae)”. The African Journal of Traditional, Complementary and Alternative medicines (AJTCAM) 6.3 (2009): 289-295.
- Philips L. Online Seminars for Municipal Arborists; Medicinal Properties of Trees (2010): 1-5.
- Aiyelaagbe OO and Osamudiamen PM. International Journal of ChemTech Research 46 (2000): 203-208.
- Middleton EJ and Kandaswami C. “The impact of plant favonoids on mammalian biology: implications for immunity, in ammation and cancer. In The Flavonoids. Advances in Research Science 1986 Ed”. Harborne, J.B London: Chapman & Hall (1994): 619-652.
- Bravo L. “Polyphenols: chemistry, dietary sources, metabolism andnutritional significance”. Nutrition Reviews 56.11 (1998): 317-333.
- Vinson JA., et al. “Phenol antioxidant quantity andquality in foods: Vegetables”. Journal of Agricultural and Food Chemistry 46 (1998): 3630-3634.
- Sun HX., et al. “Advances in saponin-based adjuvants”. Vaccine 27.12 (2009): 1787-1796.
- Setzer WN and Setzer MC. “Plant-derived triterpenoids as potential antineoplasticagents”. Mini Reviews in Medicinal Chemistry 3.6 (2003): 540–556.
- Fuchs H., et al. “Saponins as tool for improvedtargeted tumor therapies”. Current Drug Targets 10.2 (2009): 140-151.
- Roa RR., et al. “Saponins as anti-carcinogens”. The Journal of Nutrition 125 (3 Suppl) (1995): 717- 724.
- Mudi SY and Salisu A. “Studies on Brine Shrimp Lethality and Activity of Stem Bark Extract of Acacia Senegal on Respiratory Tract Pathogenic Bacteria”. International Journal of Biomedical and Health Sciences 5.3 (2009): 139-143.
- William Kemp. Organic Spectroscopy, Macmillan Education Ltd. (1987).
- Mucha H., et al. “Melliand International.” 8 (2002): 148-151.
- JR Aspland. “American association of the textile chemists and colorists.” 44 (2002): 72.
- Banupriya J and Maheshwari V. “Evaluation of anti-fungal activity of woven fabrics by herbal method”. World journal of pharmaceutical research 4.8 (2015) 1774.
- S Malpani. “Antibacterial Treatment on Cotton Fabric from Neem Oil, Aloe Vera &Tulsi” International Journal of Advance Research in Science and Engineering 2.7 (2013): 35-43.
- S Yadav. “A Study of Antimicrobial Properties of Fabric Treated with Silane and N- halamine Complex” doctoral dissertation, Eastern Michigan University (2013).
- S Kavitha and S Grace Annapoorani. “Aloe Vera Finish On Cotton and Organic Cotton Fabrics” Global Research Analysis 2.5 (2013): 104-105.
- S Sewani and M Qureshi. “Antimicrobial activity of Neem, Clove, Curry leaves, Cardamom, Tulsi stem and Tulsi leaves”. International Research Journal of Biological Sciences 5.1 (2016): 42-46.
- S Hooda., et al. “Effect of Laundering on Herbal Finish of Cotton”. International Journal of Textile and Fashion Technology 3.4 (2013): 35-42.
- D Jothi. “Experimental study on antimicrobial activity of cotton fabric treated with aloe gel extract from Aloe vera plant for controlling the Staphylococcus aureus (bacterium)”. African Journal of Microbiology Research 3.5 (2009): 228-232.
- Sa-Nguanpuag K., et al. “Ginger (Zingiber officinale) Oil as an AntimicrobialAgent for Minimally Processed Produce: A Case Study in Shredded Green Papaya”. International Journal of Agriculture & Biology 13 (2011): 895-901.
- Jaswal P., et al. “Antimicrobial activity of herbal treated cotton Fabric.” International Research Journal of Engineering and Technology 4.8 (2017): 39-43.
- N Hein., et al. “A Study on the Effect of Antimicrobial Agent from Aloe Vera Gel on Bleached Cotton Fabric”. International Journal of Emerging Technology and Advanced Engineering 4.2 (2013): 7-11.
- Jaswal P., et al. “Antimicrobial activity of herbal treated cotton Fabric”. International Research Journal of Engineering and Technology 4.8 (2017): 39-43.
- Thangamani K and Periasamy R. “Study on antimicrobial activity of cotton, bamboo and soyabean fabrics with herbal finishing.” International research journal of pharmacy 8.5 (2017): 115-119.
Citation:
N Gokarneshan., et al. “Medical Properties of Herbal Treated Textile Materials – Some Insights on Recent Research Trends”.
Chronicle of Medicine and Surgery 1.2 (2017): 51-66.
Copyright: © 2017 N Gokarneshan., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































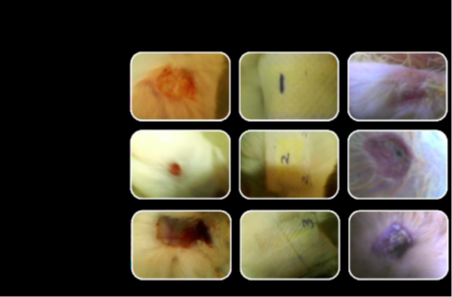


 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.